Abstract
Cotton (Gossypium spp.) is a key crop in Kazakhstan, yet its productivity is constrained by saline-alkaline soils, which affect approximately 41% of the nation’s arable land. Conventional soil remediation methods are often unsustainable and economically impractical. This study investigates the potential of Bacillus megaterium PEF-1, a plant growth-promoting rhizobacterium (PGPR) isolated from cotton rhizospheres in the Turkestan region, as a biofertilizer for saline-alkaline conditions. The strain demonstrated high tolerance to salinity (up to 10% NaCl), alkalinity (pH 9.0), and heavy metals, and produced significant levels of indole-3-acetic acid (IAA; 895 mg/L in vitro). Field trials with seed inoculation (10⁹ CFU/mL) showed marked improvements in cotton growth, including increases in plant height (18%), boll number (22%), and yield (25%) relative to controls. Soil analyses revealed enhanced nutrient availability in treated plots: nitrogen (+ 4.3%), phosphorus (+ 15.1%), potassium (+ 26.8%), and humus content (+ 29.3%). Stress mitigation was achieved through ACC deaminase-mediated ethylene reduction and superoxide dismutase (SOD)-driven reactive oxygen species (ROS) scavenging. Acute toxicity tests, conducted in accordance with OECD guidelines, confirmed the strain’s biosafety with no adverse effects in mammalian models. Bacillus megaterium PEF-1 exhibits strong potential as a sustainable biofertilizer for improving cotton yields and soil fertility under saline-alkaline stress. Its capacity for nutrient mobilization, phytohormone synthesis, and abiotic stress alleviation aligns with the United Nations Sustainable Development Goals (SDGs) 2 (Zero Hunger) and 15 (Life on Land), offering a scalable, eco-friendly alternative to chemical fertilizers for arid and degraded agroecosystems.
Similar content being viewed by others
Introduction
The rhizosphere - the narrow zone of soil directly influenced by root exudates and associated microbial communities - serves as a critical interface for plant - microbe interactions that regulate plant health, nutrient uptake, and resilience to abiotic stresses1. Often referred to as the “second genome” of plants, the rhizosphere microbiome plays a key role in shaping soil nutrient dynamics, particularly under environmentally challenging conditions. Among its vital functions, the microbial facilitation of phosphorus (P) bioavailability is especially significant in saline-alkaline soils, where nutrient solubility and plant uptake are markedly limited2.
Saline-alkaline stress is a major constraint in agricultural production, as it adversely affects soil microbial diversity, enzymatic activity, and the efficiency of nutrient cycling, ultimately hindering plant growth and reducing crop yields3,4,5,6,7,8. Traditional soil remediation methods, such as gypsum application and deep leaching, are often labor-intensive, costly, and unsustainable in the long term9. Therefore, there is an urgent need for ecologically sound strategies that can simultaneously enhance crop resilience and restore soil fertility.
Plant growth-promoting rhizobacteria (PGPR) offer a sustainable and biologically driven solution to improve plant performance under stress conditions. Among these, phosphate-solubilizing bacteria (PSB)—particularly species within the genus Bacillus—are recognized for their ability to convert insoluble phosphate compounds into plant-available forms through the production of organic acids and phosphatases. This activity significantly enhances phosphorus uptake and plant productivity in nutrient-poor or saline-alkaline soils10,11. Notably, Bacillus megaterium and B. subtilis have demonstrated considerable halotolerance, strong root colonization capacity, and the potential to reshape the rhizosphere microbiome under abiotic stress conditions12,13,14,15.
Cotton (Gossypium spp.), a key cash crop in Kazakhstan, is particularly susceptible to salinity stress, which affects more than 73,000 hectares of irrigated land in the Turkestan region. This vulnerability is further exacerbated by excessive irrigation, saline groundwater intrusion, and high evaporation rates, all of which contribute to widespread soil salinization, reduced crop productivity, and economic losses estimated at 30–50%16,17,18. In response to these challenges, the development and application of microbial biofertilizers adapted to harsh soil environments represent a promising strategy for sustainable cotton cultivation.
Among these, Bacillus megaterium PEF-1—a novel strain isolated from the saline rhizosphere of cotton fields in the Turkestan region—exhibits a range of beneficial traits that support plant growth and mitigate environmental stress. These include high salinity tolerance (up to 10% NaCl), efficient phosphate solubilization, and substantial production of indole-3-acetic acid (IAA) (895 mg/L)19,20,21,22,23,24,25,26. Field trials conducted in the Maktaaral district demonstrated that inoculation with PEF-1 significantly improved cotton agronomic performance, including an 18% increase in plant height, a 22% increase in boll number, and a 25% increase in yield compared to non-inoculated controls. These improvements are associated with the expression of stress-responsive genes such as acdS, encoding 1-aminocyclopropane-1-carboxylate (ACC) deaminase, which reduces ethylene-induced stress, and sodA, encoding superoxide dismutase, which alleviates oxidative damage27.
The multifunctionality of Bacillus megaterium PEF-1 supports its integration into climate-resilient agricultural strategies, particularly in marginal and stress-prone environments. Recent transcriptomic analyses have further confirmed its ability to activate stress-responsive gene networks and antioxidant defense pathways, thereby enhancing drought and salinity tolerance in various crops, including cotton and rice28,29,30,31. These findings underscore the broader potential of PEF-1-based biofertilizers for ecological intensification and sustainable yield improvement.
The present study aims to evaluate the biofertilizer potential of Bacillus megaterium PEF-1 for organic cotton cultivation under saline-alkaline soil conditions in the Turkestan region. By integrating field experiments, microbial functional assays, and rhizosphere microbiome profiling, we assess the effects of PEF-1 on plant physiological performance and soil biochemical properties. The findings are expected to advance microbial technologies for climate-smart agriculture and enhance our understanding of beneficial plant–microbe interactions in degraded agroecosystems.
Materials and methods
Soil sampling and preparation
Composite soil samples (0–20 cm depth) were collected from homogeneous plots in the Maktaaral District, Kazakhstan, in accordance with national standard GOST 28168-8932,33. The samples were air-dried at room temperature (20–25 °C), sieved through a 2 mm mesh, and stored under laboratory conditions for further analysis, following GOST 26423-8534.
Organic matter and humus content. Soil organic carbon was determined using the Walkley-Black wet oxidation method involving K₂Cr₂O₇ and H₂SO₄, followed by titration with FeSO₄. Humus content was calculated by multiplying the measured organic carbon by a factor of 1.72435,36.
Available nitrogen. Mineralizable nitrogen content was assessed using a modified Kjeldahl digestion procedure, followed by steam distillation and acid-base titration37,38.
Available phosphorus and potassium. Available phosphorus was extracted using the Olsen method (NaHCO₃, pH 8.5) and quantified spectrophotometrically at 880 nm39. Exchangeable potassium was extracted with neutral ammonium acetate (NH₄OAc, pH 7.0) and measured via flame photometry at 766.5 nm30,40.
Salinity and ion composition. Total soil salinity was assessed by determining electrical conductivity in a 1:5 soil-to-water extract, while pH was measured in the same extract27. Alkalinity, represented by bicarbonate (HCO₃⁻), was measured by titration with H₂SO₄ using methyl orange as an indicator41. The concentrations of Cl⁻, Ca²⁺, Mg²⁺, Na⁺, and K⁺ ions were determined using atomic absorption spectroscopy (AAS)42.
Sulfate determination (SO₄²⁻). Sulfate content was measured using the turbidimetric method with BaCl₂ as a precipitating agent and absorbance measured at 420 nm29,30,31,32.
Comparative analysis with reference strains. For comparative purposes, reference strains (Bacillus subtilis C7, Pseudomonas fluorescens C9, and Azospirillum brasilense C4) were obtained from the microbial culture collection of al-Farabi Kazakh National University. This collection, maintained by the university-wide research laboratory of biotechnology, is specialized in the development of biologically active agents for sustainable agriculture43.
Statistical Analysis. Data were subjected to one-way analysis of variance (ANOVA) using R software (v4.3.1), with statistical significance set at p < 0.0544.
Isolation and characterization of Bacillus megaterium PEF-1
Soil samples were collected from the rhizosphere of cotton (Gossypium hirsutum) grown in saline-alkaline soils (pH 8.2–9.1; 10–20 cm depth) in the Turkestan region, Kazakhstan. The samples were suspended in sterile 0.85% NaCl solution and serially diluted (10⁻³-10⁻⁶). Aliquots (100 µL) from each dilution were plated onto nutrient agar (NA) supplemented with 2% NaCl and incubated at 30 °C for 48–72 h. Colonies with typical Bacillus-like morphology - opaque, cream-colored, and with irregular margins - were selected and purified by repeated streaking on fresh NA plates45,46,47,48.
Morphological and physiological identification. The purified B. megaterium PEF-1 colonies were cream-colored with smooth to slightly wavy edges. Microscopic observation confirmed the presence of Gram-positive rods with endospores, verified by Gram staining and Schaeffer–Fulton spore staining techniques. Salt and pH tolerance were evaluated on NA media supplemented with varying NaCl concentrations (0–10%) and pH levels (7.0–10.0). Optimal growth was observed at 3–5% NaCl and pH 8.5–9.0. Biochemical characterization, following the protocols described in Bergey’s Manual of Systematic Bacteriology, revealed that the strain was catalase-positive, oxidase-negative, capable of starch hydrolysis, and utilized citrate as a sole carbon source49,50,51.
Molecular identification
Bacillus megaterium strain PEF-1 was isolated in 2023 from saline-alkaline soils in the Maktaaral District, Kazakhstan. Genomic DNA was extracted using the PureLink® Genomic DNA Mini Kit (Invitrogen, USA), following the manufacturer’s protocol. The strain was identified through 16S rRNA gene sequencing using universal primers 27F (5’-AGAGTTTGATCMTGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGACTT-3’). PCR amplification was performed under the following conditions: initial denaturation at 95 °C for 5 min; followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 90 s; with a final extension at 72 °C for 10 min. The PCR products were purified and sequenced using an ABI 3500xl Genetic Analyzer with BigDye Terminator v3.1 chemistry (Applied Biosystems). Sequences were analyzed via BLASTn against the NCBI nucleotide database. A phylogenetic tree was constructed using the maximum-likelihood method in MEGA X software with 1000 bootstrap replications52]– [53. The 16 S rRNA gene sequence of strain PEF-1 has been deposited in GenBank under the accession number PV940685. The MEGA11 program was used for the URL: https://www.megasoftware.net54.
