Abstract
This is an in vitro study aimed to develop a nano-antibiotic paste containing chloramphenicol, tetracycline, and silver and zinc oxide nanoparticles, evaluating its microbiological efficacy against four bacterial strains, determining its minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against a commercial CTZ paste. Silver and zinc oxide nanoparticles (1 × 10− 2 M) were synthesized and characterized by UV-Vis and DLS. A paste of tetracycline and chloramphenicol was prepared to a known concentration (10%), adding the synthesized nanoparticles and reducing the antibiotic percentage from the original proportions (− 4%). The commercial CTZ paste was then prepared following the manufacturer’s instructions (13% concentration). Microdilution method and Kirby-Bauer assessment were used at three different concentrations to evaluate antimicrobial activity for both pastes against Enterococcus faecalis, Staphylococcus aureus, Streptococcus mutans, and Escherichia coli. MBC for the nano antibiotic paste (10%) and commercial CTZ paste (13%) were 4.8 µg/ml and 12 µg/ml for S. aureus, 19.5 µg/ml and 12.8 µg/ml for E. coli, 19.5 µg/ml and 12.8 µg/ml for E. faecalis, 39 µg/ml and 25 µg/ml for S. mutans, respectively. For Kirby Bauer statistical difference was shown, favoring nano paste for C1 E. coli (p = 0.000262) and C2 E. coli (p = 0.000388), and for C3 S. mutans (p = 0.00749), and commercial CTZ paste, for C2 S. aureus (p = 0.0146) and C2 E. faecalis (p = 0.00033). Microbiological assays revealed that the commercial CTZ de Mexico™ paste (13%) exhibited superior bactericidal activity, with lower MBCs, than the Nano formulation (10%) containing 4% less antibiotic. Results offer in vitro doses for the strains.
Similar content being viewed by others
Introduction
With the emergence and application of the lesion sterilization and tissue repair technique (LSTR) in recent years, the use of antibiotic pastes in pulp therapy of necrotic deciduous teeth has become increasingly common. The LSTR technique was developed in 2004 at the Cariology Research Unit of Niigata University, and it bases its success on the use of antibiotic pastes placed at the entrance of the root canals, promoting their disinfection by pharmacological means, to subsequently seal the cavity with a biocompatible and long-lasting material until the deciduous tooth exfoliates1,2,3. LSTR is indicated in primary teeth with a diagnosis of irreversible pulpitis and pulp necrosis, observing clinical (80–95%) and radiographic (70–85%) success rates similar to those obtained by conventional pulpectomy obturated with iodine-formed paste4,5. Its main advantage lies in the simplification of pulpectomy, a systematic procedure involving a series of complex steps that require precision of the clinician and the cooperation of the pediatric patient in an even more complex anatomic scenario, where primary molars may exhibit accessory root canals and unpredictable resorptions that prevent a proper hermetic seal2,6. Antibacterial drugs selected to elaborate the pastes are based on several studies where bacteria have been isolated from necrotic root canals of primary teeth. Being a polymicrobial infection, it is recognized that a single antibiotic would be insufficient1,6. This is why mixtures of two or three antibiotics are reported to be the most effective. However, these pastes are made of a variety of antibiotic mixtures with different doses, without a previous in vitro study of an optimal dose and standardization of their preparation, representing a lack of consistency in their use and application, causing controversy in the efficacy of the treatment in pediatric dentistry6,7,8.
Tri-antibiotic pastes (TAP) are among the most used and studied pastes, characterized by their great variability. These may contain minocycline, ciprofloxacin, and clindamycin; minocycline, ciprofloxacin, and metronidazole; gentamicin, amoxicillin, and metronidazole, and ornidazole instead of minocycline1,6,8. Another agent used with favorable results is CTZ paste, composed of 2 broad-spectrum antibiotics (tetracycline and chloramphenicol) combined with dental type I zinc oxide and eugenol (ZOE), standing out for its antimicrobial, antiseptic, anti-inflammatory, and analgesic properties4,9,10. Currently, studies report the inclusion of other drugs to antibiotic mixtures to enhance anti-inflammatory and regenerative properties, promising to improve their efficacy11.
