Abstract
The prognosis of stroke is related to insulin resistance (IR), and post-stroke depression (PSD) is a prevalent psychological consequence following a stroke. The triglyceride-to-glucose (TyG) index and the triglyceride-to-high-density lipoprotein cholesterol (TG/HDL-C) ratio are simple and accurate markers of insulin resistance. However, the relationship between early-onset PSD and TyG index or TG/HDL-C ratio remains unknown. The Hamilton Depression Scale-17 item (HAMD-17) was used to assess the severity of depression. After two weeks of acute ischemic stroke (AIS), patients with HAMD-17 ≥ 7 were defined as early-onset post-stroke depression. Among the 543 recruited patients, a total of 206 (38%) patients were diagnosed with early-onset PSD. The logistic regression model determined that early-onset PSD was independently associated with the TyG index (odds ratio [OR], 1.915; 95% confidence interval [CI] 1.665–2.115, P < 0.001) and TG/HDL-C ratio (OR, 1.458; 95% CI 1.213–1.965, P = 0.002). The area under curve (AUC) of TyG index, TG/HDL-C ratio, and TyG index combined TG/HDL-C ratio to predict early-onset PSD was 0.765, 0.705, and 0.813, respectively. According to our research, the TG/HDL-C ratio and the TyG index could serve as independent risk factors and prediction indicators for early-onset PSD.
Similar content being viewed by others
Introduction
Post-stroke depression (PSD) is a major neuropsychiatric sequela of stroke, characterized by depressed mood, anhedonia, and in severe cases, suicidal ideation1,2. Early-onset PSD is defined as the emergence of depressive symptoms within the first two weeks following an acute stroke3,4. This condition is associated with a high incidence of pronounced depressive features and carries a significantly elevated risk of adverse clinical outcomes5. Moreover, its symptomatic presentation is often subtle and easily overlooked in early clinical assessment. Critically, early-onset PSD is linked to increased mortality and imposes substantial burdens on both caregivers and healthcare systems6. Consequently, the identification of reliable prognostic factors for early-onset PSD is of crucial clinical importance.
Insulin resistance (IR) contributes to the pathophysiology of cerebrovascular disorders primarily through thrombosis, endogenous fibrinolytic dysfunction, inflammation, and increased platelet activation7, which is associated with the prognosis of stroke8. Moreover, IR might negatively influence the prognosis of individuals with psychiatric disorders, including anxiety and depression, by impacting neuronal and synaptic function and intensifying neuroinflammation9,10,11. The triglyceride-to-high-density lipoprotein cholesterol (TG/HDL-C) ratio and the triglyceride-to-glucose (TyG) index have been shown to be accurate, affordable, and readily available surrogate indicators for IR in recent years12,13,14. Large cohort study suggest that the TyG index may serve as a valuable insulin resistance biomarker for assessing the outcome of stroke patients15. The TyG index is associated with arterial stiffness16 and adverse outcomes in cardiovascular and cerebrovascular disorders17,18. Notably, elevated TyG index has been linked to in-hospital mortality in severe stroke patients19 and early stroke recurrence20. Additionally, TyG levels are strongly associated with the onset and persistence of depression21,22. Nevertheless, the correlation between the TyG index and early-onset PSD remains ambiguous. Similarly, the TG/HDL-C ratio-a readily obtainable serum measure-is a reliable estimator of IR14. A longitudinal study indicates that the TG/HDL-C ratio may serve as an invaluable and independent biomarker for evaluating cardiovascular disease outcomes and development23. Our previous study showed that TG/HDL-C ratio can be used as a prognostic factor to predict post-thrombolysis early neurological deterioration8. Previous studies have also linked a high TG/HDL-C ratio to depressive symptoms, potentially mediated by insulin resistance, oxidative stress, endothelial dysfunction, atherosclerosis, and pro-inflammatory states24. Thus, more research is necessary to determine a connection between early-onset PSD and the TG/HDL-C ratio.
So far, research on the relationship between the TyG index, TG/HDL-C ratio, and early-onset PSD remains limited. Therefore, this study aimed to systematically evaluate this association and assess their potential utility as predictive indicators for early-onset PSD.
Materials and methods
Research methodology and subjects
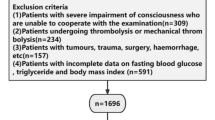
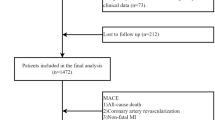
We prospectively and consecutively enrolled eligible patients with acute ischemic stroke (AIS) at Changsha Central Hospital between August 2023 and September 2024. The study protocol was approved by the Ethics Committee of Changsha Central Hospital and conducted in accordance with the principles of the revised Declaration of Helsinki (1975) and the National Institutes of Health Human Subjects Policies and Guidelines (1999). Inclusion criteria were as follows: (1) diagnosis of ischemic stroke based on the 2018 Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke; (2) age between 18 and 85 years; and (3) hospital admission within 72 h of stroke onset. Exclusion criteria included: (1) severe dysarthria, aphasia, or impaired consciousness preventing completion of assessments; (2) pre-stroke diagnosis of dementia or significant cognitive impairment (or use of dementia medications); (3) severe cardiac, hepatic, or renal insufficiency; (4) pre-existing mental illness such as depression or prior use of psychotropic drugs; (5) history of other central nervous system diseases such as Parkinson’s disease or epilepsy; and (6) current diagnosis of malignant tumors, regardless of metabolic impact. A total of 542 AIS patients were recruited during the study period (Fig. 1).