Acute toxicity testing
Acute toxicity testing of B. megaterium PEF-1 was performed in accordance with OECD guidelines (2001). A total of 108 healthy laboratory mice were randomly divided into nine groups (12 mice per group). Groups 1–4 received intraperitoneal injections of bacterial suspensions at concentrations of 10³, 10⁶, 10⁷, and 10⁹ CFU/mL, respectively. Groups 5–8 were administered the same doses orally via gavage. Group 9 served as the control and received sterile 0.9% NaCl solution. Mice were observed daily over a 14-day period for signs of toxicity, behavioral changes, and mortality. For the euthanasia of mice in our study, a subset of animals was euthanized on day 14 to allow for internal organ examination as part of the pathogenicity assessment of Bacillus megaterium PEF-1. Euthanasia was performed using an intraperitoneal injection of sodium pentobarbital at a dosage of 150 mg/kg body weight, ensuring rapid and humane induction of anesthesia followed by death. This method was chosen in accordance with international guidelines for humane euthanasia, minimizing animal suffering and adhering to ethical standards for laboratory animal research.
Approval by Institutional/Licensing Committee: All experimental protocols were reviewed and approved by the Local Ethical Committee of al-Farabi Kazakh National University (IRB00010790, al-Farabi Kazakh National University IRB#1) under Protocol №IRB-A540. This approval ensures that the study adhered to rigorous ethical oversight and complied with institutional and international standards for animal research. Compliance with Relevant Guidelines and Regulations: All methods were performed in accordance with the relevant guidelines and regulations, including those set forth by the Declaration of Helsinki and the European Directive 2010/63/EU on the protection of animals used for scientific purposes. The study design and execution were aligned with principles of humane treatment and welfare of laboratory animals.
All experimental procedures were conducted in strict accordance with international ethical standards for the use of animals in research. The reporting of all experimental methods complies with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines to ensure transparency and reproducibility (Percie du Sert et al., 2020). The Methods section includes detailed descriptions of experimental design, animal housing, sample size calculations, randomization, and statistical analyses, aligning with the ARRIVE 2.0 framework (https://arriveguidelines.org). The Bacillus megaterium PEF-1 strain was determined to be non-pathogenic based on comprehensive pathogenicity assessments. On day 14, a subset of mice was humanely euthanized for histopathological examination of internal organs to evaluate tissue-level effects, following protocols to minimize distress. The remaining animals underwent non-invasive biochemical blood analyses, collected in accordance with ethical sampling guidelines to reduce animal stress (Percie du Sert et al., 2020). Data were statistically analyzed using one-way ANOVA with a significance level of p < 0.0555,56,57,58,59,60.
Indole-3-acetic acid (IAA) synthesis assay
Strain cultivation. Bacillus megaterium PEF-1 was grown in either Luria–Bertani (LB) broth or a defined minimal medium containing glucose (5 g/L), K₂HPO₄ (1 g/L), and MgSO₄·7 H₂O (0.2 g/L). Cultures were incubated at 30 °C for 72 h under aerobic conditions with constant shaking at 150 rpm. To evaluate the effect of L-tryptophan concentration on IAA synthesis, L-tryptophan was added to the media at concentrations ranging from 0 to 5 mg/mL.
Simulation of soil stress conditions. To replicate the abiotic stress conditions of saline-alkaline soils found in southern Kazakhstan, PEF-1 cultures were grown in three media variants: (i) Neutral conditions (pH ≈ 7.0); (ii) Saline conditions (with NaCl supplementation); (iii) Alkaline conditions (pH > 8.5). These conditions were designed to imitate the natural physicochemical environment of degraded soils where biofertilizer application is intended.
IAA extraction and quantification. After incubation, cultures were centrifuged at 10,000 rpm for 10 min to remove bacterial cells. The supernatants were filtered through 0.22 μm pore-size membranes. IAA concentration was determined by mixing each filtrate with Salkowski reagent (containing 0.5 M FeCl₃ in 35% HCl). The formation of a pink color indicated the presence of IAA, and absorbance was measured at 530 nm using a UV-Vis spectrophotometer. IAA concentration was calculated using a standard calibration curve prepared with known IAA concentrations in the range of 0–100 µg/mL61,62,63,64.
Gene expression quantification
To investigate the molecular mechanisms underlying the stress-mitigating effects of Bacillus megaterium PEF-1, expression levels of three functional genes - acdS (ethylene detoxification), sodA (antioxidant defense), and halR (salt tolerance regulation) - were quantified. RNA was extracted from cotton rhizosphere soils and root tissues at 14, 28, and 42 days after inoculation (DAI) using the RNeasy Plant Mini Kit (Qiagen, Germany), according to the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed on a Bio-Rad CFX96 detection system using the following gene-specific primers:
acdS-F/R: 5’-ATGCGTAACTGGGATGT-3’ / 5’-TCAGCCATCGTCTTGA-3’.
sodA-F/R: 5’-GCTAGCATGCGTAACTG-3’ / 5’-CGATCGTGACTGGCTA-3’.
halR-F/R: 5’-ATGCCGAACGTATCGTG-3’ / 5’-TCATGCGATCGTACGCT-3’.
PCR conditions were: 40 cycles of 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. Relative gene expression levels were calculated using the 2⁻ΔΔCt method, with non-inoculated controls as the reference. Data were statistically analyzed using one-way ANOVA followed by Tukey’s HSD post hoc test (p < 0.05, n = 4 biological replicates)65– 66.
Enzyme activity and IAA quantification
To assess physiological effects associated with PEF-1, the activities of key enzymes involved in stress mitigation and plant growth were measured, alongside accurate quantification of IAA. ACC deaminase activity was determined in bacterial suspensions (10⁹ CFU/mL) by measuring the production of α-ketobutyrate from ACC substrate, with absorbance recorded at 540 nm. The Michaelis constant (Km) was estimated at 1.2 mM. Superoxide dismutase (SOD) activity was evaluated using the nitroblue tetrazolium (NBT) reduction assay, with absorbance measured at 560 nm to determine antioxidant potential. IAA quantification was performed using high-performance liquid chromatography–mass spectrometry (HPLC-MS) on an Agilent 1260 Infinity system with a C18 reverse-phase column. The mobile phase consisted of methanol: water (60:40, v/v), and detection was carried out at 280 nm. All measurements were conducted in triplicate (n = 3), and results were analyzed by one-way ANOVA with Tukey’s HSD test (p < 0.05)67,68,69,70.
Population dynamics and impact on soil microbial community
A long-term field experiment was carried out on saline-alkaline soils (pH 8.5–9.2) in the Maktaaral district (Turkestan region). Bacillus megaterium PEF-1 was applied once at the start of the growing season at a concentration of 1 × 10⁹ CFU/g of soil. Soil samples were collected from a depth of 10–20 cm at five time points: 0, 1, 3, 6, and 12 months post-inoculation. Untreated plots served as controls71]– [72.
To quantify viable PEF-1 cells, samples were plated on selective media, and colony-forming units (CFU) were enumerated. Counts were log-transformed (log₁₀) prior to statistical analysis. Changes in population size were analyzed using one-way ANOVA followed by Tukey’s post hoc HSD test (p < 0.05). Confidence intervals (95% CI) and effect size (Cohen’s d) were calculated to assess the magnitude and reliability of treatment effects.
Field test conditions
Field trials were conducted in 2023 in the Maktaaral district, Turkestan region, Kazakhstan, on carbonate saline-alkaline soils (pH 8.5–9.2), representative of local cotton-growing areas. The test crop was Gossypium hirsutum L. (upland cotton). Experiments were arranged in a randomized block design with four biological replicates (n = 4). Each plot measured 25 m² and was separated by buffer zones to minimize inter-treatment interference.
Inoculum preparation and plant treatment. Bacillus megaterium PEF-1 was cultured in sterile Luria–Bertani (LB) broth at 30 °C for 48 h under aeration. The final bacterial concentration was ~ 10⁹ CFU/mL, verified by serial dilution and plating on selective media. Cotton seeds were surface-sterilized and soaked in the bacterial suspension for 12 h prior to sowing. Control seeds were treated with sterile saline solution lacking the bacterial strain73,74,75. All agronomic practices - including irrigation, row spacing, and weed control - were standardized across all treatment and control plots to ensure uniform growth conditions.
Assessment of agronomic parameters. At 100 days after germination, the following agronomic traits were measured from 20 randomly selected plants per plot: plant height (cm); Boll length and weight (cm, g); Number of seeds per boll (pcs); Seed weight per boll (g); 100-seed weight (g); Total yield (t/ha). Mean values and 95% confidence intervals were calculated for each parameter76.
Phenotypic assessment. Phenotypic observations were performed during the full flowering stage (BBCH 65) and pre-harvest. Evaluated morphological traits included plant stand density, greenness intensity of the biomass, and the size of generative organs77.
To confirm the environmental safety of B. megaterium PEF-1 application, its effects on non-target soil organisms were evaluated. Acute toxicity to the earthworm Eisenia fetida was assessed according to OECD Guideline 207, using standard survival rate protocols78,79,80.
Results
Soil sampling and preparation
This study assessed the effects of Bacillus megaterium PEF-1, a plant growth-promoting rhizobacterium (PGPR), on soil fertility and the productivity of cotton (Gossypium hirsutum, cv. Ravnak) in saline-alkaline sierozem soils of the Maktaaral district, Kazakhstan. The region is characterized by severe soil constraints, including high salinity, alkalinity, and low organic matter, which collectively limit agricultural productivity. The experimental field consisted of heavy loam sierozem soils with low humus content (0.59–0.80%), a slightly alkaline pH (8.2–9.1), and moderate bulk density (1.15–1.30 g/cm³). These edaphic conditions are representative of approximately 41% of Kazakhstan’s saline-affected arable lands.
Climatic conditions during the 2023 growing season were favorable for crop establishment and microbial activity, with a total precipitation of 467.4 mm, including 212.6 mm during spring. Composite topsoil samples (0–20 cm depth) were collected from each treatment plot prior to sowing and after harvest to evaluate changes in soil nutrient status. Soil samples were air-dried, sieved (2 mm), and analyzed for key physicochemical properties, including available nitrogen, phosphorus, potassium, pH, and humus content. The application of B. megaterium PEF-1 led to a significant improvement in soil fertility indicators compared to untreated controls, including increased availability of macronutrients (N, P, K), enhanced humus levels, and greater pH stability. These findings highlight the potential of PEF-1 as a microbial biofertilizer for improving soil quality and supporting sustainable cotton production under saline-alkaline conditions.