Nanotechnology and nanomaterials use in dentistry has been exponential in recent years12. The application areas in dentistry include restorative, periodontal, and prosthetic treatments, orthodontics, implantology, and endodontics13. In the endodontics and pulp therapy area, the use of graphene, silver, chitosan, hydroxyapatite, titanium dioxide, zirconia, calcium oxide, and copper nanoparticles has been reported to be added to irrigants, dressings, intra-canal and obturation materials, showing great potential in the reduction of bacterial biofilm14,15,16.
Silver nanoparticles (SNPs) are recognized for their broad-spectrum antimicrobial properties against Gram-positive and Gram-negative bacteria preventing formation of bacterial biofilms, by adhering to the cell wall causing pores, and inhibiting the synthesis of proteins and ATP; the colloidal morphology shows good antibacterial activity and it is known that sizes smaller than 15 nm and with a concentration of 0.004% w/w have shown maximum success in avoiding the bacterial growth. Its benefits also include osteogenic induction and anti-inflammatory effect, decreasing vascular growth factor levels, preventing mucin secretion, and suppressing IL-12 and TNF-alpha17. Recent studies demonstrated synergism with certain antibiotics when absorbed by nanomaterials18,19,20. Khurana et al. (2016) reported synergism between tetracycline and kanamycin, as well as silver nanoparticles, which absorb these drugs, increasing their loading efficiency against certain bacterial strains, including S. aureus20,21. Zinc oxide nanoparticles exhibit antimicrobial properties similar to those of silver, with anti-inflammatory effects that inhibit the proliferation of mast cells by decreasing IL-12, 13, and TNF-alpha17.
Antibiotic pastes used in pulp therapy could benefit from nanotechnology. Specifically, the incorporation of zinc oxide and silver nanoparticles in antibiotic mixtures such as CTZ paste, mainly due to the formula per se. The original formula consists of two antibiotics (chloramphenicol and tetracycline), a combination that has demostrated antimicrobial synergy used alongside silver nanoparticles18,20 and zinc oxide type I. Zinc oxide with a uniform and controlled nano size would contribute its intrinsic anti-inflammatory and antimicrobial properties; meanwhile, the addition of silver nanoparticles could lead to a reduction in the percentage of antibiotics from the original formulation. This new nano antibiotic mixture could potentially equal or increase the microbiological efficacy of the commercial CTZ paste, with less antibiotic concentration, improving the overall performance of the paste while minimizing unwanted effects when used in the LSTR technique in pediatric dentistry.
Therefore, the present study aims to develop a novel antibiotic paste formulated with reduced concentrations of chloramphenicol and tetracycline enhanced by the incorporation of silver and zinc oxide nanoparticles, as well as performing its in vitro microbiological evaluation by determining the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against four clinically relevant bacterial strains. This approach also proposes a standardized preparation protocol to address the current inconsistency in existing formulations and eliminate dose variability.
Materials and methods
Study design
This is an experimental in vitro study carried out in the Materials Laboratory of the Faculty of Science of the Universidad Autónoma de San Luis Potosí, in San Luis Potosí, México.
Ethical aspects
This research was submitted for approval to the Research Ethics Committee of the Faculty of Stomatology, UASLP, CONBIOETHICS-24-CEI-001-20190213. Approved and assigned with the code: CEI-FE-O18- 023. This study and all resulting laboratory procedures were carried out in compliance with the requirements of the following standards:
-
NOM 087 SSA1–2002 on Environmental Protection - Environmental Health - Biologically Infectious Hazardous Waste - Classification and Handling Specifications.
-
NOM-065-SSA1- 1993 on the health specifications for culture media.
-
NOM 013 STPs – 1993 on Safety and Hygiene Conditions in Workplaces Where Non-ionizing Electromagnetic Radiation Is Generated.
