Abstract
Many fish species use acoustic signals for various purposes, with sciaenids being among the best-known vocal teleosts. Although advertisement and disturbance calls have been well studied, the dual-pulse call (or dual-knock) has been reported infrequently. Here, we recorded dual-pulse sounds in five South Atlantic sciaenid species and analyzed their acoustic features from four species in captivity and one in the wild. These stereotyped calls are emitted by both free-swimming and stationary individuals, often without apparent social interactions, although they are routinely elicited by a human feeder. The occurrence of this call type across geographically distant species in South America, North America, and Asia suggests it may represent a basal trait within the family, potentially serving important but yet undetermined functions.
Similar content being viewed by others
Introduction
Sound production is a rapid and effective means of communication in fishes1 and plays an important role both in species identification by researchers (e.g., through species-specific acoustic signatures) and in species recognition among fish, especially during social and reproductive interactions2,3,4,5,6,7,8,9,10,11,12. In a number of teleost families, such as Batrachoididae, Gadidae, Holocentridae, Sciaenidae, Pomacentridae, Ophidiidae, and Gobiidae, sound production is associated with agonistic and reproductive behaviour13,14,15,16,17,18.
Teleosts utilize a variety of mechanisms to produce sounds, including muscular vibration of the swim bladder19, expulsion of gas from the swim bladder20, muscular vibration of the peritoneum21, and stridulation (rubbing together of hard body parts such as incisor teeth, pharyngeal teeth, or fin spines1,22,23. The teleost family Sciaenidae, commonly referred to as croakers and drums due to their characteristic sound production, comprises approximately 70 genera and 270 species worldwide (Chao, 1986; Nelson, 2016)24,25. Sound production in sciaenids has been known since before the last century19,26, often in association with reproduction6,10,27,28,29,30,31,32,33,34,. Sciaenids have a diversity of sound production mechanisms, varied sounds, and structural variation in sound-detecting structures. Indeed, the swim bladders and large-sized otoliths of many sciaenids differ with other families35,36,37. They produce sounds by contracting specialized extrinsic sonic swimbladder muscles that originate on an aponeurosis in most species although intrinsic muscles that attach exclusively to the bladder occur in black drum Pogonias cromis and southern black drum Pogonias courbina, Atlantic croaker Micropogonias undulates and several other species2,38,39,40,41,42,43. Acoustic time series during seasonal reproductive periods demonstrate that quantitative patterns in sciaenid calling correlate with spawning condition10,28,31,33,44,45,46,47,48,49,50,51. Positive correlations between sound production and courtship have been documented for many sciaenids through the simultaneous collection of eggs and acoustic recordings10,28,29,31,33,35,42,47,52,53,54,55,56,57,58, and these sounds aid communication in turbid estuaries where water visibility is often minimal59. Sciaenids have provided a major focus for the field of passive acoustics using the advertisement call for many species of this family28,29,45,58,60,61,62,63.
Sciaenids emit disturbance calls, (e.g., purrs or staccatos)10,35 with different numbers of pulses that vary more widely than in the advertisement call. The disturbance call often consists of a long sequence of pulses with short intervals, resulting in a distinctive “burst” of sound10. The pulses of the advertisement calls have longer interpulse intervals, suggesting that disturbance situations result from more rapid pacing of pattern generators in the central nervous system than used for courtship vocalizations10,64.
Despite the family’s diversity, acoustic characteristics are known for only a small subset of sciaenids35. Identity of field-recorded sounds have been verified using voluntary sounds recorded in captivity from a small number of sciaenids from North and South America, Europe, Asia and Australia and include weakfish Cynoscion regalis, speckled trout Cynoscion nebulosus, Atlantic croaker Micropogonias undulatus, whitemouth croaker Micropogonias furnieri, red drum Sciaenops ocellatus, black drum P. cromis and Southern black drum P. courbina from the Atlantic coast10,22,29,33,42,52,65,66, the orangemouth corvina Cynoscion xanthulus and white seabass Atractos cionnobilis from the Pacific coast53,60,67, the meagre Argyrosomus regius and the shi drum Umbrina cirrosa from Europe34,68, Japanese croaker Argyrosomus japonicus and black spotted croaker Protonibea diacanthus, big-snout croaker, Johnius macrorhynus from Taiwan45,69,70 and A. japonicus from Australia71.
Most passive acoustics studies in fishes focus on sounds of a single species or conversely record unidentified and unseen species in a relatively unexplored habitat.
The current study, recording the same call from five species from a single family within a relatively restricted area, is therefore novel. We hypothesize that differences in the calls may aid in species recognition although this remains to be tested.
Acoustic communication is widespread among sciaenid fishes, with several species known to produce distinct call types such as rhythmic advertisement calls during reproduction and rapid burst-type disturbance calls (references above). However, little is known about vocalizations that occur in non-reproductive social contexts, especially in environments where multiple closely related species coexist. In this context, we examine a dual-pulse call — characterized by two closely spaced pulses repeated at irregular intervals — which has been rarely described in the literature and appears to differ markedly from the known sciaenid call types28,57,70. This study aims to investigate the presence and characteristics of this dual-pulse call across five sympatric sciaenid species, using recordings obtained both in captivity and in the field in previous studies by Tellechea and colleagues. We hypothesize that interspecific differences in the acoustic structure of this call are significant and may reflect species-specific traits.
Materials and methods
Sound recordings and tank experiments
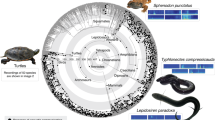
The dual-pulse call (or dual-knock) was analyzed in five sciaenid species. Sounds were obtained from recordings from previous studies published between 2010 and 2022: the whitemouth croaker Micropogonias furnieri10, striped weakfish Cynoscion guatucupa46, Argentine croaker Umbrina canosai72, southern king weakfish Macrodon atricauda57, and southern black drum P. courbina42,43 (see Fig. 1), all of which inhabit the Río de la Plata estuary and the adjacent Atlantic coast of Uruguay. Full details of the recording methods for each species are available in the original publications.
Sound recordings were conducted either in the field, in large tanks aboard a trawler, referred to as captivity, or both, depending on the species. Sounds were obtained from recordings from previous studies published between 2010 and 2022, as cited throughout the Methods section. Therefore, full technical and contextual details for each species are available in the original publications. Below, we summarize the recording protocols relevant to the comparative analysis. Specifically, for M. furnieri, C. guatucupa, and U. canosai, recordings were first made in the field at known spawning grounds, followed by complementary recordings in onboard tanks. For P. courbina and M. atricauda, recordings were conducted both from shore or small boats in the field and subsequently in tanks under controlled conditions. In these latter cases, fish were captured using hand nets and recreational fishing methods, as previously described42.
