Abstract
Respiratory syncytial virus (RSV) is a major cause of hospitalization in young children, but the impact of previous SARS-CoV-2 infection on RSV disease severity remains unclear. This study assessed whether prior or recent SARS-CoV-2 infection can influence the severity of RSV disease in children aged 0–5 years (n = 139) during the 2023/2024 season. At admission, serum samples were tested for IgG, IgA, and IgM antibodies against five SARS-CoV-2 antigens. Based on antibody profiles, children were classified into three groups: previously infected (IgG-positive), recently infected (IgM or IgA-positive only), and immunologically-naïve (negative for all antibody classes). Clinical outcomes, including duration of hospitalization, C-reactive protein (CRP) levels, need for oxygen therapy, and use of bronchodilators, antibiotics, and glucocorticoids, were compared across groups. SARS-CoV-2 seropositivity was found in 94 children (67.6%), 10 (7.2%) showed evidence of recent infection, and 35 (25.2%) were seronegative. The groups did not differ in hospitalization duration, CRP levels, or treatment needs. Although a higher proportion of children with recent infection required oxygen therapy (30%), this difference was not statistically significant. SARS-CoV-2 antibody concentrations did not correlate with disease severity. These findings suggest that prior or recent SARS-CoV-2 infection does not significantly influence RSV disease severity in young children.
Similar content being viewed by others
Introduction
Respiratory viral infections represent a significant cause of morbidity and mortality in pediatric populations worldwide, particularly among infants and young children1,2. Respiratory syncytial virus (RSV) is a leading pathogen responsible for severe lower respiratory tract infections, including bronchiolitis and pneumonia, and is one of the primary reasons for pediatric hospitalizations globally. Despite the recent introduction of new preventive strategies, such as monoclonal antibodies and maternal vaccines, RSV continues to burden healthcare systems3,4,5. Understanding the factors that influence the severity and outcomes of RSV infections remains important for mitigating this public health challenge.
The COVID-19 pandemic has introduced novel complexities into children’s epidemiology and immune responses to respiratory viruses. As SARS-CoV-2 spread globally, children were shown to be less severely affected compared to adults6. Nevertheless, exposure to SARS-CoV-2 may have long-term immunological consequences, particularly given its potential to influence host immunity through both humoral and cellular mechanisms7,8. The extent to which previous exposure to SARS-CoV-2 may modulate responses to other respiratory pathogens, including RSV, remains poorly understood and requires further studies. Such interactions between SARS-CoV-2 immunity and the pathogenesis of subsequent RSV infection may occur through multiple pathways.
Emerging studies suggest that such immunological interactions could involve alterations in the innate and adaptive immune responses, potentially affecting the clinical course of subsequent infections. For instance, some research indicates that SARS-CoV-2-induced alterations in the innate and adaptive immune systems could potentially impact the susceptibility, severity, or outcomes of RSV infections9,10,11. Additionally, the potential cross-reactivity between SARS-CoV-2 antibodies and other respiratory viruses, or broader immunomodulatory effects of SARS-CoV-2 infection, may influence the course of subsequent respiratory illnesses. Recent epidemiological data, including the current resurgence of RSV activity and reports of increased RSV severity in children following the easing of pandemic-related restrictions, further underscore the urgency of exploring these interactions in depth12,13. A recent population-based study from Poland during the 2023/2024 RSV season demonstrated widespread RSV seropositivity across age groups, but also highlighted lower antibody prevalence in infants, reflecting a post-pandemic immunity gap and continued vulnerability in this group14.
This study aimed to examine the association between prior SARS-CoV-2 exposure, as measured by the prevalence and concentration of different anti-SARS-CoV-2 antibodies, and the severity and outcomes of RSV infections in a pediatric population (up to 5 years old) during the 2023/2024 season. By investigating humoral responses and clinical outcomes, this research aimed to provide insights into the potential immunological interplay between SARS-CoV-2 and RSV. The findings may help clarify whether SARS-CoV-2 exposure alters susceptibility to severe RSV infection, contributing to a broader understanding of the post-pandemic dynamics of respiratory viral infections in children and informing future prevention and treatment strategies.