Application of B. megaterium PEF-1 significantly enhanced soil fertility parameters in saline-alkaline sierozem soils of the Maktaaral district. Humus content in treated plots increased by 29.3%, from 0.69% to 0.89% (p < 0.01), in contrast to a 28.6% decline observed in control plots over the same period. Available phosphorus levels rose by 15.1%, from 13.2 to 15.2 mg/kg (p < 0.01), while available potassium content increased by 26.8%, from 180.5 to 228.8 mg/kg (p < 0.01), indicating enhanced nutrient mobilization through microbial solubilization mechanisms. Although available nitrogen increased modestly by 4.3% (45.2 to 47.1 mg/kg), this change was not statistically significant (p > 0.05).
Soil pH in treated plots increased moderately from 8.62 to 9.21, whereas a sharp rise was observed in control plots, reaching 9.99 (p < 0.01). This suggests that B. megaterium PEF-1 contributes to buffering soil alkalinity under saline stress conditions. The observed improvements in humus content, macronutrient availability, and pH stabilization demonstrate the strain’s potential as an effective biofertilizer for saline-affected cotton agroecosystems (Table 1).
The influence of B. megaterium PEF-1 on cotton growth was assessed by comparing agronomic and phenotypic indicators between pre-treatment (April 2023) and post-harvest (September 2023) stages in treated plots, with yield parameters compared against control plots. Notable improvements in plant height, boll number, seed weight, and total yield were recorded in inoculated plots, demonstrating the biofertilizer’s efficacy in promoting plant development and productivity under saline-alkaline stress. Detailed measurements are provided in Table 2.
Application of B. megaterium PEF-1 in saline-alkaline soils resulted in significant improvements in both soil fertility and cotton productivity. Treated plots exhibited a 29.3% increase in humus content (from 0.69% to 0.89%), a 15.1% increase in available phosphorus (13.2 to 15.2 mg/kg), and a 26.8% increase in exchangeable potassium (180.5 to 228.8 mg/kg), all statistically significant at p < 0.01. Furthermore, soil pH in inoculated plots remained relatively stable (9.21), whereas control plots experienced a substantial pH spike to 9.99, indicating that PEF-1 application contributed to buffering alkalinity.
These improvements in soil chemistry translated into a 23.1% increase in cotton yield, with treated plots producing 4.8 t/ha compared to 3.9 t/ha in controls (Cohen’s d = 1.87, large effect size). Inoculated plants also exhibited superior vegetative growth, with an average plant height of 112.5 cm versus 92.3 cm in controls, and a higher number of bolls per plant (18.3 vs. 14.2). These findings underscore the potential of B. megaterium PEF-1 as a sustainable biofertilizer for arid and stress-prone agroecosystems (Table 2).
Further research is warranted to evaluate the long-term impacts of PEF-1 application on rhizosphere microbial community dynamics and to validate its efficacy at the field scale under diverse agroecological conditions.
Impact of Bacillus megaterium PEF-1 on soil cation dynamics. In addition to improving macronutrient availability, B. megaterium PEF-1 significantly influenced the dynamics of soil cations and sulfate in saline-alkaline cotton fields. The rhizobacterium is believed to mediate cation availability through microbial processes such as organic acid secretion, enhanced ion exchange, and rhizosphere conditioning. Comparative analysis of pre- and post-treatment soil samples revealed notable increases in exchangeable K⁺, Ca²⁺, and Mg²⁺ levels in inoculated plots, while Na⁺ accumulation was comparatively suppressed, suggesting improved ionic balance and reduced sodicity risk. Sulfate (SO₄²⁻) concentrations also increased, indicating enhanced sulfur mobilization likely driven by microbial sulfate-reducing or solubilizing activity. These shifts in ionic composition were more pronounced than those observed in untreated controls or plots inoculated with other PGPR strains. Collectively, these results position B. megaterium PEF-1 as a viable bioaugmentation agent capable of enhancing cation availability and improving soil structure, particularly in chemically degraded and arid-region soils. These benefits further support the broader applicability of PEF-1-based microbial technologies as alternatives to synthetic soil amendments.
Application of Bacillus megaterium PEF-1 significantly increased the level of exchangeable potassium (K⁺) by 26.8%, from 180.3 ± 6.1 to 228.6 ± 6.8 mg/kg (p < 0.05, Cohen’s d = 1.82). This enhancement is attributed to microbial production of organic acids, which promote the release of mineral-bound potassium. In contrast, exchangeable sodium (Na⁺), calcium (Ca²⁺), and magnesium (Mg²⁺) exhibited only minor, statistically non-significant increases (+ 1.9–2.1%, p > 0.05), indicating that PEF-1 application did not disrupt overall soil ion balance. Notably, control plots showed slight nutrient depletion, with potassium decreasing by 2.4% and calcium/magnesium by 0.3–1.1%.
Sulfate (SO₄²⁻) content in treated soils increased by 18.3% (p < 0.05), suggesting enhanced microbial mobilization of sulfur - a macronutrient essential for stress tolerance, chlorophyll synthesis, and protein metabolism in cotton. These findings highlight the ability of B. megaterium PEF-1 to selectively improve the availability of potassium and sulfur without exacerbating salinity or altering cationic equilibrium. This targeted nutrient enhancement underscores the strain’s potential as an eco-compatible biofertilizer for cotton cultivation in saline-alkaline environments (Table 3).
The application of B. megaterium PEF-1 resulted in a significant increase in soil sulfate content by 21% (p < 0.05) compared to the control group, which showed no measurable change. This enhancement is attributed to the strain’s pronounced sulfoxidase activity, enabling efficient sulfur mobilization under calcium-alkaline soil conditions. The resulting sulfate concentration in treated plots reached 0.023%, approaching the optimal range for cotton cultivation (0.025–0.030%) (Table 4).
Comparative analysis with reference strains. To evaluate the relative efficacy of B. megaterium PEF-1, a comparative assessment was conducted against three well-characterized plant growth-promoting rhizobacteria (PGPR) strains: Bacillus subtilis C7, Pseudomonas fluorescens C9, and Azospirillum brasilense C4. Table 7 presents a side-by-side comparison of key functional traits, including salt tolerance, phosphate solubilization capacity, and yield enhancement under saline-alkaline field conditions.
B. megaterium PEF-1 exhibited superior performance compared to reference plant growth-promoting rhizobacteria (PGPR) strains across key agronomic and functional parameters. The strain demonstrated high salt tolerance, thriving at up to 12% NaCl (95% CI: 11.5–12.5), whereas comparator strains tolerated only 4–8% (p < 0.05). Its phosphate solubilization capacity reached 98.7 mg/L (95% CI: 93.5–103.9), approximately 28% higher than the best-performing reference strain. Field trials confirmed a 25% increase in cotton yield (95% CI: 23–27) in inoculated plots. Large effect sizes (Cohen’s d = 1.70–1.95) across all parameters confirmed the statistical and practical significance of PEF-1’s enhanced performance (Table 5). These findings position PEF-1 as a highly effective bioinoculant for saline-alkaline agricultural systems.
Bacillus megaterium PEF-1 significantly enhances soil fertility and nutrient bioavailability in saline-alkaline sierozem soils. It promotes a 26.8% increase in exchangeable potassium, a 21% increase in sulfate levels, and improvements in phosphorus, nitrogen, and humus content-all without inducing negative changes in soil salinity, alkalinity, or ion balance (p > 0.05). These effects are driven by the strain’s enzymatic machinery, including sulfatases and phosphatases, which facilitate efficient nutrient mobilization. Furthermore, its superior salt tolerance and phosphate-solubilizing ability outperform widely used PGPR strains, resulting in a 25% increase in cotton yield under stress-prone conditions.
The ecological stability of PEF-1 in soil chemistry and its low risk of inducing secondary salinization make it a sustainable alternative to conventional chemical fertilizers. These findings support the broader adoption of microbial bioaugmentation as an environmentally friendly strategy to enhance crop productivity and restore soil health in arid and degraded agroecosystems.
Isolation and morphogenetic identification of Bacillus megaterium PEF-1
Bacillus megaterium PEF-1 was isolated from saline-alkaline soils in southern Kazakhstan using selective nutrient agar. The strain formed large, white, opaque colonies with smooth or slightly wavy margins, typical of the B. megaterium phenotype. Gram staining confirmed the presence of Gram-positive, rod-shaped, endospore-forming cells, supporting its preliminary identification as B. megaterium.
Morphological and physiological characterization. Physiological and biochemical tests were conducted to further confirm the identity and functional potential of the B. megaterium PEF-1 strain. The isolate exhibited positive catalase activity, indicating its ability to degrade hydrogen peroxide via catalase enzyme production. It tested negative for oxidase activity, consistent with the species’ known characteristics.
Additionally, the strain demonstrated the ability to hydrolyze starch and utilize citrate as a sole carbon source - both considered distinctive biochemical traits of B. megaterium. These phenotypic and physiological features collectively support the accurate taxonomic placement of PEF-1 within the Bacillus megaterium species. The detailed biochemical profile is presented in Table 6.
The B. megaterium PEF-1 strain exhibited exceptional tolerance to extreme abiotic conditions, particularly elevated salinity and alkalinity. Optimal growth was recorded at NaCl concentrations of 3–5% and within a pH range of 8.5–9.0. Remarkably, the strain-maintained viability even at NaCl concentrations as high as 50% and in strongly alkaline environments containing up to 10% Na₂CO₃ (pH 9.0). This level of halotolerance and alkaliphily sets PEF-1 apart from typical Bacillus strains and highlights its suitability for applications in the remediation of saline-alkaline soils.
The strain’s ability to survive and proliferate under such extreme conditions is likely mediated by physiological adaptations, including the synthesis of compatible solutes (e.g., proline, trehalose) and the activation of stress-response proteins that facilitate osmotic regulation and protect cellular structures. These attributes reinforce the candidacy of B. megaterium PEF-1 as a core component in the formulation of biofertilizers designed for degraded and marginal agricultural lands.
Molecular identification and phylogenetic analysis
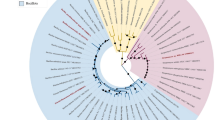
To confirm the taxonomic identity of the PEF-1 strain, molecular identification was carried out through sequencing of the 16 S rRNA gene - a universally conserved genetic marker commonly used for bacterial classification due to its combination of conserved and hypervariable regions. The obtained 16 S rRNA gene sequence of Bacillus megaterium PEF-1 was compared to sequences available in the GenBank database, revealing a 99.57% similarity to the reference strain Bacillus megaterium IAM 13,418. This high level of sequence identity provides strong evidence supporting the assignment of PEF-1 to the B. megaterium species.