For M. furnieri, C. guatucupa, and U. canosai, recordings were taken at artisanal fishing grounds in the Río de la Plata estuary and coastal Atlantic waters (for M. furnieri 34º55′01″S, 56º26′06″W; for C. guatucupa 34º54′17″S, 55º53′01″W; and for U. canosai 34º47′44″S, 54º10′50″W), as well as aboard the research vessel R.V. Aldebaran (operated by DINARA, the Ministry of Cattle Ranching, Agriculture and Fisheries of Uruguay). Sounds were first recorded in known spawning areas with the vessel engine turned off, using a hydrophone deployed at depths between 6 and 12 m for 2 h. Immediately afterward, a 20-minute trawl was conducted at 5–6 km/h (3 knots) to confirm the identity of the calling species and to collect specimens of M. furnieri, C. guatucupa, and U. canosai for each study10,46,72. Captured fish were transferred to a 3000 L onboard canvas tank filled with seawater for sound recordings and behavioral observation over a period of five days. Water temperature and salinity in the tanks were maintained at field conditions, typically 23 °C and 20‰, respectively10,46,72. Fish were allowed to acclimate for two hours before recording. Fish were then sacrificed by the crew of the fishing vessel for macroscopic gonadal inspection and sex determination, using a five-stage maturity scale adapted for sciaenid species in the Southwestern Atlantic73 and consistently applied in previous studies10,46,71. For P. courbina and M. atricauda, specimens were obtained from artisanal and recreational fishers who collaborated in this study. These fishers carried out the sacrifice of the fish, allowing us to collect samples for sex and gonadal state determination42,57,73. After sampling, the fish were returned to the fishers for commercial use.
Acoustic recordings of P. courbina and M. atricauda were obtained from the shore and from a small non-motorized rowboat in known spawning areas. Fish were captured using small nets from the shore or with the help of recreational fishers using fishing rods. After capture, individuals were transferred to holding tanks. For P. courbina, recordings were conducted along two coastal sites: the Atlantic Ocean coast at Punta del Este (34°58′S, 54°57′W), and the lower Santa Lucía River estuary near Montevideo (34°46′S, 56°25′W). This species is the largest sciaenid in the Atlantic coast and Rio de la Plata estuary. The maximum size recorded in P. courbina was 117 cm in length, with a maximum weight of 48.1kg74. Due to their large sizes specimens could only be kept in captivity for a few minutes, and the dual-pulse was not recorded in captivity42,43.
For M. atricauda, recordings were made along the Río de la Plata estuarine coast at El Pinar, Canelones Department (34°50′S, 55°55′W). The hydrophone was lowered from the small boat to depths between 1.5 and 2 m for 2 h. Immediately after the recordings, a hand net was used to capture individuals at the same location to verify the identity of the calling fish. Afterward, fish were transferred to a 1000 L tank filled with seawater, where they acclimatized for two hours before sound production.
However, despite being able to identify the calling species through post-recording fishing, the high overlap of calls produced by multiple individuals in natural aggregations prevented extracting reliable acoustic measurements at the individual level from field recordings. Therefore, the analyzed sounds possibly represent the acoustic repertoire of the species, but in situ verification was not performed, and detailed comparisons between captivity and the wild were not feasible in this study.
In the case of P. courbina, water temperature in the tanks ranged from 18 to 24 °C, and salinity from 20 to 27‰. These variations reflect studies conducted at different locations and different times of the year, specifically in October and January42. For M. atricauda, water temperature and salinity in the tanks were maintained close to field conditions, ranging between 22 and 24 °C and 20 to 23‰, respectively.
Since all research involving these species was conducted under the authority of the Ministry of Cattle Ranching, Agriculture and Fisheries of Uruguay, where the fish were collected as part of the protocol of the research fishing vessel, the Animal Care Committee of Uruguay (Honorary Commission for Animal Experimentation, CHEA) approved the scientific collection and handling of the animals, in compliance with the Uruguayan Animal Experimentation Ethics Committee. All methods are reported in accordance with ARRIVE guidelines.
For M. furnieri, P. courbina and C. guatucupa recordings were made with a calibrated omnidirectional hydrophone built in the laboratory (sensitivity of −40 dBre: 1µPa and linear frequency response from 20 Hz to 60 kHz). For U. canosai and M. atricauda recordings were made with a calibrated omnidirectional Aquarian hydrophone H1a (Useful range: <1 Hz to > 100 kHz, 100 kHz, sensitive of − 220 dB re: 1 V/µPa). M. furnieri were recorded with an analog JVC model Super ANRS/Coreless Motor Portable Stereo Cassette Deck/KD-2). In the other three species recordings were made on a digital TASCAM HDP2 recorder at a sampling rate of 44.1 kHz.
Acoustics and statistical analysis.
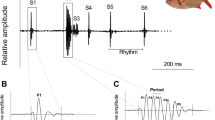
Sounds were analyzed using Audacity free software, vers. 1.2.375 and Raven Pro, Version 1.676. Spectrograms of representative dual-pulse calls were generated using the power spectrum from a 1024-point FFT with a Hanning window to illustrate the calls’ temporal and spectral structure. We qualitatively describe the number of cycles per pulse and their amplitude patterns across species (Fig. 1). These observations are for illustration only and were not included in the statistical analyses. The following acoustic parameters were measured: pulse duration, dominant frequency, the interval between the two pulses (Interval between paired pulses see Fig. 1), and the interval between successive dual pulses (Inter dual pulse time see Fig. 1).
This figure is a schematic representation of the acoustic variables; the precise definitions of pulse duration, interval between paired pulses, and inter dual pulse time are provided in the Materials and Methods section.
Descriptive statistics were performed to provide mean, standard deviation, maximum and minimum values (Fig. 1; Table 1). ANOVA followed by a Tukey post hoc test was used to compare species. To test whether the data are parametric, the Levene test for homogeneity of variances was used77. The critical ɑ level was 0.05, using PAST (version 1.95) a free statistical software package78.
Results
Dual-pulse call characteristics of each species
Dual pulse calls were recorded in the wild in all five species and in four of them in large tanks. P. courbina was only recorded in the wild, as its large size made it impractical to maintain individuals in captivity for acoustic recordings. C. guatucupa, U. canosai and M. atricauda have sonic muscles in males exclusively39,57,72, and thus their sounds are assumed to be generated by males whereas M. furnieri and P. courbina have muscles in both sexes.