Results
Characteristics of the studied group
The study included 139 children aged 15 days to 60 months (median [IQR]: 7 [3–19] months), hospitalized with confirmed RSV infection. Of these, 65 (46.8%) were male and 74 (53.2%) female (p = 0.58). The mean (± SD) duration of hospitalization was 5.04 (± 0.55) days, with a median (IQR) of 4 (3–6) days. Elevated CRP levels (> 5 mg/L) were observed in 59% of patients, with a mean CRP of 17.46 (± 4.42) mg/L. Bronchodilators were administered to 76.3% of patients, primarily salbutamol (84%), followed by adrenaline (8.5%) and fenoterol with ipratropium bromide (7.5%). Glucocorticoids were administered intravenously in 7.9% of patients and via nebulization in 6.5%. Antibiotic therapy was used in 46.8%, most commonly amoxicillin-clavulanic acid (52.3%), and passive oxygen therapy was required in 13.7%. One patient required intensive care. Across the entire cohort, only one child had a recognized risk factor for severe RSV disease (congenital heart disease); no such risk factors were identified in the remaining participants.
SARS-CoV-2 serological status
None of the patients had received a COVID-19 vaccine. Based on SARS-CoV-2 serology:
-
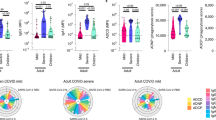
The past infection group (n = 94; 67.6%) showed IgG reactivity to at least one SARS-CoV-2 antigen, most commonly S1 and RBD (Fig. 1A), with the highest median antibody concentrations also observed for these antigens (Fig. 1B).
-
The recent infection group (n = 10; 7.2%) included IgG-seronegative children who were positive for IgM or IgA. IgM and IgA responses were robust, with high concentrations noted against all SARS-CoV-2 antigens.
-
The serologically naïve group (n = 35; 25.2%) showed no antibody response across any SARS-CoV-2 antigen or immunoglobulin class.
Association between SARS-CoV-2 status and RSV severity
Demographic and clinical characteristics across the three groups of patients are summarized in Table 1. We identified 3 SARS-CoV-2 coinfections, all within the serologically naïve subgroup; the severity and duration of illness in these cases did not differ from the remainder of that subgroup. Significant differences were observed in age distribution (p = 0.05), with the highest median age in the recent infection group (17.5 months) and the lowest in the naïve group (5 months). Gender distribution did not differ significantly (p > 0.05).
No statistically significant differences existed between groups in hospitalization duration, CRP levels, or the frequency of bronchodilator, glucocorticoid, or antibiotic use (Table 1). Oxygen therapy was most frequently required in the recent infection group (30%), followed by the past infection (13.8%) and naïve groups (8.6%); however, these differences did not reach statistical significance (p = 0.18 and p = 0.11, respectively), likely due to the small sample size of the recent infection group (Table 1).
No correlation was found between the concentration of IgG, IgM, or IgA antibodies against SARS-CoV-2 antigens and clinical markers of RSV severity, including hospitalization length and need for oxygen therapy.
Discussion
This study investigated the association between prior SARS-CoV-2 exposure, determined serologically by the presence of specific IgG, IgA, and IgM antibodies, and the clinical severity of RSV infection in children under five years of age during the 2023/2024 epidemic season. Elucidation of whether such a relation exists is crucial given that both viruses are common agents causing respiratory disease in overlapping time frames, particularly in autumn and winter seasons in temperate climates15,16. Therefore, it is plausible that over the course of a particular epidemic season, children will first be infected with SARS-CoV-2, followed by subsequent RSV infection.
Such exacerbation of the clinical severity of RSV infection could potentially result from antibody-dependent enhancement (ADE), a phenomenon in which the humoral immune response to one viral infection contributes to a more severe course of another17. For instance, prior exposure to the Zika virus is associated with a more severe course of dengue and an increased risk of fatal outcomes, largely due to cross-reactive non-neutralizing antibodies18,19. More recently, in vitro and in vivo studies have demonstrated that antibodies generated in response to SARS-CoV-2 can enhance dengue virus infection20 , though such an effect on the clinical level, at least in children, is contested21. Nevertheless, all of these observations raise theoretical concerns about similar effects with other respiratory viruses. Beyond ADE, SARS-CoV-2 infection may influence the severity of RSV infection through broader alterations in the immune system. In children, SARS-CoV-2 has been shown to induce long-term changes in both innate and adaptive immunity, including skewed cytokine responses, lymphopenia, and dysregulation of interferon signalling pathways22,23. These effects could, in theory, impair viral clearance or exacerbate inflammation during subsequent infections such as RSV. Particularly in infants, who rely heavily on the early development of mucosal and systemic immune balance, such perturbations might increase susceptibility to more severe respiratory disease24. Furthermore, SARS-CoV-2-related disruption of epithelial integrity and tissue-resident immunity in the airways could compromise frontline defence mechanisms25, potentially facilitating enhanced RSV replication or tissue damage.