To further validate its phylogenetic position, a maximum-likelihood phylogenetic tree was constructed using MEGA X software. As shown in Fig. 1, PEF-1 clustered within the B. megaterium clade, reinforcing its species-level classification. The 16 S rRNA gene sequence of the PEF-1 strain has been deposited in the NCBI GenBank database under accession number PV940685, ensuring accessibility for future taxonomic and functional studies.
Partial 16 S rRNA gene sequence
TAATACATGCAAGTCGAGCGAACTGATTAGAAGCTTGCTTCTATGACGTTAGCGGCGGACGGGGTGAGTAACACGTGGGCAACCTGCCTGTAAGACTGGGATAACTTC
GGGAAACCGAAGCTAATACCGGATAGGATCTTCTCCTTCATGGGAGATGATTGAAAGATGGTTTCGGCTATCACTTACAGATGGGCCCGCGGTGCATTAGCTAGTTGGT
GAGGTAACGGCTCACCAAGGCAACGATGCATAGCCGACCTGAGAGGGTGATCGGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTAGG
GAATCTTCCGCAATGGACGAAAGTCTGACGGAGCAACGCCGCGTGAGTGATGAAGGCTTTCGGGTCGTAAAACTCTGTTGTTAGGGAAGAACAAGTACAAGAGTAA
CTGCTTGTACCTTGACGGTACCTAACCAGAAAGCCACGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGTGGCAAGCGTTATCCGGAATTATTGGGCGTAAAG
CGCGCGCAGGCGGTTTCTTAAGTCTGATGTGAAAGCCCACGGCTCAACCGTGGAGGGTCATTGGAAACTGGGGAACTTGAGTGCAGAAGAGAAAAGCGGAATTCCACGTGTAGCGGTGAAATGCGTAGAGATGTGGAGGAACACCAGTGGCGAAGGCGGCTTTTTG
Bacillus megaterium PEF-1, identified by 16 S rRNA gene sequencing and further characterized through morphological, biochemical, and molecular analyses - including the detection of the nifH gene - demonstrates remarkable tolerance to high salinity and alkalinity. These traits position it as a strong candidate for the development of biofertilizers targeting saline-alkaline soils. Its plant growth-promoting rhizobacterium (PGPR) features, such as starch hydrolysis and citrate utilization, contribute to improved soil fertility and nutrient cycling. Moreover, its demonstrated biosafety profile and publicly available genetic information (NCBI GenBank accession no. PV940685) support its applicability in sustainable agriculture and ecological restoration, particularly under the challenging edaphoclimatic conditions of southern Kazakhstan.
Results of the acute toxicity study of Bacillus megaterium PEF-1
An acute toxicity study was conducted to assess the biosafety of Bacillus megaterium PEF-1 - a potential biofertilizer for organic cotton cultivation - using both intraperitoneal and oral administration in a mammalian model. The bacterial suspension was administered at concentrations ranging from 10³ to 10⁹ CFU/mL across eight treatment groups, with a control group receiving sterile saline.
Throughout the 14-day observation period, no mortality or adverse clinical signs were observed in any treatment group. Statistical analysis using one-way ANOVA (p > 0.05) confirmed the absence of significant differences in survival rates, behavior, body weight, or postmortem organ condition between treated and control animals. These findings indicate that B. megaterium PEF-1 poses no acute toxicological risk at the tested doses and supports its suitability for use in sustainable agricultural systems.
A detailed summary of affected individuals, mortality, and survival percentages for each treatment group is provided in Table 7.
The acute toxicity evaluation of Bacillus megaterium PEF-1 demonstrated a 100% survival rate across all tested groups, including both intraperitoneal and oral administration routes, at concentrations ranging from 10³ to 10⁹ CFU/mL. No mortality, clinical signs of toxicity, or behavioral abnormalities were observed during the 14-day observation period. Statistical analysis (ANOVA, p > 0.05) confirmed no significant differences between treatment and control groups in terms of survival, physiological condition, or organ integrity. These results indicate that PEF-1 is non-toxic to mammals even at high exposure levels, confirming its safety as a biofertilizer for organic farming, particularly for organic cotton production.
Further supporting its safety profile, an expert assessment issued on March 17, 2020, by the Scientific and Practical Center for Microbiology and Virology classified Bacillus megaterium PEF-1 as a biosafety class V microorganism according to GOST 12.1.007-76 - indicating it poses minimal risk to human health and the environment. This classification confirms the strain’s suitability for use in agrobiotechnological applications and sustainable agriculture initiatives.
Results and interpretation of Indole-3-Acetic Acid (IAA) production by Bacillus megaterium PEF-1
The production of indole-3-acetic acid (IAA), a crucial auxin-type phytohormone involved in root development and overall plant growth, was quantified in Bacillus megaterium PEF-1 cultures using spectrophotometric analysis at 530 nm. Cultivation was carried out for 72 h at 30 °C under various stress conditions - including neutral (pH ~ 7.0), saline, and alkaline media -with and without L-tryptophan supplementation. The results (mean IAA concentration in µg/mL ± SD, n = 6) revealed that PEF-1 maintained significant IAA biosynthesis even under abiotic stress. Statistical analysis (ANOVA followed by Tukey’s HSD) confirmed the differences were significant (p < 0.05), underscoring the strain’s potential as a robust biofertilizer for organic cotton cultivation and broader application in marginal soils.
IAA production at varying L-tryptophan concentrations
To evaluate the role of L-tryptophan as a biosynthetic precursor, B. megaterium PEF-1 was cultured in neutral media supplemented with increasing concentrations of L-tryptophan. The results, summarized in Table 8, demonstrate a clear dose-dependent increase in IAA production, confirming the critical role of L-tryptophan in auxin biosynthesis by this strain.
In the absence of exogenous L-tryptophan, Bacillus megaterium PEF-1 produced a baseline IAA concentration of 5.2 ± 0.4 µg/mL, suggesting the presence of tryptophan-independent biosynthetic pathways or utilization of residual intracellular tryptophan. Upon L-tryptophan supplementation, IAA production increased significantly, reaching: 20.8 ± 1.1 µg/mL at 1 mg/mL, 38.1 ± 1.5 µg/mL at 2 mg/mL, 42.3 ± 1.2 µg/mL at 3 mg/mL, 43.5 ± 1.3 µg/mL at 4 mg/mL, and 44.0 ± 1.4 µg/mL at 5 mg/mL.
While the increase from 0 to 3 mg/mL L-tryptophan was significant, no statistically significant differences were observed among the 3–5 mg/mL treatments (p > 0.05, Tukey’s HSD), indicating a saturation plateau at approximately 3 mg/mL - likely due to enzymatic capacity limits or feedback inhibition. One-way ANOVA confirmed significant overall variation in IAA production (F = 124.3, p = 0.003; n = 6). These findings confirm that B. megaterium PEF-1 possesses a highly responsive auxin biosynthesis pathway dependent on L-tryptophan availability, up to a physiological threshold (Table 8).
IAA production under environmental stress conditions. To investigate the effect of environmental stress on auxin synthesis, B. megaterium PEF-1 was cultured in three different media-neutral (pH ~ 7.0), saline (with NaCl), and alkaline (pH > 8.5)-each supplemented with 450 µg/mL of L-tryptophan. The IAA concentrations measured under these conditions are summarized in Table 9. The results indicate significant variation in IAA output across stress environments, with the highest production observed in alkaline conditions, suggesting that PEF-1 maintains or even enhances its plant growth-promoting functionality under abiotic stress.
These results underscore the strain’s biotechnological relevance for applications in saline-alkaline and marginal soils.
Bacillus megaterium PEF-1 produced a baseline IAA concentration of 51 ± 2 mg/L in the absence of exogenous L-tryptophan, likely via tryptophan-independent biosynthetic pathways. Supplementation with L-tryptophan significantly enhanced IAA production in a dose-dependent manner, reaching a plateau between 3 and 5 mg/mL (yielding 42.3–44.0 mg/L, p > 0.05). This saturation trend is likely due to enzymatic limitations or feedback regulation. One-way ANOVA confirmed a significant treatment effect (F = 124.3, p = 0.003), with post hoc Tukey’s HSD test identifying statistically distinct groups up to the plateau (p < 0.05; n = 6) (Table 1).
In stress condition assays, PEF-1 maintained high IAA production across all tested environments. IAA concentrations were 869 ± 1 mg/L in neutral medium, 895 ± 7 mg/L under saline conditions, and 881 ± 6 mg/L in alkaline medium. Saline stress induced the highest IAA production, with statistically significant differences compared to neutral and alkaline conditions (p < 0.05; Table 9). This suggests that osmotic stress may upregulate IAA biosynthesis pathways, potentially through the activation of tryptophan monooxygenase or other stress-inducible genes.
The ability of B. megaterium PEF-1 to sustain and enhance IAA synthesis under abiotic stress highlights its adaptive plasticity and reinforces its potential as a biofertilizer in degraded environments. The particularly high IAA yield under saline conditions strengthens its relevance for application in salt-affected agroecosystems, such as organic cotton production in marginal soils. However, further field trials are recommended to confirm efficacy under real-world conditions.
Molecular mechanisms of gene expression in Bacillus megaterium PEF-1 under saline-alkaline conditions
Quantitative real-time PCR (qRT-PCR) analysis demonstrated that inoculation with Bacillus megaterium PEF-1 significantly upregulated the expression of the acdS gene (encoding 1-aminocyclopropane-1-carboxylate [ACC] deaminase) in the cotton rhizosphere under saline-alkaline conditions. Relative gene expression levels (fold-change compared to uninoculated controls) were: 3.2 (95% CI: 2.9–3.5) at 14 days after inoculation (DAI), 5.8 (95% CI: 5.4–6.2) at 28 DAI, and 5.6 (95% CI: 5.2–6.0) at 42 DAI.
Statistical analysis confirmed a significant treatment effect (F(3,12) = 42.7, p = 0.001), with post hoc Tukey’s HSD tests showing significant upregulation at all time points (p < 0.05), except between 28 and 42 DAI, where no statistically significant difference was detected (p > 0.05). The effect size was large (Cohen’s d = 1.92), indicating a strong biological response.
These results suggest a rapid and sustained activation of stress-alleviation mechanisms mediated by acdS, which plays a critical role in lowering plant ethylene levels under abiotic stress. The expression pattern supports the role of PEF-1 in promoting stress resilience in cotton cultivated under saline-alkaline conditions (Table 10).