Clinical features
All participants underwent systematic evaluation of baseline characteristics including age, sex, body mass index (BMI), and vascular risk factors (diabetes mellitus, hypertension, atrial fibrillation, coronary artery disease, current smoking, and alcohol consumption). Current smoking was defined as regular consumption of ≥ 10 cigarettes per day for at least five consecutive years prior to enrollment. Similarly, active alcohol consumption was defined as sustained intake of ≥ 20 g of ethanol daily for a minimum of five years. Stroke severity was assessed by certified neurologists using the National Institutes of Health Stroke Scale (NIHSS), with evaluations completed within 24 h of hospital admission. Functional outcomes were measured at one-month follow-up using the modified Rankin Scale (mRS). Comprehensive diagnostic investigations—including computed tomography, magnetic resonance imaging, echocardiography, electrocardiography, carotid ultrasonography, and transcranial Doppler—were employed to determine lesion characteristics and classify stroke subtypes.
Subject classification and clinical evaluation
PSD was diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). To ensure objectivity and reproducibility, all patients were evaluated using the 17-item Hamilton Depression Rating Scale (HAMD-17) by trained neurologists or psychiatrists who remained blinded to the study primary hypotheses, thereby minimizing assessment bias. Early-onset PSD was defined as a HAMD-17 score ≥ 7 at two weeks following acute ischemic stroke onset, whereas patients scoring < 7 were assigned to the non-PSD group25. Depressive symptom severity was categorized as follows: mild (HAMD-17 score 7–17), moderate (18–23), and severe (≥ 24)25,26.
We defined the time of blood collection as those who were admitted 72 h after the onset of stroke and when blood was drawn at 6 or 7 a.m. the next day after at least 8 h of fasting. This minimizes the influence of intravenous fluids (especially glucose-containing fluids) and diet. The automated hematology analyzer (BZ6800, CHINA) was utilized for standard blood tests, which involved the counts of white blood cells (WBC), platelets, neutrophils, lymphocytes, and monocytes. An automated analyzer (HITACHI 7600, JAPAN) was used to perform a standard biochemical examination. The tests included measurements of creatinine (Cr), uric acid (UA), fasting blood glucose (FBG), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). We computed the TG/HDL-C ratio. Three tests of each blood type were performed. The TyG index was defined using the formula below: Ln [TG (mg/dL) × FBG (mg/dL) ÷ 2]27.
Statistical analysis
All statistical analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA) and MedCalc 15.6.0 (MedCalc Software, Ostend, Belgium). The normality of data distribution was assessed using the Kolmogorov–Smirnov test. Continuous variables with normal distribution were expressed as mean ± standard deviation and analyzed using parametric tests (Student’s t-test or ANOVA), while non-normally distributed data were presented as median (interquartile range) and analyzed with non-parametric alternatives (Mann–Whitney U test or Kruskal–Wallis test). Categorical variables were summarized as frequencies with percentages and compared using the Chi-square test or Fisher’s exact test, as appropriate. The relationships between metabolic indices (TyG index and TG/HDL-C ratio) and depression severity were visualized using scatter plots and quantified using Spearman’s rank correlation analysis. Multicollinearity among independent variables was examined through collinearity diagnostics prior to regression modeling. Binary logistic regression was employed to identify independent risk factors for early-onset PSD.The discriminative ability of the TyG index and TG/HDL-C ratio for early-onset PSD was evaluated using receiver operating characteristic (ROC) curve analysis, with area under the curve (AUC) values calculated to assess predictive performance. Regarding model validation, we have taken the following steps to ensure the robustness of our statistical models: Residual Analysis: We have examined the residuals of our models to check for homoscedasticity (constant variance) and normality of residuals. For regression models, we have performed lack-of-fit tests to assess how well the model describes the data. A two-tailed value of P < 0.05 was considered significant. The effect sizes provide important information about the magnitude of the observed effects, complementing p-values. For t-tests, we have reported Cohen’s d. For ANOVA, we have reported eta-squared (η2). For chi-squared test, we have reported Cramer’s V. For logistic regression model, we have reported the Nagelkerke R2 statistic.
Results
Clinical and demographic characteristics of early-onset PSD and Non-PSD groups
The baseline clinical and demographic characteristics of the study participants are summarized in Table 1. Of the 542 enrolled patients, 206 (38%) were diagnosed with early-onset post-stroke depression (PSD), while 336 (62%) comprised the non-PSD group. Comparative analyses revealed significant differences between the two groups. Patients in the early-onset PSD group were significantly younger (P < 0.001) and had lower HDL-C levels (P < 0.001) compared to the non-PSD group. Conversely, the early-onset PSD group demonstrated significantly higher NIHSS scores (P = 0.006), mRS scores (P < 0.001), HAMD-17 scores (P < 0.001), fasting blood glucose (P = 0.002), triglyceride levels (P < 0.001), TyG index (P < 0.001), and TG/HDL-C ratio (P < 0.001). Furthermore, as illustrated in Fig. 2, the distributions of age, NIHSS scores, mRS scores, fasting blood glucose, triglyceride levels, HDL-C levels, TyG index, and TG/HDL-C ratio were compared across patients with different severity levels of early-onset PSD.
Comparison of (A) age, (B) NIHSS score, (C) mRS score, (D) fasting blood glucose (FBG), (E) triglycerides (TG), (F) high-density lipoprotein cholesterol (HDL-C), (G) triglyceride-glucose (TyG) index, and (H) TG/HDL-C ratio across different severity levels of early-onset post-stroke depression (PSD). Data are presented as ***P < 0.001, **P < 0.01, *P < 0.05.