However, our findings do not support a clinically significant immunopathological interaction of this nature, at least within the humoral compartment detectable by current serological assays. This observation is particularly important in light of the global increase in pediatric RSV hospitalizations following the COVID-19 pandemic. For example, in Poland during the waning phase of the pandemic, hospital admission rates for children aged < 12 months, 12–24 months, and 25–60 months were approximately 2-, 4-, and fivefold higher than pre-pandemic levels, respectively26. Similar trends have been reported in other countries27,28,29. These patterns are consistent with what has been termed a "post-pandemic compensatory epidemic", also in relation to other respiratory infections30,31. RSV, a virus with strong seasonality, typically circulates from late fall through early spring, and annual exposure helps maintain a degree of population-level immunity. However, prolonged pandemic-related restrictions, including lockdowns, school closures, and reduced social contact, substantially limited RSV transmission32. As a result, many infants and toddlers missed their first natural exposure, leading to a larger pool of immunologically naïve children33. A similar phenomenon was also observed in relation to other viral infections34. Therefore, the post-pandemic resurgence of RSV likely reflects this accumulation of susceptible individuals, rather than enhanced disease severity due to prior SARS-CoV-2 exposure.
In line with our observations, clinical evidence in children suggests that there is no significant increase in the risk of RSV infection following a SARS-CoV-2 infection. A large cohort study involving children under five years old found that, after adjusting for coinfections and seasonal variations, the short-term rise in RSV cases was primarily due to coinfections rather than a true elevated risk post-COVID-1935. Additionally, case series in infants indicate that coinfection with RSV and SARS-CoV-2 does not exacerbate the severity of COVID-19 symptoms36. Interestingly, in vitro experiments suggest that SARS-CoV-2 infection can actually reduce RSV replication, suggesting possible viral interference rather than enhancement37. Consequently, in animal models, prior SARS-CoV-2 exposure does not worsen RSV disease; in fact, it may even reduce RSV replication in the lower respiratory tract38.
Nevertheless, the elevated proportion of children requiring oxygen following recent SARS‑CoV‑2 infection, though not statistically significant, suggests subtle, short‑term alterations in pulmonary or immune function. Functional imaging and lung function studies in pediatrics have documented persistent ventilation–perfusion mismatch and mild inflammatory changes several months post-infection, even in non-hospitalized cases39,40,41. Mechanistically, delayed interferon signalling and impaired epithelial regeneration following SARS‑CoV‑2 have been demonstrated in respiratory models, pointing to slower mucosal repair processes that could heighten susceptibility to hypoxia during RSV coinfection. Moreover, mild obstructive patterns and diffusion impairments observed in follow-up pulmonary function tests in children indicate ongoing airway inflammation and mucosal dysfunction42,43. Collectively, these data raise the possibility that recent SARS‑CoV‑2 exposure may transiently compromise respiratory resilience, thereby increasing oxygen support needs during subsequent RSV infection. Although our sample size limits definitive conclusions, this trend underscores the importance of longitudinal studies to explore temporal dynamics of recovery and vulnerability in pediatric mucosal immunity post-SARS‑CoV‑2.