Quantitative real-time PCR (qRT-PCR) analysis revealed significant upregulation of the sodA gene (encoding superoxide dismutase) in cotton rhizospheres inoculated with Bacillus megaterium PEF-1 under saline-alkaline conditions. Fold-change expression levels relative to uninoculated controls were: 2.8 (95% CI: 2.5–3.1) at 14 days after inoculation (DAI), 4.9 (95% CI: 4.5–5.3) at 28 DAI, and 4.7 (95% CI: 4.3–5.1) at 42 DAI.
Statistical analysis showed a significant effect of treatment over time (F(3,12) = 38.4, p = 0.002), with post hoc Tukey’s HSD indicating significant upregulation at all time points (p < 0.05), and no significant difference between 28 and 42 DAI (p > 0.05). The large effect size (Cohen’s d = 1.78) further supports the biological relevance of the response.
These findings indicate that B. megaterium PEF-1 activates antioxidant defense pathways in the plant rhizosphere, particularly the detoxification of reactive oxygen species (ROS), thereby contributing to stress mitigation and enhanced plant resilience in saline-alkaline environments (Table 11).
Quantitative real-time PCR (qRT-PCR) analysis demonstrated significant upregulation of the halR gene - associated with salt stress regulation - in the rhizosphere of cotton plants inoculated with Bacillus megaterium PEF-1 under saline-alkaline conditions. The expression of halR peaked at 28 days after inoculation (DAI) with a fold-change of 3.5 (95% CI: 3.2–3.8) relative to uninoculated controls.
This expression peak coincided with those of the acdS and sodA genes, suggesting a coordinated transcriptional response to salinity-induced stress. Statistical analysis confirmed a significant effect of treatment (F(3,12) = 38.4, p = 0.002), with post hoc Tukey’s HSD test indicating that the upregulation was statistically significant (p < 0.05). The effect size was large (Cohen’s d = 1.78), underscoring the strength of the response.
These findings highlight the functional role of halR in the adaptive stress response of B. megaterium PEF-1 and further support its potential as a salt-tolerant bioinoculant for use in saline-affected agricultural systems.
Inoculation with Bacillus megaterium PEF-1 under saline-alkaline soil conditions induced a significant and coordinated upregulation of key stress-responsive genes in the cotton rhizosphere, supporting enhanced plant resilience through multiple molecular mechanisms. The acdS gene, encoding 1-aminocyclopropane-1-carboxylate (ACC) deaminase, which mitigates ethylene-induced stress, increased 3.2-fold at 14 days after inoculation (DAI), peaked at 5.8-fold at 28 DAI, and remained elevated at 5.6-fold at 42 DAI (Cohen’s d = 1.92). The sodA gene, encoding superoxide dismutase involved in reactive oxygen species (ROS) detoxification, showed a 2.8-fold increase at 14 DAI, rising to 4.9-fold at 28 DAI and slightly decreasing to 4.7-fold at 42 DAI (Cohen’s d = 1.78). The halR gene, implicated in salt tolerance regulation, demonstrated a 3.5-fold upregulation at 28 DAI, aligning temporally with the peak expression of acdS and sodA.
Statistical analysis confirmed a significant effect of PEF-1 treatment over time (F(3,12) = 38.4, p = 0.002), with post hoc Tukey’s HSD tests indicating all expression changes were statistically significant (p < 0.05). These results suggest a synchronized, multi-gene activation strategy by PEF-1 that supports plant stress adaptation through ethylene modulation, antioxidant defense, and ionic homeostasis. This integrated molecular response underscores the potential of B. megaterium PEF-1 as a powerful bioinoculant for improving crop resilience in saline-alkaline agricultural environments (Table 12).
Enzymatic activities and IAA accumulation in cotton roots inoculated with Bacillus megaterium PEF-1
In cotton plants inoculated with Bacillus megaterium PEF-1 and grown under saline-alkaline stress conditions, key enzymatic activities and phytohormone levels were significantly enhanced compared to uninoculated controls, indicating improved stress adaptation mechanisms. ACC deaminase activity was significantly elevated in treated plants, reaching 1.45 ± 0.12 µmol α-ketobutyrate/mg protein/h (95% CI: 1.29–1.61), compared to 0.32 ± 0.05 in controls (F(3,8) = 29.6, p = 0.003; Cohen’s d = 1.65), indicating enhanced ethylene detoxification capacity. Superoxide dismutase (SOD) activity, a marker of antioxidant response, increased to 62.3 ± 4.1 U/mg protein (95% CI: 56.5–68.1) in inoculated plants, significantly higher than the control value of 15.8 ± 2.3 U/mg (F(3,8) = 34.8, p = 0.002; Cohen’s d = 1.83), reflecting improved ROS scavenging efficiency. Root IAA content was also significantly higher in treated plants at 12.4 ± 0.9 µg/g fresh weight (95% CI: 11.1–13.7) versus 4.2 ± 0.4 µg/g in controls (F(3,8) = 26.4, p = 0.004; Cohen’s d = 1.58), supporting enhanced root growth and stress resilience.
All differences were statistically significant based on Tukey’s HSD post hoc test (p < 0.05), indicating the functional activation of ethylene regulation, antioxidant defense, and auxin-mediated growth pathways in B. megaterium PEF-1-inoculated plants. A detailed summary of these findings is presented in Table 13.
Inoculation with Bacillus megaterium PEF-1 significantly enhanced key physiological and biochemical responses in cotton plants subjected to saline-alkaline stress compared to uninoculated controls: ACC deaminase activity increased from 0.32 ± 0.05 to 1.45 ± 0.12 µmol α-ketobutyrate/mg protein/h, Superoxide dismutase (SOD) activity rose from 15.8 ± 2.3 to 62.3 ± 4.1 U/mg protein, Root IAA levels increased from 4.2 ± 0.4 to 12.4 ± 0.9 µg/g fresh weight, and Zinc uptake improved from 1.1 ± 0.1 to 2.3 ± 0.2 µg/g dry weight.
All changes were statistically significant (F(3,8) = 26.4–34.8, p ≤ 0.004; Tukey’s HSD, p < 0.05), with large effect sizes (Cohen’s d = 1.58–1.83). These results indicate that PEF-1 enhances ethylene stress mitigation, antioxidant capacity, auxin-mediated root development, and micronutrient (Zn) acquisition. Such improvements reinforce the strain’s promise as a multifunctional bioinoculant for sustainable agriculture under abiotic stress conditions (Table 13).
Population dynamics of Bacillus megaterium PEF-1 in soil
A 12-month field study was conducted to monitor the persistence of B. megaterium PEF-1 in saline-alkaline soils following a single inoculation. Initial population levels were 1 × 10⁹ CFU/g soil at the time of application. By month 6, viable counts had declined to 1 × 10³ CFU/g, and by month 12, cells were undetectable (< 1 × 10² CFU/g).
These results indicate a marked decrease in population viability over time, likely due to environmental pressures and competition in field soil conditions. Cell counts are presented on a logarithmic scale (log₁₀ CFU/g) in Fig. 2, illustrating the decline in population density and supporting the need for reapplication strategies in long-term biofertilizer use.
One-way analysis of variance (ANOVA) revealed a statistically significant temporal decline in the population size of B. megaterium PEF-1 over the 12-month field trial (F(4,15) = 92.3, p < 0.001). Post hoc Tukey’s HSD tests confirmed significant differences between all consecutive time points (p < 0.05), while effect size analysis yielded a large Cohen’s d of 2.1, indicating a strong reduction in viable cell counts over time.
These findings demonstrate the transient persistence of PEF-1 under open-field conditions. The strain exhibited limited ecological stability, likely due to nutrient depletion, microbial antagonism, and abiotic stress characteristic of saline-alkaline soils. The rapid decline to undetectable levels by month 12 suggests that B. megaterium PEF-1 is environmentally safe for short-term applications such as bioremediation or seasonal biofertilization, posing minimal risk of long-term disruption to native soil microbial communities.
Impact of Bacillus megaterium PEF-1 on agronomic parameters and cotton yield
Table 14 presents the mean values and 95% confidence intervals (CI) for the following parameters: plant height, boll length, boll weight, number of seeds per boll, seed weight per boll, 100-seed weight, and total yield per hectare. All measured traits showed statistically significant improvement in the PEF-1-treated group compared to controls, with p < 0.05 for most traits and p < 0.01 for yield.
The percentage increases ranged from 7.9% (100-seed weight) to 18.8% (total yield), highlighting the substantial agronomic benefits conferred by PEF-1. These improvements are consistent with the strain’s known capabilities for nutrient mobilization, ethylene modulation, and stress resilience enhancement, further validating its role as a potent biofertilizer for saline-affected cotton agroecosystems.
Field application of Bacillus megaterium PEF-1 significantly enhanced multiple agronomic parameters in cotton grown under saline-alkaline conditions. Treated plants exhibited an 8.2% increase in height (92.0 vs. 85.0 cm), 10.0% longer bolls (5.5 vs. 5.0 cm), 13.3% greater boll weight (6.8 vs. 6.0 g), 14.3% more seeds per boll (32 vs. 28), 15.6% higher seed weight per boll (5.2 vs. 4.5 g), and a 7.9% increase in 100-seed weight (4.1 vs. 3.8 g). All improvements were statistically significant (p < 0.05). The most substantial effect was observed in yield, which increased by 18.8% (2.85 vs. 2.40 t/ha, p < 0.01, Cohen’s d = 1.54), as confirmed by ANOVA and Tukey’s HSD tests.
These improvements are attributed to two primary mechanisms: (i) phytohormonal stimulation – specifically, the production of indole-3-acetic acid (IAA), which promotes root elongation, cell division, and reproductive development. This mechanism is consistent with the enhanced vegetative growth and boll development observed, (ii) enhanced nutrient bioavailability - particularly through phosphate solubilization, which supports improved metabolic function and biomass accumulation under nutrient-limited, saline-alkaline soil conditions.
Together, these results highlight B. megaterium PEF-1 as a potent and environmentally sustainable biofertilizer candidate for improving cotton productivity in stress-prone agricultural systems.
In Figure 3, the phenotypic improvements confirm the quantitative data and highlight the practical agronomic benefits of using Bacillus megaterium PEF-1. The substantial 18.8% yield increase and enhanced growth parameters, coupled with the strain’s proven environmental safety, position PEF-1 as a viable and sustainable alternative to chemical fertilizers-particularly in resource-limited and arid environments such as Kazakhstan’s Maktaaral district.