Laboratory processes
Synthesis of silver nanoparticles (SNPs)
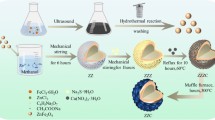
The synthesis was carried out by chemical reduction using silver nitrate (AgNO3) following the technique reported by Martínez-Castañón et al. in 200822 modified with tannic acid as a reducing agent23. 500 mL of a solution of silver nanoparticles was prepared at a concentration of 1 × 10− 2 M. 0.84935 g of silver nitrate was weighed and dissolved in 450 mL of water under constant stirring; then 1 g of tannic acid previously dissolved in 50 mL of deionized water was added, and the pH was adjusted to 11 with sodium hydroxide. The mixture was left in agitation for 30 min and proceeded to its characterization. To recover the nanoparticle powder, the pH was lowered to 2 with nitric acid so that nanoparticles flocculated and precipitated; once the precipitate was obtained, it was dried at room temperature, weighed, and stored until its use.
Synthesis of zinc oxide nanoparticles (ZnONPs)
100 mL of ZnONPs solution was prepared at 1 × 10− 2M concentration. 0.1362 g of zinc chloride was weighed and made up to 100 mL with deionized water. Two drops of hydrochloric acid were added, and the contents were poured into a beaker with a magnetic bar and placed on a heating plate. The temperature was controlled until it reached 80 °C, and pH was adjusted to 10 using sodium hydroxide. The precipitate was left to dry until the powder was obtained, which was weighed and stored until use.
Structural characterization of silver nanoparticles, zinc oxide nanoparticles, and CTZ de México™ paste
-
a.
UV-Vis spectroscopy.
SNPs were characterized by UV-Vis spectroscopy using the OceanOptics S2000 equipment, to observe the surface plasmon belonging to silver. For ZnONPs and CTZ de México™ paste, powder spectroscopy was used to determine the characteristic plasmon of zinc oxide in both powders.
-
b.
Dynamic Light Scattering (DLS).
The size of the SNP`s and ZnONPs and the zeta potential (greater than + 30mV or less than − 30mV) were determined by dynamic light scattering using the Malvern model Zetasizer Nano ZS.
-
c.
Scanning electron microscopy (SEM).
A capsule of the CTZ de México™ paste was observed by scanning electron microscopy to observe its components, as well as its particle size.
-
d.
Transmission electron microscopy (TEM).
ZnONPs were characterized by this means. Samples were prepared by placing a drop of the original dispersion obtained on polymer-coated copper grids. TEM images were obtained in a JEOL microscope model JEM 1230 operated at 120 kV.
-
e.
X-ray diffraction technique.
ZnONPs were characterized by this means using a Rigaku (DMAX 2200) powder diffractometer.
Samples preparation
Preparation of the nano antibiotic paste
The original proportion proposed by Soller and Cappiello in 1959, of tetracycline 500 mg, chloramphenicol 500 mg, and zinc oxide 1 g, was used9 . For the formulation with the nanoparticles, the reagents used were Tetracycline Hydrochloride (T76660, Sigma Aldrich™, USA) and Chloramphenicol (C0378, Sigma Aldrich™, USA) in the following percentages: Tetracycline (23%), Chloramphenicol (23%), Zinc Oxide (50%), Silver Nanoparticles (4%). 2% of the original percentage of each antibiotic was substituted for 4% of the SNP´s. For the final mixture, 100 mg were prepared following the above ratio: Tetracycline 0.023 g, Chloramphenicol 0.023 g, Zinc Oxide Nanoparticles 0.05 g, and Silver Nanoparticles 0.004 g. This mixture was dissolved in 10mL of deionized water, to obtain an antibiotic paste concentration of 10%, which was used for microbiological evaluation.
Preparation of CTZ de México™ paste
A capsule was opened and weighed. Since it was the dose proposed by the manufacturer, the entire amount was used. The total weight was 0.1317 g. It was dissolved in 10 mL of deionized water, obtaining a concentration of 13%. It is important to mention that each capsule presented a variation in its weight.
Preparation of the SNPs and ZnONPs
The samples were prepared with an initial concentration of 0.107 mg/mL for both nanoparticles. The powder of each nanoparticle was weighed and reconstituted with deionized water following the given concentration.
Evaluation of antimicrobial activity
Four ATCC strains: Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (ATCC 29213), Streptococcus mutans (ATCC 25175), Escherichia coli (ATCC 25922) were used to determine bactericidal susceptibility.
The following experimental groups were determined (Table 1):
Bactericidal susceptibility
According to the guidelines established by the Clinical and Laboratory Standards Institute (CLSI), the microdilution plate method was used to determine the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC) of the compounds evaluated24. The tests were performed against reference strains in quadruplicate, including Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (ATCC 29213), Streptococcus mutans (ATCC 25175), Escherichia coli (ATCC 25922).