The strength of the present study is the comprehensive serological profiling using a validated immunoblot microarray targeting multiple SARS-CoV-2 antigens, which allowed us to distinguish between recent and past infections with greater granularity than single-antigen assays. Furthermore, all children included in the analysis were hospitalized with confirmed RSV infection, allowing for robust comparisons of clinical severity parameters. However, the study also has limitations. First, while serological markers provide important evidence of past exposure, they cannot determine the exact timing of infection, and waning antibody levels may lead to underestimation of prior infections. Second, cellular immunity, which plays a crucial role in modulating responses to respiratory viruses, was not assessed. Third, all children included in the study were not vaccinated against SARS-CoV-2, and were not born to mothers vaccinated against RSV during pregnancy. Therefore, the results do not imply how vaccine-induced immunity, either through direct pediatric vaccination or transplacental maternal antibodies44,45, might interact with the severity of RSV infections. Fourth, the relatively small number of children classified as recently infected with SARS-CoV-2 limited the statistical power to detect subtle differences in clinical outcomes and may have masked associations of potential relevance—further studies targeting this group are required. The study cohort was drawn from a single hospital, which may limit generalizability to broader populations with differing sociodemographic or healthcare characteristics. Moreover, RSV infection was confirmed primarily based on rapid antigen testing, not routine PCR, and no PCR testing for SARS-CoV-2 infection was performed. Finally, it is essential to interpret our findings in light of the evolving epidemiology of SARS-CoV-2. The study was conducted during the Omicron-dominant era, when the clinical image of COVID-19 differed from earlier pandemic phases, during which variants such as Alpha or Delta were associated with more systemic complications46, including the now rarely observed multisystem inflammatory syndrome in children47. Moreover, Omicron appears to continue towards lower clinical significance48. Accordingly, the risk profile of RSV/COVID-19 coinfections may have shifted over time. In addition, it would be of interest to establish whether interactions between SARS-CoV-2 and RSV impact the clinical severities in other age groups, including the elderly, who constitute another risk group for both viral infections49.
Conclusions
In the studied cohort of children hospitalized with RSV, no significant association was found between prior SARS-CoV-2 exposure, as assessed by serological markers, and the severity or clinical outcomes of RSV infection. The immune imprint of SARS-CoV-2, as reflected in antibody profiles, did not appear to affect key metrics such as length of hospital stay, inflammatory markers, or respiratory support requirements. While a non-significant trend toward more frequent oxygen therapy was observed in recently infected patients, larger studies are needed to clarify whether this reflects a true biological effect or random variation. These findings suggest that prior SARS-CoV-2 infection does not markedly alter the clinical trajectory of RSV infection in young children.
Methods
Patients and sample collection
This was a retrospective cohort study. In our department, a small blood sample is routinely banked for research at admission (with parental consent) under a standing biobanking SOP. For the present analysis, we identified eligible hospitalizations retrospectively using ICD-10 codes consistent with RSV infection and then linked those admissions to the banked samples for serological testing. The study included patients up to 5 years who were hospitalized at the Department of Pediatrics, Bielański Hospital in Warsaw, with the diagnoses confirmed according to ICD-10 classification:
-
J12.1—Respiratory syncytial virus pneumonia,
-
J20.5—Acute bronchitis due to respiratory syncytial virus,
-
J21.0—Acute bronchiolitis due to respiratory syncytial virus.
In Poland, there are no fully clear, unified national guidelines for RSV admissions; decisions are largely based on clinical assessment. In practice, the following indications are used: (i) signs of severe disease—agitation and episodes of apnea as markers of hypoxemia; (ii) tachypnea > 60/min, subcostal retractions, SpO₂ < 92%; (iii) age < 6 months (especially < 3 months); (iv) feeding difficulties/poor oral intake and risk of dehydration; (v) risk factors influencing the decision to admit: comorbidities (bronchopulmonary dysplasia, congenital heart disease, cystic fibrosis), prematurity < 32 weeks’ gestation, and adverse socioeconomic conditions.
The enrolment period covered the 2023/2024 RSV epidemic season of RSV, from September 2023 to March 2024. RSV infection was confirmed by antigen testing using nasopharyngeal swabs. In cases with strong clinical suspicion but negative antigen test results, reverse transcription polymerase chain reaction (RT-PCR) testing was additionally performed. Upon hospital admission, routine blood samples were collected for laboratory evaluation, and additional blood samples were obtained for research purposes. These samples were then stored in a biobank and frozen for subsequent determination of the presence and concentration of antibodies against SARS-CoV-2. Patient medical records were reviewed to assess clinical outcomes, including the length of hospital stay, C-reactive protein (CRP) levels, the need for oxygen therapy, frequency of antibiotic use, and administration of bronchodilators and/or glucocorticoids (via nebulization or systemically).
The study was approved by the local Ethics Committee at the Centre of Postgraduate Medical Education in Warsaw (permission number 85/2025). It has been performed according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from the legal tutors of all patients involved in the study.