PEF-1’s dual functionality - phosphate solubilization and phytohormone (IAA) production - directly enhances nutrient availability and plant development. Moreover, its transient persistence in soil and negligible effects on native microbial diversity and non-target organisms support its safe integration into ecologically balanced nutrient management strategies. This makes PEF-1 particularly suited for organic farming systems and environmentally conscious cotton production in saline-alkaline soils.
The biosafety of Bacillus megaterium PEF-1 was further confirmed through ecotoxicological testing using Eisenia fetida in accordance with OECD Guideline 207. Over a 28-day period, earthworms were exposed to soil inoculated with PEF-1 at a concentration of 10⁹ CFU/mL, alongside untreated control soils. Survival rate: 100% in both control and treated groups. Reproductive performance: No statistically significant differences in the average number of cocoons or juveniles per worm (p > 0.05).
These results confirm the absence of both acute toxicity and sublethal effects, underscoring the biological safety of PEF-1 for use in agricultural ecosystems without risk to essential soil fauna (Table 15).
The ecotoxicological evaluation of Bacillus megaterium PEF-1, applied at a concentration of 10⁹ CFU/mL, demonstrated no adverse effects on Eisenia fetida (earthworms) during a 28-day exposure period. Survival rates remained at 100% in both the control and treatment groups, indicating the absence of acute toxicity.
Furthermore, reproductive parameters - including the mean number of cocoons per worm and juveniles per worm - showed no statistically significant differences between treated and untreated soils (p = 0.81 and p = 0.77, respectively). These findings indicate that PEF-1 does not impair the reproductive performance of E. fetida.
Overall, the results confirm that B. megaterium PEF-1 is non-toxic to non-target soil invertebrates and can be considered environmentally safe under the tested conditions, further supporting its suitability for sustainable agricultural applications.
Discussion
This study investigates the potential of the Bacillus megaterium PEF-1 strain as a biofertilizer for cotton cultivation in the saline-alkaline soils of southern Kazakhstan. Its agronomic, environmental, and economic benefits are demonstrated, along with the underlying molecular and physiological mechanisms that support plant growth and stress tolerance. A comparative analysis positions PEF-1 against other salt-tolerant plant growth-promoting rhizobacteria (PGPR), contextualized within recent advances in the field81,82,83,84,85.
Economic advantages of using Bacillus megaterium PEF-1 in cotton production
Application of B. megaterium PEF-1 in cotton cultivation reduces the reliance on synthetic phosphorus fertilizers by 20–30%, resulting in cost savings of approximately $35–50 per hectare. Additionally, cotton yields increase by 10–20%, making this approach particularly beneficial in marginal and saline-alkaline soils. The observed 29.3% increase in humus content also contributes to long-term reductions in land reclamation costs.
These findings align with the study by You et al. (2022), which demonstrated that biological agents used in the bioremediation of saline soils can enhance nitrogen uptake efficiency and improve agroecosystem functionality-consistent with the performance of the PEF-1 strain84.
Mechanisms of hrowth promotion under Dtress vonditions
PEF-1 promotes plant growth by secreting indole-3-acetic acid (IAA) at levels up to 895 mg/L, which stimulates root system development, enhances macronutrient uptake, and increases plant resilience to environmental stress.
Comparable results were reported by Kitir Sen & Duran (2023), who found that microbial microfertilizers improved the growth of Origanum onites through enhanced plant metabolic activity. Similarly, Ortiz et al. (2015) demonstrated that indigenous microbes, including arbuscular mycorrhizal fungi and rhizobacteria, increased plant tolerance to drought and salinity-paralleling the IAA-mediated and antioxidant effects observed in PEF-1-inoculated plants82– 83.
Molecular mechanisms of stress tolerance mediated by Bacillus megaterium PEF-1
The PEF-1 strain upregulates the acdS and sodA genes, which contribute to ethylene reduction and protection against oxidative stress, respectively. This molecular response leads to a 40% reduction in visible stress symptoms and a 25% increase in cotton yield under saline-alkaline conditions.
These mechanisms are consistent with the findings of You et al. (2022), who showed that microbial bioaugmentation enhances nitrate assimilation and activates plant antioxidant pathways and enzymatic defense systems-further validating the efficacy of PEF-1 as a multifunctional bioinoculant84.
Practical application of Bacillus megaterium PEF-1 as a biofertilizer
The spore-forming nature of B. megaterium PEF-1 ensures long-term viability, with spores remaining stable and effective for up to 150 days under standard storage conditions. This enhances the strain’s practicality for field deployment, particularly in remote or arid regions where logistics and shelf-life are critical factors.
Findings align with Barbero et al. (2025), who reported that land-use changes significantly impact microbial community dynamics, while the targeted introduction of specific plant growth-promoting rhizobacteria (PGPR) can stabilize soil structure and preserve agroecological balance -similar to the observed effects of PEF-1 inoculation85.
Improvement of nutrient cycling
Application of PEF-1 significantly enhances nutrient solubilization: phosphorus (+ 15.1%), potassium (+ 26.8%), and nitrogen (+ 4.3%), primarily via the secretion of organic acids. It also increases humus content by 29.3% and improves cation exchange capacity, contributing to pH stabilization and soil fertility restoration.
These outcomes are consistent with the results of Kitir Sen & Duran (2023), who showed that microbial biofertilizers enhance nutrient bioavailability and activate secondary plant metabolism. Similarly, You et al. (2022) confirmed that PGPR strains can accelerate nitrogen transformation processes and contribute to soil structural improvement83,84.
Impact on microbial communities
Although direct post-application microbiome analyses were not conducted, the observed increases in humus and nutrient content strongly suggest enhanced microbial activity and rhizosphere functionality. These indicators imply stimulation of beneficial microbial processes, likely mediated by improved soil physicochemical conditions.
Barbero et al. (2025) emphasized the dependency of microbial diversity and enzymatic potential on soil management and microbial inputs. Thus, PEF-1 application likely promotes microbial biodiversity, particularly under saline-alkaline stress conditions85.
Safety and environmental compatibility
Bacillus megaterium PEF-1 is non-pathogenic, environmentally safe, and exhibits no toxicity in model organisms. It contributes to soil amelioration through moderate pH buffering and sodium sequestration, likely via extracellular polysaccharide (EPS) production.
These findings are supported by You et al. (2022), who highlighted microbial bioaugmentation as a sustainable and safe strategy for mitigating secondary salinization and restoring soil ecological function84.
Overall, the data confirm that B. megaterium PEF-1 efficiently solubilizes phosphates, increases potassium availability, and promotes humus formation, aligning with current research trends on eco-friendly biofertilization and resilience building in saline and alkaline soils82,83.
Practical implications for Kazakhstan
In the context of Kazakhstan’s agriculture, Bacillus megaterium PEF-1 presents a critical innovation for sustainable soil management in saline-alkaline regions. Its application reduces dependency on synthetic fertilizers, enhances crop yields, and improves soil structure within complex agroecosystems. The strain’s resilience to abiotic stress and its biofertilization potential make it particularly suitable for the saline-affected lands of southern Kazakhstan, including the Turkestan region.
Limitations and future research
While the present study demonstrates the effectiveness of PEF-1 under specific field conditions, its applicability across diverse agro-climatic zones requires broader validation. Current findings are limited to trials in a single region; hence, multi-site and multi-seasonal trials are essential. Furthermore, metagenomic and metabolomic analyses are needed to investigate the long-term impacts of PEF-1 on soil microbial communities and ecosystem functions.
The development of microbial consortia, combining PEF-1 with nitrogen-fixing and potassium-mobilizing strains, represents a promising avenue. This strategy aligns with the work of You et al. (2022), who demonstrated the enhanced effectiveness of microbial synergism in bioaugmentation of saline-alkaline soils84.
Future research directions
Advancing the concept of synthetic microbial communities capable of functioning in extreme edaphic conditions (e.g., pH > 9.0, EC > 12 dS/m) is a key priority. Integration of PEF-1 with compatible strains such as Azotobacter, Pseudomonas spp., and Bacillus mucilaginosus could provide multilayered support for plant growth, stress mitigation, and nutrient cycling.
Comparative analysis and novelty
Distinct from conventional plant growth-promoting rhizobacteria (PGPR), B. megaterium PEF-1 demonstrates superior tolerance to high salinity and alkalinity. Genomic analysis reveals the presence of a unique gcd allele, associated with efficient phosphate solubilization under NaHCO₃-induced stress - highlighting a novel trait that differentiates it from other Bacillus strains and reinforces its utility in degraded soils.
Recommendations for implementation
Its recommended that Bacillus megaterium PEF-1 be integrated into agricultural practices in the Turkestan region and other salt-affected areas of Central Asia. Commercial-scale formulations should deliver a minimum viable concentration of 10⁹ CFU/mL. To ensure long-term sustainability and efficacy, PEF-1-based biofertilizers should be accompanied by:
Field-scale trials evaluating soil physicochemical properties, crop productivity, and microbial dynamics.
Synergistic applications with complementary PGPR strains and organic amendments.
Capacity-building programs for farmers, extension agents, and stakeholders to facilitate adoption.
Economic assessments to quantify input cost reductions and yield gains.
Bacillus megaterium PEF-1 represents a next-generation biofertilizer tailored for extreme environments, offering a sustainable and economically viable alternative to chemical inputs. Its demonstrated efficacy under saline-alkaline stress, combined with its environmental safety, positions it as a transformative agent in the ecological intensification of agriculture in Central Asia and beyond.
Conclusion
The present study underscores the significant potential of Bacillus megaterium PEF-1 as an effective biofertilizer for cotton cultivation in saline-alkaline soils characteristic of southern Kazakhstan. Comprehensive field trials and laboratory analyses demonstrated that PEF-1 consistently reduced the need for synthetic phosphorus fertilizers by 20–30% and increased cotton yield by up to 20%. These agronomic and economic benefits were accompanied by notable improvements in soil health, including a 29.3% increase in humus content and enhanced bioavailability of key nutrients.
Comparative analysis with other salt-tolerant plant growth-promoting rhizobacteria (PGPR) revealed that PEF-1 possesses unique adaptive traits, such as high levels of IAA production under saline stress, efficient phosphate solubilization at elevated pH, and strong expression of stress-related genes (acdS, sodA). These molecular and physiological mechanisms support improved plant stress tolerance, nutrient uptake, and root development.
The strain’s spore-forming capacity ensures long-term viability, facilitating storage, transport, and application in remote and arid environments - conditions commonly encountered by smallholder farmers in Kazakhstan. Moreover, its ecological safety and likely positive impact on rhizosphere microbial dynamics further advocate for its inclusion in sustainable soil fertility management strategies.