The following culture media and solutions were used for the preparation and development of the experiment: Mueller-Hinton agar (BD Bioxon™, México), Mueller-Hinton broth (BD Difco™, France), Enterococcocel Agar (Condalab™, Spain), phosphate buffer composed of Na₂HPO₄ and KH₂PO₄ (Fermont™, México), and sodium chloride (NaCl) (CTR Scientific™, México). This experimental approach ensures the accuracy and reproducibility of the results obtained in the antimicrobial evaluation. The method is described in Supplement 1.
Kirby Bauer assay
This test followed the methodology suggested by CLSI24. The strains tested were Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (ATCC 29213), Streptococcus mutans (ATCC 25175) and Escherichia coli (ATCC 25922). The bacterial suspension was adjusted to McFarland scale 0.5 and sterile swabs were used to seed the bacteria in Petri dishes. The experimental groups contained three different concentrations of each sample C1 = basal C2 = 7.5%, C3 = 5%. Discs were placed on top of each plate and 10 µL were added to each group (n = 3). Plates were incubated at 37 °C for 24 h. Growth diameter inhibition zones were measured horizontally and vertically with a vernier (Mitutoyo Corp., Kawasaki, Japan). The methods are described in Supplement 1. Figure 1 shows a graphical summary of the methodology followed.
Results
Synthesis and characterization of SNPs and ZnONPs
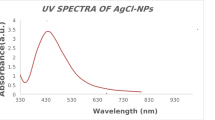
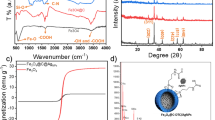
SNPs were synthesized as planned and characterized by DLS, observing a size of 10.08 nm (Fig. 2) and a zeta potential of -23mV, as well as by UV-Vis spectroscopy observing the plasmon of silver nanoparticles at 429 nm (Fig. 3).
The ZnONPs were characterized by DLS to observe a nanoparticle size of 14.98 nm (Fig. 4) with a zeta potential of -19mV. Also, they were observed by TEM, confirming the nanoscale of the sample (Figs. 5 and 6).
The powder was recovered and observed by UV-Vis spectroscopy; an absorption spectrum was recorded with wavelengths between 280 and 400 nm.
Through the X-ray diffraction technique, the representative peaks of the Wurzite crystalline structure were obtained (Fig. 7), the main diffraction planes found in order of intensity were (101), (002), (100), (110), (103), (112) and (102).
CTZ de México™ paste characterization
The content of a capsule was weighed and dilutions were performed for its characterization by DLS, obtaining a particle size of 471.5 nm. By powder spectroscopy, wavelengths between 280 and 400 nm corresponding to ZnO were observed. By scanning electron microscopy (SEM), particles of different sizes and irregular shapes were observed (Fig. 8), belonging to ZnO type I particles and the antibiotics involved, with a weight% for O of 19.53%, and Zn of 80.47%.
Preparation of the nano paste
100 mg of the modified antibiotic paste was prepared with the following proportions: Tetracycline (23%), Chloramphenicol (23%), ZnONPs (50%), and SNPs (4%). The weighed powder was diluted in 10 ml of deionized water.
Microbiological tests on ATCC strains
Five experimental groups were obtained with an initial concentration as shown in Table 1. Microdilution tests were performed to obtain the MIC and BMC of each strain used. The data obtained are summarized in Table 2.
Results showed that, due to its higher concentration (13%), the CTZ de Mexico™ paste generally displayed greater antimicrobial efficacy than the Nano paste, which was formulated at a lower concentration (10%). This trend was observed across most bacterial strains tested. For Escherichia coli and Enterococcus faecalis, CTZ de Mexico™ paste achieved bactericidal effects at 12.8 µg/ml, whereas the Nano paste required 19.5 µg/ml. Similarly, for Streptococcus mutans, the CTZ paste showed an MBC of 25 µg/ml compared to 39 µg/ml for the Nano formulation. Interestingly, Staphylococcus aureus was the exception, where the Nano paste demonstrated superior activity with an MBC of 4.8 µg/ml, outperforming the CTZ paste’s 12 µg/ml. These results suggest that while the Nano paste benefits from nanoparticle synergy, the higher concentration in the CTZ formulation remains a critical factor in achieving lower MBC values for most strains.