Whitemouth croaker micropogonias furnieri
Dual-pulse calls were recorded on the coast of the Rio de la Plata and the mouth of the Pando stream (Pinar-Canelones, location on the Uruguayan coast). Dual pulses were recorded throughout the year but with greater frequency of occurrence and intensity during the reproductive season. This sound was also recorded at the mouth of the Pando stream outside the reproductive season (October to March).
In the large tank, M. furnieri (7 females and 3 males; male total length: 22, 26, and 27 cm) emitted the dual-pulse sound without any obvious stimulus. Because calls were recorded in a mixed-sex group, it could not be determined whether females also contributed to sound production. To further assess this, males and females were subsequently separated and placed in individual 100 L tanks. In these conditions, only males produced the dual-pulse call, beginning approximately 30 min after isolation. No dual-pulse sounds were recorded from isolated females. Dual pulses had interpulse intervals averaging 1.7 ± 1.1 s. The average duration of pulse one and pulse two was 64 ± 0.1 ms and 68 ± 0.2 ms, respectively. The interval between pulse one and two (i.e., the interval between paired pulses) was 26 ± 2.9 ms, and the dominant frequency was 344 ± 0.6 Hz. Data were obtained from 11 calls (Table 1; Fig. 2), and the measured acoustic parameters are summarized in Fig. 1.
Oscillograms (above) and spectrograms (below) of dual-pulse calls from five sciaenid species: M. furnieri, C. guatucupa, U. canosai, M. atricauda, and P. courbina. Insets show an expanded view of a single pulse. For M. furnieri and C. guatucupa, each pulse consists of 2–3 cycles, with amplitude peaking in the second cycle and the third cycle is strongly attenuated. In U. canosai, pulses exhibit a gradual increase in amplitude over 5–6 cycles before decreasing. M. atricauda produces pulses of 2–3 cycles, with the second cycle typically showing the highest amplitude. In P. courbina, pulses consist of multiple cycles (12–15), with amplitude also reaching its maximum in the second cycle. Spectrograms were generated using a 1024-point FFT and a Hanning window; darker shading indicates higher amplitude. Fish drawings modified from Menezes & Figueiredo97.
Striped weakfish Cynoscion guatucupa
Dual-pulse calls were recorded in the wild during the reproductive season (October and April), with activity peaks in spring and early autumn79, both before and after spawning46. In captivity, seven dual-pulse calls were recorded from five males (total length: 25, 28, 32, 33, and 34 cm). Postmortem gonadal analysis indicated that these specimens were either not in spawning condition or had already spawned. The dual-pulse sound was emitted by stationary and free-swimming fish throughout the day during the observation period (Fig. 2). Inter dual pulse intervals averaged 1 ± 3.9 s, and the interval between pulse one and two was 26 ± 2.0 ms. The average duration of pulse one and pulse two was 29 ± 0.1 ms and 32 ± 0.1 ms, respectively, and the dominant frequency was 328 ± 2.3 Hz (Table 1).
Argentine croaker Umbrina canosai
Dual-pulse calls were recorded in the wild during and outside of the reproduction season. The three male specimens in captivity (total length: 24, 28, 29 cm) were not spawning or had already spawned as indicated by gonadal analysis. The dual pulse was emitted in stationary and free-swimming fish at various times of day (Fig. 2). Three calls were obtained in captivity for each individual fish. The dual-pulse sounds had an inter dual-pulse interval of 1.4 ± 1.3 s; the interval between pulse one and pulse two was 32 ± 1.7 ms. The average duration of pulse one and pulse two were 28.1 ± 0.9 ms and 28 ± 0.7 ms respectively, and the dominant frequency was 299 ± 0.8 Hz (Table 1).
Southern king weakfish Macrodon Atricauda
The dual-pulse was recorded from five males in captivity (total length: 26, 27, 28, 28, and 29 cm). Eight calls were recorded over 2 to 3 h during the day, starting one day after spawning (Fig. 2). The average inter-individual pulse time was 1.1 ± 0.9 s, and the interval between the two pulses was 15 ± 2.8 ms. The average duration of pulse one was 23 ± 1.2 ms and of pulse two was 23 ± 1.1 ms, with a dominant frequency of 504 ± 4.2 Hz. The presence of dual-pulse calls in this species was also confirmed in the wild within the spawning area57.
Southern black drum Pogonias courbina
P. courbina dual-pulse calls were recorded only in the wild. However, by targeting known recurrent spawning sites, we obtained these calls on several occasions before, during, and after the species characteristic advertisement calls at the Maldonado stream mouth off the coast of Uruguay42. The call was recorded at various times during the day and night. On several occasions, the dual-pulse and the advertisement calls were recorded. Behavior during sound production was not observed in this species. The dual-pulse sound has longer pulses than in the other species, and it sounds like a doubled advertisement call (Fig. 2). Table 1 shows data from 5 min recordings of double-pulse calls (Fig. 2) from an area where sport fishers were fishing for P. courbina. It is not known whether the calls were made by the same specimen.
For M. furnieri, C. guatucupa, U. canosai and M. atricauda double pulses were also recorded every time the fish were fed dead shrimp and when the person in charge of this task approached the edge of the tank.
Acoustic parametercomparison
For the four species recorded in captivity, no specific time of day was associated with the emission of dual-pulse sound. Similarly, these calls were variably produced during day and night in the wild in all five species. Since the data for P. courbina were obtained only in the wild, they were not included in the statistical analysis. Acoustic parameters for each species are summarized in Fig. 3.
Significant differences were found among species for pulse duration 1 (F₃,₅₀ = 21.49, p < 0.001) and pulse duration 2 (F₃,₅₀ = 14.47, p < 0.001). The Tukey test revealed that the species with significant pairwise differences for pulse duration 1 were: M. furnieri vs. U. canosai (p < 0.001), C. guatucupa vs. U. canosai (p < 0.001), and U. canosai vs. M. atricauda (p < 0.001). For pulse duration 2, significant differences were also found: M. furnieri vs. U. canosai (p < 0.001), C. guatucupa vs. U. canosai (p < 0.001), and U. canosai vs. M. atricauda (p < 0.001).
For the interval between paired pulses (see Fig. 1), significant differences were found among species (F₃,₅₁ = 106, p < 0.001). Pairwise comparisons revealed significant differences between M. furnieri vs. M. atricauda (p < 0.001), C. guatucupa vs. M. atricauda (p < 0.001), and U. canosai vs. M. atricauda (p < 0.001).