Determination of anti-SARS-CoV-2 antibodies
The presence and concentration of anti-SARS-CoV-2 antibodies in serum samples were determined using the CE-IVD (Conformité Européenne—In Vitro Diagnostics) certified immunoblot microarray assays (Microblot-Array COVID-19; TestLine Clinical Diagnostics, Brno, Czech Republic), testing the IgM, IgA, and IgG against SARS-CoV-2's nucleocapsid (anti-NP), receptor binding domain (anti-RBD), epitopes of subunit 1 of the spike protein other than RBD (anti-S1), epitopes of subunit 2 of the spike protein (anti-S2), and envelope protein (anti-E). In all assays, the recombinant, purified native antigens are immobilized on nitrocellulose membrane spots at the bottom of the 96-well microplate50,51. According to the information provided by the manufacturer, the assay demonstrates a diagnostic sensitivity and specificity of 98.7% and 99.3%, respectively. The concentrations of all antibodies were reported in U/mL and deemed positive if they exceeded 210 U/mL, as per the manufacturer’s guidelines. Concentrations below this threshold were classified as non-seropositive and excluded from calculating mean antibody concentrations.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 10 and Statistica v.13 (StatSoft, USA). The analyzed groups did not meet the assumptions of normality (evaluated with the Shapiro–Wilk test); therefore, statistical significance was consequently assessed using the Kruskal–Wallis test. A p-value below 0.05 was deemed statistically significant.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Teoh, Z. et al. Burden of respiratory viruses in children less than 2 years old in a community-based longitudinal US birth cohort. Clin. Infect. Dis. 77, 901–909 (2023).
Dallmeyer, L. K. et al. Epidemiology of respiratory viruses among children during the SARS-CoV-2 pandemic: A systematic review and meta-analysis. Int. J. Infect. Dis. 138, 10–18 (2024).
Rzymski, P. & Gwenzi, W. Respiratory syncytial virus immunoprophylaxis: Novel opportunities and a call for equity. J. Med. Virol. 96, e29453 (2024).
Mazur, N. I., Caballero, M. T. & Nunes, M. C. Severe respiratory syncytial virus infection in children: burden, management, and emerging therapies. Lancet 404, 1143–1156 (2024).
Wang, X. et al. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: A systematic review and meta-analysis of aggregated and individual participant data. Lancet 403, 1241–1253 (2024).
Kang, C. K., Shin, H. M., Park, W. B. & Kim, H.-R. Why are children less affected than adults by severe acute respiratory syndrome coronavirus 2 infection?. Cell. Mol. Immunol. 19, 555–557 (2022).
Cheong, J.-G. et al. Epigenetic memory of coronavirus infection in innate immune cells and their progenitors. Cell 186, 3882-3902.e24 (2023).
Saydah, S. H., Campbell, A. P. & Randolph, A. G. Consequences beyond acute SARS-CoV-2 infection in children. Sci. Transl. Med. 16, eado2099 (2024).
Liu, J. et al. Analysis of the long-term impact on cellular immunity in COVID-19-recovered individuals reveals a profound NKT cell impairment. MBio 12, 10–1128 (2021).
Jing, Y. et al. SARS-CoV-2 infection causes immunodeficiency in recovered patients by downregulating CD19 expression in B cells via enhancing B-cell metabolism. Signal Transduct. Target. Ther. 6, 345 (2021).
Abu-Raya, B., Viñeta Paramo, M., Reicherz, F. & Lavoie, P. M. Why has the epidemiology of RSV changed during the COVID-19 pandemic?. EClinicalMedicine 61, 102089 (2023).
Korsun, N. et al. Resurgence of respiratory syncytial virus with dominance of RSV-B during the 2022–2023 season. Front. Microbiol. 15, 1376389 (2024).
Liu, H.-F. et al. Comparison of characteristics of children hospitalized for respiratory syncytial virus infection during the pre- and post-COVID-19 eras: a multicenter retrospective study. BMC Infect. Dis. 24, 1009 (2024).
Poniedziałek, B. et al. Seroprevalence of RSV IgG antibodies across age groups in poland after the COVID-19 pandemic: Data from the 2023/2024 epidemic season. Vaccines https://doi.org/10.3390/vaccines13070741 (2025).
Del Riccio, M. et al. Global analysis of respiratory viral circulation and timing of epidemics in the pre-COVID-19 and COVID-19 pandemic eras, based on data from the global influenza surveillance and Response System (GISRS). Int. J. Infect. Dis. 144, 107052 (2024).