Correlation between clinical parameters and depression severity in the study
Spearman correlation analysis revealed significant associations between HAMD scores and multiple metabolic and clinical parameters. As illustrated in Fig. 3, HAMD scores exhibited significant positive correlations with NIHSS score (r = 0.116, P = 0.007), fasting blood glucose (r = 0.487, P < 0.001), triglyceride levels (r = 0.569, P < 0.001), TyG index (r = 0.561, P < 0.001), and TG/HDL-C ratio (r = 0.527, P < 0.001). Conversely, significant negative correlations were observed between HAMD scores and both age (r = -0.178, P < 0.001) and HDL-C levels (r = -0.313, P < 0.001).
Scatter plots illustrating correlations between HAMD scores and various parameters. Positive correlations were observed with (B) NIHSS score (r = 0.116, P = 0.007), (C) fasting blood glucose (FBG; r = 0.487, P < 0.001), (D) triglycerides (TG; r = 0.569, P < 0.001), (F) TyG index (r = 0.561, P < 0.001), and (G) TG/HDL-C ratio (r = 0.527, P < 0.001). Negative correlations were observed with (A) age (r = -0.178, P < 0.001) and (E) HDL-C (r = -0.313, P < 0.001).
Logistic regression analysis of risk factors for early-onset PSD
A binary logistic regression model was constructed to identify independent risk factors for early-onset PSD, utilizing all variables that demonstrated significant associations in the initial univariate analysis (Table 1). Age, NIHSS score, mRS score, FBG, TG, HDL-C, TyG index, and TG/HDL-C ratio were all associated with early-onset PSD (P < 0.05), according to crude models (Table 2). The TG/HDL-C ratio and the TyG index did not exhibit collinearity. However, due to their collinearity with the TyG index and the TG/HDL-C ratio, FBG (VIF = 44), TG (VIF = 65), and HDL-C (VIF = 87) were excluded from the model. TyG index (OR, 1.915; 95% CI 1.665–2.115, P < 0.001) and TG/HDL-C ratio (OR, 1.458; 95% CI 1.213–1.965, P = 0.002) were revealed to be independent predictors for early-onset PSD after adjusting for all other confounders (Table 2).
Subgroup analyses and interaction test
We performed stratified analyses to investigate potential effect modification by key demographic and clinical characteristics on the associations of the TyG index and TG/HDL-C ratio with early-onset PSD (Fig. 4). The results demonstrated that the positive associations persisted without significant modification across all subgroups, including those stratified by alcohol consumption, smoking status, coronary artery disease, atrial fibrillation, diabetes mellitus, hypertension, sex and age. In comprehensive subgroup analyses, the significant associations of both the TyG index and TG/HDL-C ratio with early-onset PSD persisted consistently, validating the robustness and generalizability of our conclusions across diverse clinical populations. Formal interaction tests further validated these observations, showing no statistically significant interaction effects (all P for interaction > 0.05).
The diagnostic performance of the TyG index and TG/HDL-C ratio for early-onset PSD was evaluated using ROC analysis.
As shown in Fig. 5, the TyG index achieved an AUC of 0.765 (95% CI: 0.727–0.800; P < 0.001), with an optimal cutoff value of 7.06 yielding a sensitivity of 83.98% and specificity of 69.94%. The TG/HDL-C ratio demonstrated an AUC of 0.705 (95% CI: 0.664–0.743; P < 0.001), with a cutoff of 1.82 corresponding to 69.42% sensitivity and 67.56% specificity. Notably, the combination of both biomarkers significantly enhanced predictive accuracy, achieving an AUC of 0.813 (95% CI: 0.778–0.845; P < 0.001). At the optimal combined cutoff of 0.32, sensitivity reached 84.95% with specificity of 65.77%. These results indicate that while both indices alone show significant discriminative power for early-onset PSD, their combination provides superior predictive capability.
Discussion
TyG index and TG/HDL-C ratio are surrogate markers of IR, which is closely related to the prognosis of a variety of diseases. A substantial body of research has well-established the strong association of the TyG index and TG/HDL-C ratio with both stroke incidence and clinical outcomes. Nonetheless, the predictive significance of the TyG index and TG/HDL-C ratio in individuals with early-onset PSD is still uncertain. This investigation yielded some novel discoveries. Initially, compared to patients in the non-PSD group, patients in the early-onset PSD group had a greater TG/HDL-C ratio and TyG index. Additionally, the TG/HDL-C ratio and TyG index were shown to be independent predictors of early-onset PSD using the logistic regression model. Lastly, the TyG index combined TG/HDL-C ratio showed decent early-onset PSD discriminating power, according to ROC analysis. All of these results point to a relationship between early-onset PSD and the TG/HDL-C ratio and the TyG index.
IR is more than just a basic pathophysiological feature of diabetes, it also serves as a metabolic basis for depression and depression-related diseases. Insulin-responsive neurons have constant insulin receptors on their plasma membranes, making the brain sensitive to glucose levels28. This sensitivity partially explains depression-related glucose control issues29, highlighting the relationship between IR and depression. Previous studies demonstrate that insulin resistance is associated with a twofold increase in the risk of developing major depressive disorder (MDD). Notably, even among individuals without baseline depressive symptoms, those with insulin resistance remained at a significantly elevated risk of incident MDD during follow-up30. A meta-analysis revealed a significant association between insulin resistance and depression severity scores, suggesting that insulin resistance may serve as a biomarker for an acute depressive state31. Although each unit increase in the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) is associated with an elevated risk of PSD32,33, its reliance on fasting serum insulin-a non-routine measurement-restricts its clinical applicability. The TyG index may serve as an effective measure for assessing the degree of IR. A growing body of research has established a correlation between the TyG index and the prognosis of AIS patients, as well as depression. Elevated TyG index is associated with poor functional outcome at 90 days after thrombolysis in AIS patients34. According to several earlier studies, people who have a higher TyG index are far more prone to suffer from depressed symptoms20,35,36, and that raised IR and TyG index worsen depression and decrease the effectiveness of antidepressant treatments37,38. Based on recent research, patients with coronary heart disease who also have depression may be at risk for significant adverse cardiovascular and cerebrovascular events if their TyG index is elevated39. Prior research has demonstrated a nonlinear correlation between depression and the TyG index in hypertensive people in the United States40. Suicidal thoughts and actions are more common in those with higher TyG scores36. In addition, depression may exacerbate the severity of IR and raise the TyG index41. In our study, 206 patients (38%) developed early-onset PSD, a prevalence concordant with previous reports42,43,44,45. We observed a significant positive correlation between TyG index levels and the severity of early-onset PSD. Moreover, after comprehensive adjustment for potential confounders, binary logistic regression analysis confirmed that the TyG index remained independently associated with early-onset PSD, aligning with emerging evidence in this field46. Our research further confirmed the significant association between the TyG index and the risk of early-onset PSD.