For the interval between successive dual pulses (inter dual pulse time), differences among species were marginally significant (F₃,₄₆ = 2.79, p = 0.05). Finally, call duration showed significant differences among species (F₃,₃₆ = 8.08, p = 0.004), with pairwise differences between M. furnieri and C. guatucupa (p = 0.007), M. furnieri and U. canosai (p = 0.003), and M. furnieri and M. atricauda (p = 0.009) (Fig. 3).
Differences in dominant frequency were highly significant among the four analyzed species (F₃,₈₀ = 792.71, p < 0.001). Post hoc Tukey tests revealed that all four species differed significantly from each other in dominant frequency (p < 0.05 for all pairwise comparisons; Fig. 3).
To differentiate dual-pulse calls from advertisement and disturbance calls, we analyzed the acoustic variables of these three call types in U. canosai as a representative species (Fig. 4). Pulse duration showed partial overlap among call types, although dual-pulse calls exhibited more consistent pulse durations across pulses one and two. However, interpulse intervals displayed clear distinctions: advertisement calls presented short intervals (~ 100–300 ms) within repetitive trains, disturbance calls consisted of isolated pulses with long and irregular intervals (> 1000 ms), while dual-pulse calls exhibited a consistent short interval (~ 25–30 ms) between the paired pulses and longer intervals (~ 1000–2000 ms) between successive dual pulses. Dominant frequency overlapped across all call types. These results indicate that dual-pulse calls possess a distinct and stereotyped temporal structure, supporting their classification as a separate call type within the acoustic repertoire of U. canosai.
Comparison of acoustic parameters among the studied species. Box plots show the median (center line), first and third quartiles (box edges), and range (whiskers) of the mean values per individual for each acoustic parameter. Standard deviations (SD) based on these individual means are reported in Table 1. Parameters shown are: (1) Inter dual pulse time (s), (2) Pulse duration 1 (ms), (3) Pulse duration 2 (ms), (4) Interval between paired pulses (ms), (5) Dominant frequency (Hz), and (6) Call duration (s). Call duration and P. courbina were excluded from statistical comparisons, because it is not known whether the calls were made by the same specimen; the latter is shown only for illustration and has no letter assigned. Different letters above boxes indicate statistically significant differences between species (Tukey post hoc test, p < 0.05).
Box plot comparison of acoustic parameters among advertisement calls, disturbance calls, and dual-pulse calls in U. canosai. The plots display the median (center line), interquartile range (boxes), and whiskers representing minimum and maximum values excluding outliers. For advertisement calls, the interval represents interpulse time within trains; for disturbance calls, the interval represents time between isolated pulses; and for dual-pulse calls, the interval represents the time between paired pulses. Data from72.
Acoustic behavior associated with dual-pulse calls
In M. furnieri, C. guatucupa, U. canosai, and M. atricauda, dual-pulse calls were recorded in captivity under various conditions. These calls were emitted both when fish were stationary and while freely swimming, and were consistently observed during feeding routines, particularly when dead shrimp were introduced or when the person responsible for feeding approached the tanks.
In M. furnieri, only males produced dual-pulse calls when isolated, while no sounds were recorded from isolated females. In group settings, the calls were produced spontaneously, without evident agonistic interactions or structured courtship behaviors.
Although detailed behavioral sequences were not quantified, these observations indicate that the dual-pulse call occurs in diverse group contexts, both in wild aggregations and in community tanks, suggesting a broad behavioral flexibility in its emission. The specific function of these calls outside reproductive aggregations remains undetermined.
Discussion
The occurrence of dual-pulse calls in sciaenids had only been reported by Mok and Gilmore28, Lin et al.70, and Tellechea57. Mok & Gilmore28 mention that the spotted seatrout (C. nebulosus) produces double pulses designated as dual knocks in spawning aggregations. Double-beats of the Taiwanese croaker J. taiwanensis are often placed at the initial phase of a series of calls and do not appear to be a fear response70. Tellechea57 reported the dual pulse in M. atricauda occurred in free-swimming captive fish. In all three species (C. nebulosus, J. taiwanensis and M. atricauda), dual-pulse calls have been reported only in males, as only males possess sonic muscles.
Our comparative analysis of U. canosai call types provided clear evidence that dual-pulse calls are structurally distinct from advertisement and disturbance calls, particularly in their temporal patterning (significant differences in inter-dual-pulse time). While pulse duration and frequency overlap between call types, the interpulse intervals in dual-pulse calls consistently follow a fixed structure: a short interval (~ 25–30 ms) between the two pulses of a pair, followed by a long interval (~ 1000–2000 ms) before the next pulse pair. This supports the classification of dual-pulse calls as a discrete call type within the species’ acoustic repertoire.
Of the five sciaenid species in this study that emitted dual-pulse sounds, M. furnieri and P. courbina have sonic muscles in both sexes10,42,43, whereas C. guatucupa, U. canosai and M. atricauda have sonic muscles only in males46,57,72. For P. courbina, it is unclear whether males and females emit this sound, as recordings were made in the wild. However, the advertisement call in this species is produced only by males42,43.
Significant differences were found in acoustic variables between species, except for the inter-dual-pulse time in M. furnieri, C. guatucupa, U. canosai, and M. atricauda, which suggests this variable may play an important role in message exchange. Although several calls were analyzed per species, statistical comparisons were conducted using individual means (i.e., the average of calls per fish), which were then used to calculate species-level averages, resulting in relatively small sample sizes for some parameters. Despite this, significant interspecific differences were found in most variables. The inter-dual-pulse time, however, showed higher variability, likely because it reflects the interval between successive calls, which may involve different individuals swimming freely. In contrast, parameters measured within each call, such as pulse duration or frequency, are less influenced by inter-individual variation, explaining the greater dispersion observed in inter-dual-pulse time.
In environments where multiple sciaenid species coexist, subtle temporal variations in the structure of dual-pulse calls may facilitate species recognition and reduce acoustic interference, as has been observed in other fish families where temporal coding supports species-level discrimination80. Another potential source of variability is the recording environment. In several fish species, including sciaenids, acoustic features of the same call type can differ between wild and captive conditions28,81. Factors such as confinement, water acoustics, temperature or behavioral context in captivity may modify sound characteristics. In our study, although efforts were made to standardize recording conditions, some of the interspecific variability observed may partially reflect differences arising from calls recorded in both wild and captive settings.
In P. courbina, double pulses are much longer than in the other species in this study and in reports on C. nebulosus and J. taiwanensis28,70. Its sound pulses resemble those used in the advertisement call42 in duration but are longer than those in its disturbance calls42,43.