Pratt, G. W., Wong, C. L. & Rao, L. V. Prevalence and co-detection rates of SARS-CoV-2, influenza, and respiratory syncytial virus: A retrospective analysis. APMIS 133, e70010 (2025).
Wells, T. J., Esposito, T., Henderson, I. R. & Labzin, L. I. Mechanisms of antibody-dependent enhancement of infectious disease. Nat. Rev. Immunol. 25, 6–21 (2025).
Bonheur, A. N. et al. A fatal case report of antibody-dependent enhancement of dengue virus type 1 following remote Zika virus infection. BMC Infect. Dis. 21, 749 (2021).
Estofolete, C. F. et al. Influence of previous Zika virus infection on acute dengue episode. PLoS Negl. Trop. Dis. 17, e0011710 (2023).
Jakhar, K. et al. SARS-CoV-2 spike antibodies cross-react with dengue virus and enhance infection in-vitro and in-vivo. bioRxiv 2023.10.09.557914 (2023) https://doi.org/10.1101/2023.10.09.557914.
Ravikumar, N. et al. Impact of recent SARS-CoV-2 infection on the course and severity of dengue in children: A prospective observational study from north India. Am. J. Trop. Med. Hyg. 105, 751–755 (2021).
Loske, J. et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 1–6 (2021).
Chou, J., Thomas, P. G. & Randolph, A. G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 23, 177–185 (2022).
Simon, A. K., Hollander, G. A. & McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 282, 20143085 (2015).
Gonzalez-Rubio, J. et al. SARS-CoV-2 particles promote airway epithelial differentiation and ciliation. Front. Bioeng. Biotechnol. 11, 1268782 (2023).
Mazela, J. et al. Epidemiology of respiratory syncytial virus hospitalizations in Poland: An analysis from 2015 to 2023 covering the entire polish population of children aged under five years. Viruses 16, 704 (2024).
Lastrucci, V. et al. Seasonality and severity of respiratory syncytial virus during the COVID-19 pandemic: a dynamic cohort study. Int. J. Infect. Dis. 148, 107231 (2024).
Rios-Guzman, E. et al. Deviations in RSV epidemiological patterns and population structures in the United States following the COVID-19 pandemic. Nat. Commun. 15, 3374 (2024).
Maglione, M. et al. Changes in respiratory viruses’ activity in children during the COVID-19 pandemic: A systematic review. J. Clin. Med. 14, 1387 (2025).
Rzymski, P. et al. Tracking clinical severity of influenza in adult hospitalized patients in 2024: Data from the FluTer registry in Poland. Vaccine 61, 127443 (2025).
Rzymski, P. et al. Unraveling Poland’s unprecedented influenza surge in early 2025: increased viral severity or post-pandemic vulnerability?. Pharmacol. Rep. https://doi.org/10.1007/s43440-025-00731-8 (2025).
Billard, M.-N. et al. International changes in respiratory syncytial virus (RSV) epidemiology during the COVID-19 pandemic: Association with school closures. Influenza Other Respi. Viruses 16, 926–936 (2022).
Billard, M.-N. & Bont, L. J. Quantifying the RSV immunity debt following COVID-19: a public health matter. Lancet. Infect. Dis 23, 3–5 (2023).
Rzymski, P. et al. The burden of infectious diseases throughout and after the COVID-19 pandemic (2020–2023) and Russo-Ukrainian war migration. J. Med. Virol. 96, e29651 (2024).
Rao, S. et al. P-727. Is there increased risk of RSV infection following SARS-CoV-2 infection in children? An EHR-based cohort study from the RECOVER Program. Open Forum Infect. Dis. 12, (2025).
Giannattasio, A. et al. Silent RSV in infants with SARS-CoV-2 infection: A case series. Pediatr. Pulmonol. 56, 3044–3046 (2021).
Fage, C., Hénaut, M., Carbonneau, J., Piret, J. & Boivin, G. Influenza A(H1N1)pdm09 virus but not respiratory syncytial virus interferes with SARS-CoV-2 replication during sequential infections in human nasal epithelial cells. Viruses 14, 395 (2022).