While the precise mechanism connecting the TyG index to early-onset PSD is not yet fully elucidated, several pathophysiological pathways may explain this association. Firstly, inflammation is postulated as a key pathophysiological link between the TyG index—a surrogate marker of IR—and PSD. This hypothesis is supported by several lines of evidence. On the one hand, proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), have been confirmed to be closely related to the occurrence and development of PSD47,48. Inhibition of TNF-α has been shown to improve glucose tolerance and insulin sensitivity, which provides direct evidence that inflammation leads to IR49. Furthermore, the strong correlation between TyG index and peripheral blood inflammatory markers, such as white blood cell count and high-sensitivity C-reactive protein, further supports that inflammation is an important mechanism mediating the association between TyG index and PSD50. Secondly, oxidative stress is another important pathophysiological mechanism linking IR and PSD. Oxidative stress is closely related to IR51. Meanwhile, oxidative stress plays a key role in the pathophysiological process of PSD. Animal experiments have shown that superoxide dismutase activity in the hippocampus of PSD model rats is significantly changed52. Moreover, serum levels of malondialdehyde, one of the biomarkers of oxidative stress, were positively correlated with HAMD scores in stroke patients53. These findings suggest that chronic oxidative stress, exacerbated by insulin resistance, may contribute to PSD by directly impairing neurological function. Finally, hypothalamic–pituitary–adrenal (HPA) axis dysfunction is considered to be another common mechanism linking IR and PSD. A stroke event initiates a cascade of neuroendocrine alterations, resulting in dysregulation of the HPA axis54. Concurrently, previous studies have demonstrated a strong association between excessive activation of the HPA axis and the development of insulin resistance syndrome55. In addition, the HPA axis activity indexes, such as plasma cortisol concentration and its peak value, are positively correlated with HOMA-IR index in elderly patients with depression56. Taken together, HPA axis dysfunction constitutes a critical physiological bridge that may simultaneously promote insulin resistance and PSD.
A previous study found that an elevated TG/HDL-C ratio was indicative of higher risk and accelerated arterial stiffness development in the hypertensive group57. The TG/HDL-C ratio may be a unique biomarker for cardiovascular disease outcomes and progression23. Moreover, a higher risk of cardiovascular disease was linked to an elevated TG/HDL-C ratio14. Nevertheless, it is still unknown how the TG/HDL-C ratio and early-onset PSD are related. Evidence suggests that dysregulation of lipid profiles is associated with the risk of PSD. Specifically, our findings demonstrated a significant association between elevated LDL-C/HDL-C ratios, decreased HDL-C levels, and increased PSD risk26. Another cross-sectional study further demonstrated that an elevated monocyte-to-HDL cholesterol ratio was strongly associated with a higher PSD risk, indirectly supporting the role of reduced HDL-C in PSD pathogenesis58. However, conflicting findings have been reported; a retrospective study indicated that higher HDL-C levels were significantly associated with an increased risk of depressive symptoms59. These inconsistencies highlight the translational need for novel biomarkers that can accurately identify high-risk individuals, thereby enabling the development of stratified management approaches for early-onset PSD. In our study, the TG/HDL-C ratio in the early-onset group was significantly higher than that in the non-PSD group. In addition, our study shows that the TG/HDL-C ratio had a positive relationship with the severity of early-onset PSD. Finally, the TG/HDL-C ratio was identified as an independent factor for early-onset PSD after adjustment for potential confounder, which is consistent with the latest study60. According to previous research, bipolar disorder and metabolic syndrome share endocrine abnormalities, sympathetic nervous system dysregulation, and behavioral tendencies like inactivity and overeating61. Adults who had their first severe depressive episode had lower HDL-C and higher TG than healthy controls62. Furthermore, a number of studies have discovered a correlation between high TC, LDL-C, TG, and TG/HDL-C ratio and depressive episodes and symptoms63,64,65. The precise mechanism through which the TG/HDL‑C ratio elevates the risk of early‑onset PSD remains incompletely elucidated. However, several plausible pathophysiological pathways may account for this relationship. Firstly, lipids modulate serotonin activity by influencing receptor–ligand binding, internalization, and signal transduction, thereby potentially contributing to mood dysregulation66,67,68. In addition, dyslipidemia-particularly low HDL‑C and elevated LDL‑C-may initiate or amplify inflammatory cascades. In the acute phase of stroke, inflammatory responses provoke the release of multiple pro‑inflammatory cytokines, which have been associated with an increased risk of depressive symptoms69. Finally, oxidative stress plays a central role in PSD pathogenesis. It disrupts neurotransmitter homeostasis by promoting peroxidation of lipids and proteins, ultimately leading to impaired neural function70,71. Notably, by compromising critical protective functions against oxidation and inflammation, diminished HDL-C levels may increase the brain’s susceptibility to damage, thereby exacerbating neurodegenerative processes72,73.The findings of our study have enhanced our comprehension of the TG/HDL-C ratio’s significance in early-onset PSD and offered novel insights into future treatment options. However, more study is needed to examine the connection between the development of early-onset PSD and the TG/HDL-C ratio.