In four of the five species (M. furnieri, C. guatucupa, U. canosai, and M. atricauda), acoustic recordings were accompanied by behavioral observations in captivity. The dual-pulse call was emitted in various contexts, including free swimming, stationary positions, and during feeding events in aquaria. Typically, these calls were produced without obvious interactions with tank mates, although subtle social cues cannot be ruled out. Lin et al.70 also reported dual knocks from isolated fish in aquaria with no apparent external stimulus. Similarly, streaked gurnards have been found to make competitive feeding sounds82, which appear to be agonistic behaviors differing from calls directed towards a human feeder, as observed in our study.
The dual-pulse call exhibited a stereotyped acoustic structure within each species, characterized by low variability in key temporal and spectral parameters. Specifically, the interpulse interval between the two pulses showed minimal variation, as did the duration and amplitude envelope of each pulse. The dominant frequency was also consistent within species, reflecting a stable harmonic structure. This stereotypy is evident in the narrow standard deviations reported for these parameters (Table 1; Figs. 3 and 4), indicating that the dual-pulse call is a highly conserved vocal pattern in terms of its acoustic features, even though it may occur in diverse behavioral contexts.
This stereotyped acoustic structure suggests that the dual-pulse call may serve one or several functions, including social communication or group cohesion. The trains of pulses emitted by most sciaenids exhibit little frequency modulation within calls, and their dominant frequencies typically fall within the lower range of fish sounds (100–1,000 Hz36, similar to the low-frequency swimbladder sounds reported in other species. Winn3,83 concluded that fish calls encode information—such as species identity or behavioral state—primarily through temporal patterns. Although several studies have characterized the temporal acoustic features of sciaenid calls, no experimental tests have been conducted to determine whether sciaenids can discriminate between species or individuals based on these temporal characteristics. However, data from other teleost groups suggest this possibility. Myrberg and colleagues79,84,85, demonstrated species discrimination based on temporal call features in damselfish, while Crawford86,87 showed similar findings in mormyrids (elephant-nose fishes).
Given its conserved acoustic structure and subtle interspecific differences, the dual-pulse call may facilitate species recognition or individual identification. Its occurrence beyond the reproductive season suggests potential utility for passive acoustic monitoring of sciaenid populations year-round, as has been proposed for other sound-producing fishes6,61,88.
Although the exact function of the dual-pulse call remains unclear, its emission across multiple contexts hints at a multifunctional role. The literature on sound-producing behaviors in fishes within natural habitats is scarce, and generalized patterns are difficult to establish, likely due to the multimodal nature of fish communication89. For example, playback experiments in oyster toadfish demonstrated context-dependent vocal responses83,90,91,92, suggesting that acoustic stimuli can modulate call production in complex ways. While other fish species, such as croaking gouramis (Trichopsis spp.), also produce double-pulse sounds, the underlying mechanisms differ substantially. In gouramis, dual pulses arise from specialized anatomical structures, specifically the friction of two hypertrophied tendons against fin rays93. In contrast, sciaenid double pulses are produced through sequential neural activation of the sonic muscles, with no specialized anatomical adaptations for pulse doubling. Thus, although the resulting acoustic pattern is superficially similar, the physiological basis of sound production is fundamentally different.
Sciaenids inhabit turbid, low-visibility waters where acoustic communication becomes particularly important. The fact that dual-pulse calls are produced in contexts such as proximity to human feeders and in non-reproductive periods suggests these vocalizations may function as contact or arousal-related calls, reflecting anticipatory behaviors linked to feeding or social cohesion. However, the specific behavioral categories associated with dual-pulse production remain unclear and warrant further investigation. Similar context-dependent vocalizations have been reported in other taxa94,95, where calls are used flexibly depending on situational cues.
From a phylogenetic perspective, sciaenids are widespread across tropical and temperate regions, concentrated in four main areas: the Eastern Pacific, Western Atlantic, Eastern Atlantic, and Indo-West Pacific24,95. Molecular analyses by Lo et al.96 divided the sciaenid family into 11 groups, supporting a New World origin. The South American species in our study belong to several of these lineages, including both basal groups (e.g., P. courbina) and more derived groups (e.g., M. atricauda), providing a phylogenetic framework to hypothesize that dual-pulse calls may represent an ancestral (plesiomorphic) trait within Sciaenidae. However, independent evolutionary origins of this call type in distinct lineages cannot be ruled out.
A qualitative comparison of dual-pulse calls across geographic regions would be valuable to assess whether key acoustic features are conserved, providing insights into the evolutionary origins and diversification of acoustic communication in this family.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tavolga, W. N. Sound production and detection. In Fish Physiology (eds Hoar, W. S. & Randall, D. J.) 135–205 (Academic, (1971).
Tavolga, W. N. Sonic characteristics and mechanisms in marine fishes. In Marine bio-acoustics Vol. 1, 195–211Pergamon, (1964).
Winn, H. E. The biological significance of fish sounds. In Marine bio-acoustics (ed. Tavolga, W. N.) 213–231 (Pergamon, 1964).
Fine, M. L., Winn, H. E. & Olla, B. Communication in fishes. In How Animals Communicate (ed Sebeok, T. A.) 472–518 (Indiana University Press, (1977).
Ladich, F., Collin, S. P., Moller, P. & Kapoor, B. G. (eds) Communication in fishes. Science Publishers (2006).
Rountree, R. A. et al. Listening to fish: applications of passive acoustics to fisheries science. Fisheries 31, 433–446 (2006).
Amorim, M. C. P. Species differences in courtship acoustic signals among five lake Malawi cichlid species (Pseudotropheus spp). J. Fish. Biol. 72, 1355–1368 (2008).
Parmentier, E., Lecchini, D., Frederich, B., Brie, C. & Mann, D. Sound production in four damselfish (Dascyllus) species: phyletic relationships? Biol. J. Linn. Soc. 97, 928–940 (2009).
Gannon, D. P. Acoustic behavior of Atlantic croaker, Micropogonias undulatus (Sciaenidae). Copeia 193–204 (2007). (2007). https://doi.org/10.1643/0045-8511(2007)7[193:ABOACM]2.0.CO;2.
Tellechea, J. S., Martinez, C., Fine, M. L. & Norbis, W. Sound production in Whitemouth croaker (Micropogonias furnieri – Sciaenidae) and relationship between fish size and disturbance call parameters. Environ. Biol. Fish. 89 (2), 163–172 (2010a).
Bolgan, M. et al. Acoustic complexity of vocal fish communities: A field and controlled validation. Sci. Rep. 8, 10559. https://doi.org/10.1038/s41598-018-28771-6 (2018).