Morris, D. R. et al. The impact of RSV/SARS-CoV-2 coinfection on clinical disease and viral replication: insights from a BALB/c mouse model. bioRxivorg (2023) https://doi.org/10.1101/2023.05.24.542043.
Heiss, R. et al. Pulmonary dysfunction after pediatric COVID-19. Radiology 306, e221250 (2023).
Shmueli, E. et al. Pulmonary evaluation in children with post-COVID-19 condition respiratory symptoms: A prospective cohort study. J. Clin. Med. 12, 6891 (2023).
Bakhtiari, E. & Moazzen, N. Pulmonary function in children post -SARS-CoV-2 infection: a systematic review and meta-analysis. BMC Pediatr. 24, 87 (2024).
Magalhães, V. G. et al. Immune-epithelial cell cross-talk enhances antiviral responsiveness to SARS-CoV-2 in children. EMBO Rep. 24, e57912 (2023).
Bragazzi Cunha, J. et al. Type I interferon signaling induces a delayed antiproliferative response in respiratory epithelial cells during SARS-CoV-2 infection. J. Virol. 97, e0127623 (2023).
Kampmann, B. et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N. Engl. J. Med. 388, 1451–1464 (2023).
Rumfelt, K. E. et al. Maternal-infant respiratory syncytial virus and influenza A virus antibody transfer in preterm and full-term infants. Open Forum Infect. Dis. 12, ofae723 (2025).
Dobrowolska, K. et al. Differences between the course of SARS-CoV-2 infections in the periods of the delta and omicron variant dominance in Poland. Pol. Arch. Intern. Med. 133, 16403 (2023).
Sangwha, K. et al. Frozen sections in decision-making regarding the axillary procedures in breast conserving surgery for intraductal carcinoma at preoperative diagnosis. J. Korean Med. Sci. 38, e224 (2023).
Flisiak, R. et al. Change in the clinical picture of hospitalized patients with COVID-19 between the early and late period of dominance of the omicron SARS-CoV-2 variant. J. Clin. Med. 12, 5572 (2023).
Rzymski, P., Poniedziałek, B., Zarębska-Michaluk, D., Tomasiewicz, K., Flisiak, R. High seroprevalence and high risk: Why are older adults more prone to respiratory syncytial virus?. J Virol:e01432–25. https://doi.org/10.1128/jvi.01432-25
Montesinos, I. et al. Neutralizing antibody responses following natural SARS-CoV-2 infection: Dynamics and correlation with commercial serologic tests. J. Clin. Virol. 144, 104988 (2021).
Brydak, L. et al. Association between the seroprevalence of antibodies against seasonal alphacoronaviruses and SARS-CoV-2 humoral immune response, COVID-19 severity, and influenza vaccination. J. Clin. Med. 12, 1733 (2023).
Funding
This study was supported by the Centre of Postgraduate Medical Education in Warsaw, grant number 501-1-020-19-25, and funds of the Department of Environmental Medicine (Poznan University of Medical Sciences).
Author information
Authors and Affiliations
Contributions
Conceptualisation: Teresa Jackowska, Piotr Rzymski. Investigation: Barbara Poniedziałek,, Marcin Rozwadowski, Katarzyna Zabłocka, Teresa Jackowska, Piotr Rzymski. Methodology: Barbara Poniedziałek, Teresa Jackowska, Piotr Rzymski. Visualisation: Piotr Rzymski. Writing—original draft: Barbara Poniedziałek, Marcin Rozwadowski, Piotr Rzymski. Writing—review & editing: Katarzyna Zabłocka, Teresa Jackowska. Supervision: Teresa Jackowska, Piotr Rzymski.
Corresponding author
Ethics declarations
Competing interests
T.J. reports lecture fees from Pfizer. P.R. reports grants, advisory, and lecture fees from Pfizer, Moderna, and Sanofi. The other authors declare no conflict of interest in relation to this work.
Ethics approval
The study was approved by the local Ethics Committee at the Centre of Postgraduate Medical Education in Warsaw (permission number 85/2025) and conducted in accordance with the Helsinki Declaration with later amendments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Poniedziałek, B., Rozwadowski, M., Zabłocka, K. et al. Impact of previous SARS-CoV-2 infection on the severity of respiratory syncytial virus infection in hospitalized children. Sci Rep 15, 44570 (2025). https://doi.org/10.1038/s41598-025-28414-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28414-7