Additionally, consistent with previous research, patients with early-onset PSD showed more severe neurological deficits (NIHSS) and functional disability (mRS)1,43. This elevated disability burden likely contributes to the worsening of depressive symptoms and other psychological comorbidities74. Some previous studies have noted that PSD was strongly associated with the lesion location of brain, suggesting that the left side prefrontosubcortical circuits played a role in development of PSD75,76. No significant associations were observed in the present analysis. We speculate that these null findings may be attributed to population heterogeneity in ethnicity, limited sample size, variations in medication status, and differences in baseline disease severity across studies.
ROC analysis demonstrated that both the TyG index and TG/HDL-C ratio exhibited favorable discriminatory ability for early-onset PSD. The TyG index showed superior diagnostic performance compared to the TG/HDL-C ratio, suggesting its potential utility as a predictive tool for early-onset PSD. Moreover, the results of our research showed the combined effects of the TyG index and TG/HDL-C ratio exhibited superior discriminatory power for early-onset PSD, with an AUC of 0.813. This value surpassed that of the individual markers and suggests that the combination of these two indicators may be more beneficial in predicting early-onset PSD.
This research has a number of shortcomings, which are as follows: (1) as a cross-sectional study conducted exclusively in a Chinese population, the findings may be subject to inherent selection biases and require validation in other ethnic groups through future longitudinal studies; (2) direct assessment of insulin resistance was not feasible due to constraints in our clinical setting; (3) the exclusion of patients with severe aphasia, coma, or dementia may have affected the estimated prevalence of early-onset PSD; (4) potential confounding factors such as chronic life stress, educational attainment, and social support were not considered; (5) findings from this short-term observational study warrant verification in long-term investigations; (6) as with any observational study, residual confounding by unmeasured factors (e.g., social support, genetic predisposition) remains a possibility; and (7) this result was applied at only a mild stroke patient, because median NIHSS score was 2 and a half of patients was SAO.
Conclusion
Our study identified the TyG index and TG/HDL-C ratio as independent risk factors for early-onset PSD, supporting their utility as predictive biomarkers. Furthermore, the combination of these indices demonstrated superior predictive value compared to either marker alone. The identification of an association between the TyG index, TG/HDL-C ratio, and early-onset PSD holds substantial clinical implications. It would facilitate proactive screening for high-risk individuals, inform the development of personalized treatment plans, contribute to the elucidation of underlying pathophysiological mechanisms, and improve prognostic evaluations. The integration of these readily available biomarkers into standard assessment protocols is expected to enhance risk stratification and ultimately lead to improved overall clinical outcomes for stroke patients. Nevertheless, these findings warrant further validation in larger, multi-center cohorts, and the underlying pathophysiology of early-onset PSD requires more comprehensive investigation.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Deng, M. et al. Higher homocysteine and fibrinogen are associated with early-onset post-stroke depression in patients with acute ischemic stroke. Front. Psychiatry 15, 1371578. https://doi.org/10.3389/fpsyt.2024.1371578 (2024).
Zhou, J., Fangma, Y., Chen, Z. & Zheng, Y. Post-stroke neuropsychiatric complications: Types, pathogenesis, and therapeutic intervention. Aging Dis. 14, 2127–2152. https://doi.org/10.14336/ad.2023.0310-2 (2023).
Huang, J. et al. Predictors of remission of early-onset poststroke depression and the interaction between depression and cognition during follow-up. Front. Psych. 9, 738. https://doi.org/10.3389/fpsyt.2018.00738 (2018).
Lin, W. et al. Severe periodontitis is associated with early-onset poststroke depression status. J. Stroke Cerebrovasc. Dis. 28, 104413. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104413 (2019).
Zeng, Y. Y. et al. Early-onset depression in stroke patients: effects on unfavorable outcome 5 years post-stroke. Front. Psychiatry 12, 556981. https://doi.org/10.3389/fpsyt.2021.556981 (2021).
Cai, W., Mueller, C., Li, Y. J., Shen, W. D. & Stewart, R. Post stroke depression and risk of stroke recurrence and mortality: A systematic review and meta-analysis. Ageing Res. Rev. 50, 102–109. https://doi.org/10.1016/j.arr.2019.01.013 (2019).
Zhang, B. et al. Association between triglyceride-glucose index and early neurological outcomes after thrombolysis in patients with acute ischemic stroke. J. Clin. Med. 12, 3471. https://doi.org/10.3390/jcm12103471 (2023).
Deng, M. et al. Association of higher triglyceride-glucose index and triglyceride-to-high-density lipoprotein cholesterol ratio with early neurological deterioration after thrombolysis in acute ischemic stroke patients. Front. Neurol. 15, 1421655. https://doi.org/10.3389/fneur.2024.1421655 (2024).
Püschel, G. P., Klauder, J. & Henkel, J. Macrophages, low-grade inflammation, insulin resistance and hyperinsulinemia: A mutual ambiguous relationship in the development of metabolic diseases. J. Clin. Med. 11, 4358. https://doi.org/10.3390/jcm11154358 (2022).