Bass, A. H., Rice, A. N. & Feng, N. Y. Singing behavior in fishes: Hormones, neurons, and evolution. In Encyclopedia of Animal Behavior (ed. Choe, J. C.) 340–351Academic Press, (2019).
Hawkins, A. D. & Rasmussen, K. J. The calls of gadoid fish. J. Mar. Biol. Assoc. U K. 58 (4), 891–911 (1978).
Ladich, F. Agonistic behaviour and significance of sounds in vocalizing fish. Mar. Freshw. Behav. Physiol. 29 (1–4), 87–108 (1997).
Amorim, M. C. P. Diversity of sound production in fish. In Communication in Fishes (eds Ladich, F., Collin, S. P., Moller, P. & Kapoor, B. G.) 71–105 (Science, (2006).
Fine, M. L. & Parmentier, E. Mechanisms of fish sound production. In Sound Communication in Fishes, Animal Signals and Communication Vol. 4 (ed. Ladich, F.) 77–126 (Springer, 2015); https://doi.org/10.1007/978-3-7091-1846-7_3
Parmentier, E. & Fine, M. L. Fish sound production. In Handbook of Auditory Research (eds Suthers, R. A. & Fitch, T.) 19–49 (Springer, (2016).
Kaatz, I. M., Lobel, P. S. & Rice, A. N. Sound production and communication in catfishes. In Catfishes, a Highly Diversified Group, Vol.1: Their Outstanding Biology, 299–320 (Science Publishers, Enfield, NH, 2024).
Tower, R. W. The production of sound in the drumfishes, the sea-robin and the toadfish. Ann. N Y Acad. Sci. 18 (5), 149–180 (1908).
Wilson, B., Batty, R. S. & Dill, L. M. Pacific and Atlantic herring produce burst pulse sounds. Proc. R. Soc. Lond. B 271(Suppl 3), S95-S97 (2004). https://doi.org/10.1098/rsbl.2003.0107
Takemura, A., Takita, T. & Mizue, K. Studies on the underwater sound-VII underwater calls of the Japanese marine drum fishes (Sciaenidae). Nippon Suisan Gakkaishi. 44 (2), 121–125 (1978).
Fish, M. P. & Mowbray, W. H. Sound of the Western North Atlantic Fishes (Johns Hopkins, 1970).
Parmentier, E. et al. Sound production in the clownfish amphiprion Clarkii. Science 316 (5837), 1006. https://doi.org/10.1126/science.1138379 (2007).
Chao, L. N. & Sciaenidae In Fishes of the North-eastern Atlantic and the Mediterranean Vol. 2 (eds. Whitehead, P. J. P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J. & Tortonese, E.) 865–874 (UNESCO, 1986).
Nelson, J. S., Grande, T. C. & Wilson, M. V. H. Fishes of the world. John Wiley Sons. https://doi.org/10.1002/9781119174844 (2016). 5th ed.
Smith, H. M. The drumming of the drum-fishes (Sciaenidae). Science 22, 376–378 (1905).
Guest, W. C. & Lasswell, J. L. A note on courtship behavior and sound production of red drum. Copeia 1978 (2), 337–339 (1978).
Mok, H. K. & Gilmore, R. G. Analysis of sound production in estuarine aggregations of pogonias cromis, Bairdiella chrysoura, and Cynoscion nebulosus (Sciaenidae). Bull. Inst. Zool. Acad. Sinica. 22, 157–186 (1983).
Saucier, M. H. & Baltz, D. M. Spawning site selection by spotted seatrout, Cynoscion nebulosus, and black drum, pogonias cromis, in Louisiana. Environ. Biol. Fish. 36, 257–272 (1993).
Luczkovich, J. J., Mann, D. A. & Rountree, R. A. Passive acoustics as a tool in fisheries science. Trans. Am. Fish. Soc. 137 (2), 533–541 (2008a).
Locascio, J. V. & Mann, D. A. Diel and seasonal timing of sound production by black drum (Pogonias cromis). Fish. Bull. 109, 327–338 (2011).
Borie, A., Mok, H. K., Chao, N. L., Fine, M. L. & Coleman, M. J. Spatiotemporal variability and sound characterization in silver croaker plagioscionsquamosissimus (Sciaenidae) in the central Amazon. PLoS ONE. 9, e99326 (2014).
Montie, E. W., Kehrer, C., Yost, J. & Brenkert, K. Long-term monitoring of captive red drum Sciaenops ocellatus reveals that calling incidence and structure correlate with egg deposition. J. Fish. Biol. 88, 1776–1795 (2016).
Picciulin, M., Bolgan, M., Corò, A. B., Calcagno, G. & Malavasi, S. Sound production by the Shi drum umbrina cirrosa and comparison with the brown meagre Sciaena umbra: A passive acoustic monitoring perspective. J. Fish. Biol. 88, 1655–1660 (2016).
Ramcharitar, J., Gannon, D. P. & Popper, A. N. Bioacoustics of fishes of the family Sciaenidae (croakers and drums). Trans. Am. Fish. Soc. 135 (5), 1409–1431 (2006).
Ramcharitar, J. U., Higgs, D. M. & Popper, A. N. Audition in Sciaenid fishes with different swim bladder-inner ear configurations. J. Acoust. Soc. Am. 119 (1), 439–443 (2006).
Verocai, J. E., Lombarte, A. & Norbis, W. Ontogenetic changes in sagitta otoliths of Whitemouth croaker micropogonias furnieri (Acanthuriformes: Sciaenidae) and its implication in acoustic communication. Anim. Biol. 73 (2), 195–211. https://doi.org/10.1163/15707563-bja10105 (2023).
Ono, R. D. & Poss, S. G. Structure and innervation of the swimbladder musculature in the weakfish, Cynoscion regalis (Teleostei: Sciaenidae). Can. J. Zool. 60, 1955–1967 (1982).
Hill, G. L., Fine, M. L. & Musick, J. A. Ontogeny of the sexually dimorphic Sonic muscle in three Sciaenid species. Copeia 1987 (3), 708–713 (1987).
Connaughton, M. A., Fine, M. L. & Taylor, M. H. The effects of seasonal hypertrophy and atrophy on fiber morphology, metabolic substrate concentration, and sound characteristics of the weakfish Sonic muscle. J. Exp. Biol. 200, 2449–2457 (1997).
Ladich, F. & Fine, M. L. Sound-generating mechanisms in fishes: a unique diversity in vertebrates. In Communication in Fishes (ed Ladich, F.) 3–43 (Science, (2006).
Tellechea, J. S., Olsson, D., Norbis, W. & Fine, M. L. Calls of the black drum (Pogonias cromis – Sciaenidae): geographical differences in sound production between Northern and Southern hemisphere populations. J. Exp. Zool. A. 315 (1), 48–55 (2010b).