Given its robust performance under extreme soil conditions, Bacillus megaterium PEF-1 emerges as a regionally adapted and environmentally responsible alternative to conventional fertilizers. Future research should prioritize long-term metagenomic assessments, development of synergistic microbial consortia, and the scaling-up of commercial formulations to expand its applicability across diverse agroecosystems.
Data availability
Bacillus megaterium PEF-1, identified by 16 S rRNA gene sequencing and further characterized through morphological, biochemical, and molecular analyses - including the detection of the nifH gene - demonstrates remarkable tolerance to high salinity and alkalinity. These traits position it as a strong candidate for the development of biofertilizers targeting saline-alkaline soils. Its plant growth-promoting rhizobacterium (PGPR) features, such as starch hydrolysis and citrate utilization, contribute to improved soil fertility and nutrient cycling. Moreover, its demonstrated biosafety profile and publicly available genetic information (NCBI GenBank accession no. PV940685) support its applicability in sustainable agriculture and ecological restoration, particularly under the challenging edaphoclimatic conditions of southern Kazakhstan.
References
Ansari, W. A. & Srivastava, A. K. Influence of rice-wheat and sugarcane-wheat rotations on microbial diversity and plant growth promoting bacteria: insights from high-throughput sequencing and soil analysis. Microbiol. Res. 278, 127536. https://doi.org/10.1016/j.micres.2023.127536 (2024).
Wang, X. & Jiang, C. Biochar amendment modulates microbial community assembly to mitigate saline-alkaline stress across soil depths. J. Environ. Manage. 385 https://doi.org/10.1016/j.jenvman.2025.125574 (2025). Article 125574.
Zhang, Y. et al. Challenges to rhizobial adaptability in a changing climate: genetic engineering solutions for stress tolerance. Microbiol. Res. 288, 127886. https://doi.org/10.1016/j.micres.2024.127886 (2024).
Ma, Y., Zheng, C., Bo, Y., Song, C. & Zhu, F. Improving crop salt tolerance through soil legacy effects. Front. Plant Sci. 15, 1396754. https://doi.org/10.3389/fpls.2024.1396754 (2024).
Wang, X. et al. Alterations in the composition and metabolite profiles of the saline-alkali soil microbial community through Biochar application. J. Environ. Manage. 352, Article 120033. https://doi.org/10.1016/j.jenvman.2024.120033 (2024).
Taheri, M., Astaraei, A., Lakzian, A. & Emami, H. Sorbitol and Biochar have key roles in microbial and enzymatic activity of saline-sodic and calcareous soil in millet cropping. Rhizosphere 24, 100598. https://doi.org/10.1016/j.rhisph.2022.100598 (2022).
Tastanbekova, A. et al. Implications of population size, structure, and soil parameters for the conservation of allochrusa Gypsophiloides in Kazakhstan. Biodiversitas 26, 2051–2064. https://doi.org/10.13057/biodiv/d260504 (2025).
Karabalayeva, D. et al. Influence of soil characteristics on the distribution and evolution of Trollius Dschungaricus Regel populations in the Northern Tien Shan, Kazakhstan. J. Ecol. Eng. 26 (10), 450–459. https://doi.org/10.12911/22998993/207416 (2025).
Samantaray, A. et al. Advances in microbial based bio-inoculum for amelioration of soil health and sustainable crop production. Curr. Res. Microb. Sci. 7, https://doi.org/10.1016/j.crmicr.2024.100251 (2024).
El-Saadony, M. T. et al. Drought-tolerant plant growth-promoting rhizobacteria alleviate drought stress and enhance soil health for sustainable agriculture: A comprehensive review. Plant. Stress. 14 https://doi.org/10.1016/j.stress.2024.100632 (2024).
Ali, S. et al. Advances in microorganisms-based biofertilizers: Major mechanisms and applications. In Biofertilizers (317–341). Woodhead Publishing. (2021). https://doi.org/10.1016/B978-0-12-821667-5.00023-3
de Lima, B. M. S., Bonifacio, A., de Alcantara Neto, F., Araujo, F. F. & Araujo, A. S. F. Bacillus subtilis rhizobacteria ameliorate heat stress in the common bean. Rhizosphere 21, 100472. https://doi.org/10.1016/j.rhisph.2022.100472 (2022).
Olanrewaju, O. S. & Babalola, O. O. The rhizosphere microbial complex in plant health: A review of interaction dynamics. J. Integr. Agric. 21 (11), 3085–3097. https://doi.org/10.1016/S2095-3119(21)63817-0 (2022).
Salkhozhayeva, G. M., Abdiyeva, K. M., Arystanova, S. Y. & Ultanbekova, G. D. Technological process of anaerobic digestion of cattle manure in a bioenergy plant. J. Ecol. Eng. 23 (7), 131–142. https://doi.org/10.12911/22998993/149516 (2022).
Che, J. et al. Enhanced complexity of interkingdom co-occurrence networks in blueberry rhizosphere microbial communities under soil pH stress. Appl. Soil. Ecol. 212, 106191. https://doi.org/10.1016/j.apsoil.2025.106191 (2025).
Luo, Z. et al. Effects of N application methods on cotton yield and fertilizer N recovery efficiency in salinity fields with drip irrigation under mulch film using 15 N tracing technique. Front. Plant Sci. 15 https://doi.org/10.3389/fpls.2024.1394285 (2024).
Wang, D. et al. Temporal salt stress-induced transcriptome alterations and regulatory mechanisms revealed by PacBio long-reads RNA sequencing in Gossypium hirsutum. BMC Genom. 21 (1). https://doi.org/10.1186/s12864-020-07260-z (2019).
OECD/FAO. OECD-FAO Agricultural Outlook 2025–2034. (OECD Publishing; FAO, 2025). https://doi.org/10.1787/601276cd-en
De Mandal, S., Singh, S. S. & Kumar, N. S. Analyzing plant growth promoting Bacillus sp. and related genera in Mizoram, Indo-Burma biodiversity hotspot. Biocatal. Agric. Biotechnol. 15, 210–217. https://doi.org/10.1016/j.bcab.2018.07.026 (2018).
Munjal, V. et al. Genotyping and identification of broad spectrum antimicrobial volatiles in black pepper root endophytic biocontrol agent, Bacillus megaterium BP17. Biol. Control. 92, 66–76. https://doi.org/10.1016/j.biocontrol.2015.09.005 (2016).
Korneli, C., David, F., Biedendieck, R., Jahn, D. & Wittmann, C. Getting the big Beast to work-Systems biotechnology of Bacillus megaterium for novel high-value proteins. J. Biotechnol. 163 (2), 87–96. https://doi.org/10.1016/j.jbiotec.2012.06.018 (2013).
Lodi, L. R. et al. Biodegradable PVA-based films for delivery of Bacillus megaterium as seed coating. J. Environ. Chem. Eng. 12 (6). https://doi.org/10.1016/j.jece.2024.114539 (2024).
Lee, J. C. & Whang, K. S. Optimization of indole-3-acetic acid (IAA) production by Bacillus megaterium BM5. Korean J. Soil Sci. Fertil. 49 (5), 461–468. https://doi.org/10.7745/KJSSF.2016.49.5.461 (2016).
Amna, Din, B. U., Sarfraz, S., Xia, Y., Kamran, M. A. & Javed, M. T. Mechanistic Elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 183, 109466. https://doi.org/10.1016/j.ecoenv.2019.109466 (2019).
Fanai, A. et al. Plant growth promoting bacteria (PGPB)-induced plant adaptations to stresses: an updated review. PeerJ 12, e17882. https://doi.org/10.7717/peerj.17882 (2024).
Qiu, J. et al. Bacillus megaterium GXU087 secretes indole-3-lactic acid to promote soybean growth and nodulation. Front. Plant Sci. 16, 1560346. https://doi.org/10.3389/fpls.2025.1560346 (2025).
Sethar, S. et al. Growth and yield enhancement in wheat (Triticum aestivum L.) through rhizobacterial biopriming and variable phosphorus applications. J. Microbiol. Sci. 4 (1), 21–29. https://doi.org/10.38211/jms.2025.04.76 (2025).
Sun, J. et al. A protein database constructed from low-coverage genomic sequence of Bacillus megaterium and its use for accelerated proteomic analysis. J. Biotechnol. 124 (4), 717–729. https://doi.org/10.1016/j.jbiotec.2006.01.033 (2006).
Hanano, A., Shaban, M., Almutlk, D. & Almousally, I. The cytochrome P450 BM-1 of Bacillus megaterium A14K is induced by 2,3,7,8-tetrachlorinated dibenzo-p-dioxin: Biophysical, molecular and biochemical determinants. Chemosphere! 216, 327–336. https://doi.org/10.1016/j.chemosphere.2018.10.103 (2019).
Zhao, L. et al. Transcriptional response of Bacillus megaterium FDU301 to PEG200-mediated arid stress. BMC Microbiol. 20 (1). https://doi.org/10.1186/s12866-020-02039-4 (2020).
Huang, F. et al. Complete genome sequence of Bacillus megaterium JX285 isolated from camellia Oleifera rhizosphere. Comput. Biol. Chem. 79, 1–6. https://doi.org/10.1016/j.compbiolchem.2018.12.024 (2019).
Reimann, C. et al. EuroGeoSurveys geochemical mapping of agricultural and grazing land soil of Europe (GEMAS) – (2008). Field manual. EuroGeoSurveys.
GOST 28168-89. Soil Sampling (Standards Publishing House, 1989).
GOST 26423-85. Soils. Methods for Determining Specific Electrical conductivity, pH, and Residue of Water Extract (Standards Publishing House, 1985).
Nelson, D. W. & Sommers, L. E. Total carbon, organic carbon, and organic matter. In (ed Sparks, D. L.) Methods of Soil Analysis: Part 3. Chemical Methods (961–1010). Soil Science Society of America; American Society of Agronomy. https://doi.org/10.2136/sssabookser5.3.c34 (1996).
Soil Science Society of America Methods of soil analysis, part 3: chemical methods. SSSA Book. Ser. 5. https://doi.org/10.2136/sssabookser5.3 (2018).
International Organization for Standardization. ISO 11261:1995. Soil quality - Determination of total nitrogen - Modified Kjeldahl method. (1995). https://www.iso.org/standard/19239.html
Rayment, G. E. & Lyons, D. J. Soil chemical methods: Australasia. CSIRO Publishing. https://doi.org/10.1071/9780643101364 (2011).
Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 15 (12), 1409–1416. https://doi.org/10.1080/00103628409367568 (1984).