Song, J. Amygdala activity and amygdala-hippocampus connectivity: Metabolic diseases, dementia, and neuropsychiatric issues. Biomed. Pharmacother. 162, 114647. https://doi.org/10.1016/j.biopha.2023.114647 (2023).
McIntyre, R. S. Surrogate markers of insulin resistance in predicting major depressive disorder: Metabolism metastasizes to the brain. Am. J. Psychiatry 178, 885–887. https://doi.org/10.1176/appi.ajp.2021.21080814 (2021).
Wang, J. et al. Association of triglyceride-glucose index with early neurological deterioration events in patients with acute ischemic stroke. Diabetol. Metab. Syndr. 15, 112. https://doi.org/10.1186/s13098-023-01091-0 (2023).
He, J. et al. Triglyceride-glucose index as a suitable non-insulin-based insulin resistance marker to predict cardiovascular events in patients undergoing complex coronary artery intervention: A large-scale cohort study. Cardiovasc. Diabetol. 23, 15. https://doi.org/10.1186/s12933-023-02110-0 (2024).
Che, B. et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: An analysis of UK biobank data. Cardiovasc. Diabetol. 22, 34. https://doi.org/10.1186/s12933-023-01762-2 (2023).
Si, S. et al. Causal Effect of the triglyceride-glucose index and the joint exposure of higher glucose and triglyceride with extensive cardio-cerebrovascular metabolic outcomes in the UK Biobank: A Mendelian randomization study. Front. Cardiovasc. Med. 7, 583473. https://doi.org/10.3389/fcvm.2020.583473 (2020).
Lee, S. B. et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc. Diabetol. 17, 41. https://doi.org/10.1186/s12933-018-0692-1 (2018).
Ma, X. et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc. Diabetol. 19, 31. https://doi.org/10.1186/s12933-020-01006-7 (2020).
Kim, J., Shin, S. J. & Kang, H. T. The association between triglyceride-glucose index, cardio-cerebrovascular diseases, and death in Korean adults: A retrospective study based on the NHIS-HEALS cohort. PLoS ONE 16, e0259212. https://doi.org/10.1371/journal.pone.0259212 (2021).
Zhang, B. et al. Triglyceride-glucose index linked to hospital mortality in critically Ill stroke: An observational multicentre study on eICU database. Front. Med. 7, 591036. https://doi.org/10.3389/fmed.2020.591036 (2020).
Nam, K. W., Kwon, H. M. & Lee, Y. S. High triglyceride-glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci. Rep. 11, 15335. https://doi.org/10.1038/s41598-021-94631-5 (2021).
Shi, Y. Y., Zheng, R., Cai, J. J. & Qian, S. Z. The association between triglyceride glucose index and depression: Data from NHANES 2005–2018. BMC Psychiatry 21, 267. https://doi.org/10.1186/s12888-021-03275-2 (2021).
Liu, J. et al. Association between triglyceride glucose index and suicide attempts in patients with first-episode drug-naïve major depressive disorder. Front. Psychiatry 14, 1231524. https://doi.org/10.3389/fpsyt.2023.1231524 (2023).
Salazar, M. R. et al. Comparison of two surrogate estimates of insulin resistance to predict cardiovascular disease in apparently healthy individuals. Nutr. Metab. Cardiovasc. Dis. 27, 366–373. https://doi.org/10.1016/j.numecd.2016.12.002 (2017).
Liu, F. et al. Combined influence of depression symptoms and ratio of triglyceride to high-density lipoprotein cholesterol on cardiometabolic multimorbidity: Findings from the China Health and Retirement Longitudinal Study 2011–2018. J. Affect. Disord. 360, 242–248. https://doi.org/10.1016/j.jad.2024.05.159 (2024).
Zhang, Y. et al. Diabetes mellitus is associated with late-onset post-stroke depression. J. Affect. Disord. 221, 222–226. https://doi.org/10.1016/j.jad.2017.06.045 (2017).
Shen, H. et al. Serum lipid profiles and post-stroke depression in acute ischemic stroke patients. Neuropsychiatr. Dis. Treat. 15, 1573–1583. https://doi.org/10.2147/ndt.S204791 (2019).
Simental-Mendía, L. E., Rodríguez-Morán, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 6, 299–304. https://doi.org/10.1089/met.2008.0034 (2008).
Szablewski, L. Glucose transporters in brain. In health and in Alzheimer’s disease. J. Alzheimers Dis. JAD 55, 1307–1320. https://doi.org/10.3233/jad-160841 (2017).
Jin, M. et al. Association of triglyceride-glucose index with major depressive disorder: A cross-sectional study. Medicine 102, e34058. https://doi.org/10.1097/md.0000000000034058 (2023).
Watson, K. T. et al. Incident major depressive disorder predicted by three measures of insulin resistance: A Dutch cohort study. Am. J. Psychiatry 178, 914–920. https://doi.org/10.1176/appi.ajp.2021.20101479 (2021).
Fernandes, B. S. et al. Insulin resistance in depression: A large meta-analysis of metabolic parameters and variation. Neurosci. Biobehav. Rev. 139, 104758. https://doi.org/10.1016/j.neubiorev.2022.104758 (2022).
Qiu, H. C., Liu, H. Z., Li, X., Zeng, X. & Zhao, J. Z. Insulin resistance as estimated by homeostasis model assessment predicts incident post-stroke depression in Chinese subjects from ischemic stroke. J. Affect. Disord. 231, 1–7. https://doi.org/10.1016/j.jad.2018.01.023 (2018).
Yi, X. et al. The Combination of insulin resistance and serum interleukin-1β correlates with post-stroke depression in patients with acute ischemic stroke. Neuropsychiatr. Dis. Treat. 17, 735–746. https://doi.org/10.2147/ndt.S291164 (2021).