Tellechea, J. S. et al. Sound variation by hypertrophy and atrophy Sonic muscle in the male Southern black drum (Pogonias courbina). J. Acoust. Soc. Am. 152 (1), 429–436 (2022).
Luczkovich, J. J., Pullinger, R. C., Johnson, S. E. & Sprague, M. W. Identifying Sciaenid critical spawning habitats by the use of passive acoustics. Trans. Am. Fish. Soc. 137 (2), 576–605. https://doi.org/10.1577/T06-135.1 (2008b).
Mok, H. K., Yu, H. Y., Ueng, J. P. & Wei, R. C. Characterization of sounds of the blackspotted croaker Protonibea diacanthus (Sciaenidae) and localization of its spawning sites in estuarine coastal waters of Taiwan. Zool. Stud. 48, 325–333 (2009).
Tellechea, S. J. & Norbis, W. Sexual dimorphism in sound production and relationship between fish size and call characteristics in the striped weakfish Cynoscion Guatucupa. Zool. Stud. 51 (7), 946–955 (2012).
Montie, E. W. et al. Acoustic monitoring indicates a correlation between calling and spawning in captive spotted seatrout (Cynoscion nebulosus). J. Fish. Biol. 90 (1), 203–221. https://doi.org/10.1111/jfb.13218 (2017).
Monczak, A., Ji, Y., Soueidan, J. & Montie, E. W. Automatic detection, classification, and quantification of Sciaenid fish calls in an estuarine soundscape in the Southeast united States. PLoS ONE. 14 (1), e0209914. https://doi.org/10.1371/journal.pone.0209914 (2019).
Vieira, M. et al. Seasonal variation of captive meagre acoustic signalling: A manual and automatic recognition approach. Fishes 4 (2), 28. https://doi.org/10.3390/fishes4020028 (2019).
Picciulin, M., Fiorin, R., Facca, C. & Malavasi, S. Sound features and vocal rhythms as a proxy for locating the spawning ground of Sciaena umbra in the wild. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 1299–1312 (2020).
Pereira, B. et al. Sound production in the Meagre, Argyrosomus regius (Asso, 1801): intraspecific variability associated with size, sex and context. PeerJ 8, e8559. https://doi.org/10.7717/peerj.8559 (2020).
Luczkovich, J. J., Sprague, M. W., Johnson, S. E. & Paullinger, R. C. Delimiting spawning areas of weakfish, Cynoscion regalis (family Sciaenidae) in Pamlico Sound, North Carolina using passive hydroacoustic surveys. Bioacoustics 10 (2–3), 143–160 (1999).
Aalbers, S. A. & Drawbridge, M. A. White Seabass spawning behavior and sound production. Trans. Am. Fish. Soc. 137, 542–550 (2008).
Lowerre-Barbieri, S. K. et al. Use of passive acoustics to determine red drum spawning in Georgia waters. Trans. Am. Fish. Soc. 137 (2), 562–575. https://doi.org/10.1577/T06-223.1 (2008).
Locascio, J. V., Burghart, S. & Mann, D. A. Quantitative and Temporal relationships of egg production and sound production by black drum pogonias Cromis. J. Fish. Biol. 81 (4), 1175–1191. https://doi.org/10.1111/j.1095-8649.2012.03391.x (2012).
Rice, A. N. et al. Spatial and Temporal patterns of toadfish and black drum chorusing activity in the South Atlantic bight. Environ. Biol. Fish. 99 (7–8), 705–716. https://doi.org/10.1007/s10641-016-0511-z (2016).
Tellechea, J. S. The acoustic behavior of Southern King weakfish (Macrodon atricauda-Sciaenidae). Environ. Biol. Fish. 102, 1253–1264 (2019).
Bolgan, M. et al. Calling activity and calls’ Temporal features inform about fish reproductive condition and spawning in three cultured Sciaenidae species. Aquaculture 524, 735243. https://doi.org/10.1016/j.aquaculture.2020.735243 (2020).
Holt, S. A. Distribution of red drum spawning sites identified by a towed hydrophone array. Trans. Am. Fish. Soc. 137 (2), 551–561 (2008).
Fish, J. F. & Cummings, W. C. A 50-dB increase in sustained ambient noise from fish (Cynoscion xanthulus). J. Acoust. Soc. Am. 52 (4B), 1266–1270 (1972).
Tellechea, J. S., Bouvier, D. & Norbis, W. Spawning sounds in Whitemouth croaker (Sciaenidae): seasonal and daily cycles. Bioacoustics 20 (2), 159–168. https://doi.org/10.1080/09524622.2011.9753641 (2011).
Parsons, M. J., McCauley, R. D. & Mackie, M. C. Characterisation of Mulloway Argyrosomus japonicus advertisement sounds. Acoust. Aust. 41 https://doi.org/10.1007/s40857-017-0112-9 (2013).
Picciulin, M. et al. Passive acoustic monitoring of Sciaena umbra on Rocky habitats in the Venetian Littoral zone. Fish. Res. 145, 76–81. https://doi.org/10.1016/j.fishres.2013.02.008 (2013).
Bass, A. H. & McKibben, J. R. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 69, 1–26. https://doi.org/10.1016/S0301-0082(03)00004-2 (2003).
Fine, M. L., Schrinel, J. & Cameron, T. M. The effect of loading on disturbance sounds of the Atlantic croaker micropogonius undulatus: air versus water. J. Acoust. Soc. Am. 116, 1271–1275 (2004).
Parmentier, E., Tock, J., Falguière, J. C. & Beauchaud, M. Sound production in Sciaenops ocellatus: preliminary study for the development of acoustic cues in aquaculture. Aquaculture 432, 204–211 (2014).
Aalbers, S. A. & Sepulveda, C. A. The utility of a long-term acoustic recording system for detecting white Seabass Atractoscion nobilis spawning sounds. J. Fish. Biol. 81, 1859–1870. https://doi.org/10.1111/j.1095-8649.2012.03399.x (2012).
Lagardère, J. P. & Mariani, A. Spawning sounds in meager Argyrosomus regius in the Gironde estuary. France J. Fish. Biol. 69 (6), 1697–1708. https://doi.org/10.1111/j.1095-8649.2006.01237.x (2006).
Ueng, J. P., Huang, B. Q. & Mok, H. K. Sexual differences in spawning sounds of the Japanese croaker Argyrosomus japonicus (Sciaenidae). Zool. Stud. 46, 103–110 (2007).