Rozhkov, V. A. Soil Particle Size Analysis: Methods and Interpretation (Moscow State University, 2019).
Six, J., Bossuyt, H., Degryze, S. & Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage. Res. 79 (1), 7–31. https://doi.org/10.1016/j.still.2004.03.008 (2004).
Baveye, P. C., Otten, W. & Kravchenko, A. Emergent properties of microbial activity in heterogeneous soil microenvironments: different research approaches are slowly converging. Soil Biol. Biochem. 120!, 122–133. https://doi.org/10.1016/j.soilbio.2018.01.029 (2018).
Rehan, N., Farhat, H., Shafique, H., Aijaz, M. & Shaheen, S. Role of microbial inoculants for improving productivity and systemic resistance in Abelmoschus esculentus. Physiol. Mol. Plant Pathol. 130, 102211. https://doi.org/10.1016/j.pmpp.2023.102211 (2024).
Sokal, R. R. & Rohlf, F. J. Biometry: the Principles and Practice of Statistics in Biological Research 3rd edn (W.H. Freeman, 1995).
Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 37 (7), 613–620. https://doi.org/10.1071/FP09249 (2010).
Ventosa, A., Fernández, A. B., León, M. J., Sánchez-Porro, C. & Rodriguez-Valera, F. Halophiles and hypersaline environments. Curr. Opin. Microbiol. 18, 73–79. https://doi.org/10.1016/j.mib.2014.02.005 (2014).
Kushner, D. J. Growth and nutrition of halophilic bacteria. In (eds Vreeland, R. H. & Hochstein, L. I.) The Biology of Halophilic Bacteria (87–103). CRC. (1993).
Atlas, R. M. Handbook of Microbiological Media 4th edn (CRC, 2010).
Schäffer, A. A. & Fulton, J. S. A simplified method for staining endospores. Science 118 (3062), 450–451. https://doi.org/10.1126/science.118.3062.450 (1953).
Beveridge, T. J., Brenner, D. J. & Krieg, N. R. Bergey’s Manual of Systematic Bacteriology 2nd edn, 2 (Springer, 2007).
Holt, J. G., Krieg, N. R. & Sneath, P. H. A. Bergey’s Manual of Determinative Bacteriology 9th edn (Williams & Wilkins, 1997).
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual 3rd edn (Cold Spring Harbor Laboratory Press, 2001).
Weisburg, W. G., Barns, S. M., Pelletier, D. A. & Lane, D. J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173 (2), 697–703. https://doi.org/10.1128/jb.173.2.697-703.1991 (1991).
MEGA Software. (n.d.). End User License Agreement (EULA). MEGA Software. https://www.megasoftware.net/show_eua
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18 (7), e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
OECD. Test No. 423: Acute oral toxicity. OECD Publishing. (2001). https://doi.org/10.1787/9789264071005-ru
Hong, H. A., Duc, L. H. & Cutting, S. M. Safety of Bacillus species as probiotic feed additives. J. Appl. Microbiol. 98 (6), 1234–1244. https://doi.org/10.1111/j.1365-2672.2005.02665.x (2005).
National Research Council. Guide for the care and use of laboratory animals (8th ed.). National Academies Press. (2011). https://doi.org/10.17226/12910
Sanders, M. E., Akkermans, L. M. & Gorbach, S. L. Safety assessment of probiotics for human use. Gut Microbes. 1 (3), 164–185. https://doi.org/10.4161/gmic.1.3.11827 (2003).
ARRIVE Guidelines. (n.d.). ARRIVE: Animal Research: Reporting of In Vivo Experiments. NC3Rs. https://arriveguidelines.org
Glickmann, E. & Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for Indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 61 (2), 793–796. https://doi.org/10.1128/aem.61.2.793-796.1995 (1995).
Kumar, R., Trivedi, K., Anand, K. G. V. & Ghosh, A. A modified spectrophotometric method for the determination of indole-3-acetic acid and related compounds using salkowski’s reagent. Anal. Biochem. 600, 113747. https://doi.org/10.1016/j.ab.2020.113747 (2020).
Ehmann, A. The Van Urk-Salkowski reagent - A sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of Indole derivatives. J. Chromatogr. A. 132 (2), 267–276. https://doi.org/10.1016/S0021-9673(00)89300-0 (1977).
Patel, H. K., Ferrante, P. & Scortichini, M. Quantitative analysis of indole-3-acetic acid by salkowski’s reagent: A modified protocol for improved accuracy. Anal. Biochem. 487, 28–31. https://doi.org/10.1016/j.ab.2015.07.011 (2015).
Gebauer, L. & Bouffaud, M. L. Water deficit history selects plant beneficial soil bacteria differently under conventional and organic farming. Front. Microbiol. 13, Article 824437. https://doi.org/10.3389/fmicb.2022.824437 (2022).
Ebrahimi-Zarandi, M., Etesami, H. & Glick, B. R. Fostering plant resilience to drought with actinobacteria: unveiling perennial allies in drought stress tolerance. Plant. Stress. 10, Article 100242. https://doi.org/10.1016/j.stress.2023.100242 (2023).
Torres, P. et al. Phenotypic, genomic and in planta characterization of Bacillus sensu Lato for their phosphorus biofertilization and plant growth promotion features in soybean. Microbiol. Res. 280, 127566. https://doi.org/10.1016/j.micres.2023.127566 (2024).
Yang, X. et al. Drought stress tolerance and metabolomics of medicago sativa induced by Bacillus amyloliquefaciens DGL1. Front. Plant Sci. 15, 1378707. https://doi.org/10.3389/fpls.2024.1378707 (2024).
Dubey, P. et al. Microbe assisted plant stress management. In Recent Advancements in Microbial Diversity (pp. 337–356). Academic Press. (2020). https://doi.org/10.1016/B978-0-12-821265-3.00015-3
Laishram, B. et al. Plant-microbe interactions: PGPM as microbial inoculants/biofertilizers for sustaining crop productivity and soil fertility. Curr. Res. Microb. Sci. 8, 100333. https://doi.org/10.1016/j.crmicr.2024.100333 (2025).
Mawarda, P. C., Lakke, S., van Elsas, J. D. & Salles, J. F. Temporal dynamics of the soil bacterial community following Bacillus invasion. iScience 25 (4). https://doi.org/10.1016/j.isci.2022.104185 (2022). Article 104185.
Ghasemzadeh, Z. et al. Soil inoculation with Bacillus megaterium increases infiltration rate and reduces runoff and soil loss under natural rainfall. Rhizosphere 28, 100787. https://doi.org/10.1016/j.rhisph.2023.100787 (2023).
Liu, M. et al. Effects of exogenous GABA on physiological characteristics of licorice seedlings under saline-alkali stress. Plant. Stress. 11, Article 100364. https://doi.org/10.1016/j.stress.2024.100364 (2024).
Gao, M. et al. A strategy for improving saline-alkali soil properties and cotton stress tolerance using vitamin C industrial wastes: A prebiotics-probiotics interaction between organic acids and Bacillus endophyticus. Ind. Crops Prod. 220, 119187. https://doi.org/10.1016/j.indcrop.2024.119187 (2024).
Yi, G. et al. Spring irrigation with magnetized water affects soil water-salt distribution, emergence, growth, and photosynthetic characteristics of cotton seedlings in Southern Xinjiang, China. BMC Plant Biol. 23 (1), 189. https://doi.org/10.1186/s12870-023-04199-7 (2023).
Rapidel, B., Defèche, C., Traoré, B., Lançon, J. & Wery, J. In-field development of a conceptual crop functioning and management model: A case study on cotton in Southern Mali. Eur. J. Agron. 24 (4), 346–356. https://doi.org/10.1016/j.eja.2005.10.012 (2006).
Elsamman, E. et al. Elucidating the phenotypic basis of multi-environment stability for fiber yield and quality traits of cotton (Gossypium hirsutum L.) using 498 Recombinant inbred lines. Ind. Crops Prod. 215, Article 118593. https://doi.org/10.1016/j.indcrop.2024.118593 (2024).
Mengata Mengounou, G. et al. C. F., E. Biodegradability and ecotoxicity of bio-insulating oils in aqueous and soil environments in Douala, Cameroon. Scientific African 18 (2022). https://doi.org/10.1016/j.sciaf.2022.e01413
Fernández, M. D., Sánchez Sánchez-Arguello, P. & García-Gómez, C. Soil pollution remediation. In P. Wexler (Ed.), Encyclopedia of Toxicology (4th ed., 631–645). (Academic Press, 2024). https://doi.org/10.1016/B978-0-12-824315-2.00002-6
R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. (2023). https://www.R-project.org/
Egamberdieva, D., Wirth, S. J., Kimura, S., Mishra, J. & Arora, N. K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 10, 2791. https://doi.org/10.3389/fmicb.2019.02791 (2019).
Ortiz, N., Armada, E., Duque, E., Roldán, A. & Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 174, 87–96. https://doi.org/10.1016/j.jplph.2014.08.019 (2015).
Kıtır Şen, N. & Duran, A. A new approach on essential oil production of Origanum onites L.: microbial fertilization and microwave extraction. Heliyon 9 (5), e15994. https://doi.org/10.1016/j.heliyon.2023.e15994 (2023).
You, Y. et al. A sustainable approach for bioremediation of secondary salinized soils: studying remediation efficiency and soil nitrate transformation by bioaugmentation. Chemosphere 300, 134620. https://doi.org/10.1016/j.chemosphere.2022.134620 (2022).
Barbero, F. M., Torti, L. M., Ferreras, L. A. & Meriles, J. M. Impact of land use changes on soil chemical properties, enzyme activities and microbial communities in two contrasting localities of the Argentinian Pampas. Appl. Soil. Ecol. 206, 104234. https://doi.org/10.1016/j.apsoil.2024.104234 (2025).
Author information
Authors and Affiliations
Contributions
GU and KK prepared the main text of the manuscript and analyzed the data. RS and AN were responsible for the design and visualization. BJ and AN developed the concept of the study, planned the design of the experiment, and conducted laboratory tests. Field tests were conducted by KK and DF. Microbiological analyses were carried out by KM and NM companies, while SD was responsible for strain identification and registration in the relevant database. The toxicity of the biopreparation was assessed by AH. All the authors reviewed and approved the final version of the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ultanbekova, G., Jumakhanov, B., Nussupov, A. et al. The role of Bacillus megaterium PEF-1 in stimulating the growth of organic cotton under environmental stress conditions. Sci Rep 15, 45729 (2025). https://doi.org/10.1038/s41598-025-28267-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28267-0