Lin, S. F. et al. Triglyceride-glucose index and intravenous thrombolysis outcomes for acute ischemic stroke: A multicenter prospective-cohort study. Front. Neurol. 13, 737441. https://doi.org/10.3389/fneur.2022.737441 (2022).
Wan, W. & Yu, Y. Association between the triglyceride glucose index and depression: a meta-analysis. Front. Psychiatry 15, 1390631. https://doi.org/10.3389/fpsyt.2024.1390631 (2024).
Behnoush, A. H. et al. The importance of assessing the triglyceride-glucose index (TyG) in patients with depression: A systematic review. Neurosci. Biobehav. Rev. 159, 105582. https://doi.org/10.1016/j.neubiorev.2024.105582 (2024).
Liu, J. et al. Association between triglyceride glucose index (TyG) and psychotic symptoms in patients with first-episode drug-naïve major depressive disorder. Front. Psychiatry 15, 1342933. https://doi.org/10.3389/fpsyt.2024.1342933 (2024).
Zheng, L. et al. Longitudinal association between triglyceride glucose index and depression progression in middle-aged and elder adults: A national retrospective cohort study. Nutr. Metab. Cardiovasc. Dis. 33, 507–515. https://doi.org/10.1016/j.numecd.2022.11.015 (2023).
Zhao, W. et al. Triglyceride-glucose index as a potential predictor of major adverse cardiovascular and cerebrovascular events in patients with coronary heart disease complicated with depression. Front. Endocrinol. 15, 1416530. https://doi.org/10.3389/fendo.2024.1416530 (2024).
Zhang, X., Zhao, D., Guo, S., Yang, J. & Liu, Y. Association between triglyceride glucose index and depression in hypertensive population. J. Clin. Hypertens. 26, 177–186. https://doi.org/10.1111/jch.14767 (2024).
Gruber, J. et al. Impact of insulin and insulin resistance on brain dopamine signalling and reward processing - An underexplored mechanism in the pathophysiology of depression?. Neurosci. Biobehav. Rev. 149, 105179. https://doi.org/10.1016/j.neubiorev.2023.105179 (2023).
Liu, L. et al. Prevalence and natural history of depression after stroke: A systematic review and meta-analysis of observational studies. PLoS Med. 20, e1004200. https://doi.org/10.1371/journal.pmed.1004200 (2023).
Zhou, H. et al. Lesion location and serum levels of homocysteine are associated with early-onset post-stroke depression in acute ischemic stroke. Brain Behav. 13, e3210. https://doi.org/10.1002/brb3.3210 (2023).
Deng, M. et al. Association of atherogenic index of plasma with early-onset post-stroke depression: A prospective study. Front. Psychiatry 16, 1563289. https://doi.org/10.3389/fpsyt.2025.1563289 (2025).
Deng, M. et al. Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and the risk of early-onset post-stroke depression: A prospective study. Front. Neurol. 16, 1645765. https://doi.org/10.3389/fneur.2025.1645765 (2025).
Liang, F., Shan, X., Chen, X. & Yang, B. The association between triglyceride-glucose index and its combination with post-stroke depression: NHANES 2005–2018. BMC Psychiatry 25, 243. https://doi.org/10.1186/s12888-025-06676-9 (2025).
Wang, L., Chunyou, C., Zhu, J., Bao, X. & Tao, X. Prediction of post-stroke depression with combined blood biomarkers IL-6, TNF-a, and fatty acid binding protein: A prospective study. J. Med. Biochem. 42, 638–644. https://doi.org/10.5937/jomb0-43904 (2023).
Mu, Y., Wang, Z., Zhou, J., Tan, C. & Wang, H. Correlations of post-stroke depression with inflammatory response factors. Iran. J. Public Health 47, 988–993 (2018).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 259, 87–91. https://doi.org/10.1126/science.7678183 (1993).
Nam, K. W. et al. High triglyceride-glucose index is associated with subclinical cerebral small vessel disease in a healthy population: A cross-sectional study. Cardiovasc. Diabetol. 19, 53. https://doi.org/10.1186/s12933-020-01031-6 (2020).
Hurrle, S. & Hsu, W. H. The etiology of oxidative stress in insulin resistance. Biomed. J. 40, 257–262. https://doi.org/10.1016/j.bj.2017.06.007 (2017).
Hou, X. et al. CDDO-Im exerts antidepressant-like effects via the Nrf2/ARE pathway in a rat model of post-stroke depression. Brain Res. Bull. 173, 74–81. https://doi.org/10.1016/j.brainresbull.2021.05.008 (2021).
Liu, Z. et al. Malondialdehyde: A novel predictive biomarker for post-stroke depression. J. Affect. Disord. 220, 95–101. https://doi.org/10.1016/j.jad.2017.05.023 (2017).
Datta, A. et al. Neuroendocrine regulation in stroke. Trends Endocrinol. Metab. 34, 260–277. https://doi.org/10.1016/j.tem.2023.02.005 (2023).
Phillips, D. I. et al. Elevated plasma cortisol concentrations: A link between low birth weight and the insulin resistance syndrome?. J. Clin. Endocrinol. Metab. 83, 757–760. https://doi.org/10.1210/jcem.83.3.4634 (1998).
Yokoyama, K. et al. Relationship between hypothalamic-pituitary-adrenal axis dysregulation and insulin resistance in elderly patients with depression. Psychiatry Res. 226, 494–498. https://doi.org/10.1016/j.psychres.2015.01.026 (2015).
Wu, Z. et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc. Diabetol. 20, 134. https://doi.org/10.1186/s12933-021-01330-6 (2021).