Lin, Y. C., Mok, H. K. & Huang, B. Q. Sound characteristics of big-snout croaker Johnius macrorhynus (Sciaenidae). J. Acoust. Soc. Am. 121 (1), 586–593. https://doi.org/10.1121/1.2384844 (2007).
Parsons, M. J. G., McCauley, R. D., Paulus, M. C. M. & Siwabessey, J. In situ source levels of Mulloway (Argyrosomus japonicus) calls. J. Acoust. Soc. Am. 132, 3559–3568 (2012).
Tellechea, J. S., Fine, M. L. & Norbis, W. Passive acoustic monitoring, development of disturbance calls and differentiation of disturbance and advertisement calls in the Argentine croaker umbrina canosai (Sciaenidae). J. Fish. Biol. 90 (4), 1631–1643 (2017).
Saborido Rey, F. & Macchi, G. J. (eds) Ecología reproductiva y pesquerías en el contexto iberoamericano. Red INVIPESCA (2021).
Urteaga, J. R. & Perrotta, R. G. Estudio preliminar de La edad, El crecimiento, área de distribución y Pesca de La Corvina negra, pogonias cromis, En El litoral de La provincia de Buenos Aires. Informes técnicos INIDEP. 43, 1–22 (2001).
Mazzoni, D. Audacity 1.2.6. (2006). Available from: http://audacity.sourceforge.net/
K. Lisa Yang Center for Conservation Bioacoustics. Raven Pro: Interactive Sound Analysis Software (Version 1.6.1) [computer Software] (The Cornell Lab of Ornithology, 2019).
Levene, H. Stanford University Press,. Robust tests for equality of variances. In Contributions to probability and statistics (ed. Olkin, I.) 278–292 (1960).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: paleontological statistic software package for education and data analysis. Palaeontol. Electron. 4 (1), 1–9 (2001).
Militelli, M. I. & Macchi, G. J. Spawning and fecundity of striped weakfish, Cynoscion guatucupa, in the Rio de La Plata estuary and adjacent marine waters, Argentina-Uruguay. Fish. Res. 77 (1), 110–114 (2006).
Myrberg, A. A. & Riggio, R. J. Acoustically mediated individual recognition by a coral reef fish (Pomacentrus partitus). Anim. Behav. 33 (2), 411–416. https://doi.org/10.1016/S0003-3472(85)80065-8 (1985).
Amorim, M. C. P., Vasconcelos, R. O. & Fonseca, P. J. Fish sounds and mate choice. In Sound Communication in Fishes. Animal Signals and Communication. Vol. 4 (ed. Ladich, F.) (Springer, 2015). https://doi.org/10.1007/978-3-7091-1846-7_1.
Amorim, M. C. P. & Hawkins, A. Growling for food: acoustic emissions during competitive feeding in the streaked Gurnard. J. Fish. Biol. 57, 895–907 (2000).
Winn, H. E. Acoustic discrimination by the toadfish with comments on signal systems. In Behavior of Marine Animals Vol. 2 (eds Winn, H. E. & Olla, B.) 361–385 (Plenum, 1972).
Myrberg, A. A. J. Ocean noise and the behaviour of marine animals: relationships and implications. In Effects of Noise on Wildlife (eds Fletcher, J. L. & Busnel, R. G.) 169–208 (Academic, (1978).
Myrberg, A. A. J., Ha, S. J. & Shamblott, M. J. The sounds of bicolor damselfish (Pomacentrus partitus): predictors of body size and a spectral basis for individual recognition and assessment. J. Acoust. Soc. Am. 94 (6), 3067 (1993).
Crawford, J. D. Feature-detecting auditory neurons in the brain of a sound-producing fish. J. Comp. Physiol. A. 180, 439–450 (1997a).
Crawford, J. D. Hearing and acoustic communication in the Mormyrid electric fishes. Mar. Freshw. Behav. Physiol. 29, 1–21 (1997b).
Locascio, J. V. & Mann, D. A. Diel periodicity of fish sound production in Charlotte Harbor, Florida. Trans. Am. Fish. Soc. 137 (2), 606–615 (2008).
Banse, M. et al. Same calls, different meanings: acoustic communication of holocentridae. PLoS ONE. 19, e0312191. https://doi.org/10.1371/journal.pone.0312191 (2024).
Winn, H. E. Vocal facilitation and the biological significance of toadfish sounds. In Marine bio-acoustics Vol. 2 (ed. Tavolga, W. N.) 283–304 (Pergamon, 1967).
Fish, J. F. The effect of sound playback on the toadfish. In Behaviour of Marine Animals (eds Winn, H. E. & Olla, B.) 386–434 (Plenum, 1972).
Thorson, R. F. & Fine, M. L. Acoustic competition in the Gulf toadfish Opsanus beta: acoustic tagging. J. Acoust. Soc. Am. 111, 2302–2307. https://doi.org/10.1121/1.1466865 (2002).
Ladich, F. Females whisper briefly during sex: Context- and sex-specific differences in sounds made by croaking gouramis. Anim. Behav. 73, 379–387 (2007).
Brown, J. L. The Evolution of Behavior (W.W. Norton, 1975).
Remage-Healey, L., Nowacek, D. P. & Bass, A. H. Dolphin foraging sounds suppress calling and elevate stress hormone levels in a prey species, the Gulf toadfish. J. Exp. Biol. 209 (22), 4444–4451. https://doi.org/10.1242/jeb.02525 (2006).
Lo, P. C. et al. A multi-gene dataset reveals a tropical New World origin and Early Miocene diversification of croakers (Perciformes: Sciaenidae). Mol. Phylogenet Evol. 88, 132–143 https://doi.org/10.1016/j.ympev.2015.03.02 (2015).
Menezes, N. A. & Figueiredo, J. L. Família Phosichthyidae. In Catálogo Das espécies De Peixes Marinhos Do Brasil (eds Menezes, N. A., Buckup, P. A., Figueiredo, J. L. & Moura, R. L.) 160 (Museu de Zoologia da Universidade de São Paulo, (2003).
Acknowledgements
We would like to thank the sport fishermen who allowed us to record their fish and collect the necessary data for this investigation. We thank José for his invaluable help during fieldwork, logistics, and analytical support. Your contribution was deeply appreciated, and you will be greatly missed, RIP. The authors also thank the ANII (Agencia Nacional de Investigación e Innovación) for support through the National Research System (ANII–SIN–Uruguay).
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tellechea, J.S., Mok, HK. & Fine, M.L. Characterizing dual-pulse calls in five Sciaenid species. Sci Rep 15, 42442 (2025). https://doi.org/10.1038/s41598-025-26648-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26648-z