Li, Y., Zhang, M., Xue, M., Liu, D. & Sun, J. Elevated monocyte-to-HDL cholesterol ratio predicts post-stroke depression. Front. Psychiatry 13, 902022. https://doi.org/10.3389/fpsyt.2022.902022 (2022).
Shin, H. Y. et al. Relationships between high-density lipoprotein cholesterol and depressive symptoms: Findings of the Korean National Health and Nutrition Examination Survey (KNHANES). Psychiatry Res. 241, 172–174. https://doi.org/10.1016/j.psychres.2016.05.003 (2016).
Wang, P. et al. Association between four non-insulin-based insulin resistance indices and the risk of post-stroke depression. Clin. Interv. Aging 20, 19–31. https://doi.org/10.2147/cia.S501569 (2025).
McElroy, S. L. & Keck, P. E. Jr. Metabolic syndrome in bipolar disorder: A review with a focus on bipolar depression. J. Clin. Psychiatry 75, 46–61. https://doi.org/10.4088/JCP.13r08634 (2014).
Wei, Y. G. et al. Cholesterol and triglyceride levels in first-episode patients with major depressive disorder: A meta-analysis of case-control studies. J. Affect. Disord. 266, 465–472. https://doi.org/10.1016/j.jad.2020.01.114 (2020).
Gohar, S. M. et al. Association between serum lipid levels, osteoprotegerin and depressive symptomatology in psychotic disorders. Eur. Arch. Psychiatry Clin. Neurosci. 269, 795–802. https://doi.org/10.1007/s00406-018-0897-z (2019).
Kalelioğlu, T. et al. Atherogenic index of plasma as a cardiovascular risk marker in manic, depressive, and euthymic stages of bipolar disorder. Turk Kardiyol. Dern. Ars. 46, 32–38. https://doi.org/10.5543/tkda.2017.23350 (2018).
Moreira, F. P. et al. Metabolic syndrome in subjects with bipolar disorder and major depressive disorder in a current depressive episode: Population-based study: Metabolic syndrome in current depressive episode. J. Psychiatr. Res. 92, 119–123. https://doi.org/10.1016/j.jpsychires.2017.03.025 (2017).
Persons, J. E. & Fiedorowicz, J. G. Depression and serum low-density lipoprotein: A systematic review and meta-analysis. J. Affect. Disord. 206, 55–67. https://doi.org/10.1016/j.jad.2016.07.033 (2016).
da Graça Cantarelli, M., Tramontina, A. C., Leite, M. C. & Gonçalves, C. A. Potential neurochemical links between cholesterol and suicidal behavior. Psychiatry Res. 220, 745–751. https://doi.org/10.1016/j.psychres.2014.10.017 (2014).
Björk, K., Sjögren, B. & Svenningsson, P. Regulation of serotonin receptor function in the nervous system by lipid rafts and adaptor proteins. Exp. Cell Res. 316, 1351–1356. https://doi.org/10.1016/j.yexcr.2010.02.034 (2010).
Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y. & Rosenberg, G. A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 16, 142. https://doi.org/10.1186/s12974-019-1516-2 (2019).
Zhang, H. & Dhalla, N. S. The role of pro-inflammatory cytokines in the pathogenesis of cardiovascular disease. Int. J. Mol. Sci. 25, 1082. https://doi.org/10.3390/ijms25021082 (2024).
Alsbrook, D. L. et al. Neuroinflammation in acute ischemic and hemorrhagic stroke. Curr. Neurol. Neurosci. Rep. 23, 407–431. https://doi.org/10.1007/s11910-023-01282-2 (2023).
Immanuel, J. & Yun, S. Vascular inflammatory diseases and endothelial phenotypes. Cells 12, 1640. https://doi.org/10.3390/cells12121640 (2023).
Morris, G. et al. The role of high-density lipoprotein cholesterol, apolipoprotein A and paraoxonase-1 in the pathophysiology of neuroprogressive disorders. Neurosci. Biobehav. Rev. 125, 244–263. https://doi.org/10.1016/j.neubiorev.2021.02.037 (2021).
Thabrew, H. et al. Psychological therapies for anxiety and depression in children and adolescents with long-term physical conditions. Cochrane Database Syst. Rev. 12, Cd012488. https://doi.org/10.1002/14651858.CD012488.pub2 (2018).
Robinson, R. G. & Price, T. R. Post-stroke depressive disorders: A follow-up study of 103 patients. Stroke 13, 635–641. https://doi.org/10.1161/01.str.13.5.635 (1982).
Vataja, R. et al. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch. Gen. Psychiatry 58, 925–931. https://doi.org/10.1001/archpsyc.58.10.925 (2001).
Funding
This study was funded by the Natural Science Foundation of Hunan Province (grant no. 2023JJ40072), the Science and Technology Planning Project of Hunan Health Committee (grant no. D202303076376), the Medical Clinical Research 4310 Project of University of South China (grant no. KJZX2021078), and Hunan Science and Technology Innovation Key Project (grant no. 2020SK1012).
Author information
Authors and Affiliations
Contributions
Design of the work: M.D., F.L., Z.W., and K.S.; methodology, investigation, and data analysis: Z.W., X.M., S.C., and W.X.; manuscript writing and validation: Z.W., M.D., and F.L. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Informed consent was obtained from all subjects and/or their legal guardian(s) and allowed investigators to measure their blood sample levels.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deng, M., Wang, Z., Zhao, W. et al. Association of higher triglyceride glucose index and triglyceride to high density lipoprotein cholesterol ratio with early-onset post-stroke depression. Sci Rep 15, 43935 (2025). https://doi.org/10.1038/s41598-025-27632-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-27632-3