For the Kirby Bauer Method, it was observed that the initial concentration (C1), for Nano Paste (10%) and CTZ de México™ paste (13%), showed inhibition halos of almost 100%, so dilutions were made at 7.5% for concentration two (C2), and 5% for concentration three (C3). The results are shown in Table 3.
The Kirby Bauer sensitivity test showed that Staphylococcus aureus, Escherichia coli, and Streptococcus mutans exhibited clear zones of inhibition at all three tested concentrations for both the Nano paste and the CTZ de Mexico™ paste. In contrast, Enterococcus faecalis demonstrated sensitivity only at C1 and C2 concentrations, while at C3, the lowest concentration, both pastes yielded intermediate halos, suggesting reduced susceptibility. Figure 9 shows the comparative performance of the Nano paste against the four bacterial strains, highlighting its effectiveness despite a lower antibiotic load.
Statistical analysis
Statistical analysis was performed to determine the differences between the study groups using the R program version 4.4.125. The normality of the model residuals was analyzed using the Shapiro-Wilk test, and the Student’s t-test was applied for the variables with normality of the model residuals, while the Mann Whitney U test was applied for those that showed a distribution of the residuals different from normal. A statistically significant difference in the Kirby Bauer evaluation method was determined between the groups Nano paste and CTZ de México™ paste using the Student’s T-test, at the following concentrations; for C2 S. aureus with a (p = 0.0146), for C1 E. coli (p = 0.000262) and C2 E. coli (p = 0.000388), for C2 E. faecalis (p = 0.00033) and for C3 S. mutans (p = 0.00749). For the other concentrations tested in the different bacterial strains, there was no statistically significant difference.
Discussion
Several studies have demonstrated clinical and radiographic efficacy of CTZ paste when applied to the LSTR technique4,9,10. In the present work, an antibiotic paste was prepared, composed of tetracycline and chloramphenicol, and modified by adding zinc oxide and silver nanoparticles to equal or improve the original properties of the CTZ pastes available in the current market. The addition of ZnONPs and SNPs aims to reduce the undesirable effects, like the appearance of reinfections, internal and/or external root resorptions that may suggest the presence of proinflammatory cells in apical tissues, and to decrease the antibiotic percentage following the ideal concentration of SNPs reported by Carrouel et al. in 202017.
One of the main problems observed when reviewing the literature was the diversity of antibiotic mixtures currently used in the LSTR technique, and their application without a previous report or an in vitro study that justifies the dose used4,7,9,11,26,27. Studies report the preparation of the paste based on proportions, some even without weighing the required powder, obtaining the antibiotic from the tablet, removing the enteric coating, and adding the agents in equal parts. Even in the commercial paste, we confirmed that each capsule had a variation in its weight, suggesting that its manufacture may not be standardized. In this study, the components were accurately weighed, regulating their use in a known concentration (10%) to determine a minimum inhibitory and bactericidal concentration, information that may be of great value to create an effective dose to be tested in future studies.
Although pulp necrosis is a polymicrobial infection with broad-spectrum pathogens, we acknowledge the role of E. faecalis. A gram-positive coccus, a facultative anaerobe, the main target in endodontics, and recognized as the principal cause of recurrences and failures in pulp therapy of deciduous and permanent teeth; resistant to many antibiotics and capable of surviving in hostile environments, such as highly alkaline pH28. Therefore, we tested 4 ATCC bacterial strains, including E. faecalis, due to its implications already mentioned, and S. aureus, S. mutans, and E. Coli, recognized for their clinical pathogenicity and microbiological relevance in causing important infections in the human body.
In a recent review, Casals et al. in 2025 reported a promising synergy between SNPs and antibiotics, based on recent studies that have shown how controlled silver ion release enhances the efficacy of common antibiotics18. Following the same line, Khurana et al. since 2016 observed an antimicrobial synergy by promoting the absorption of tetracycline into the synthesized SNPs, increasing its antimicrobial power measured by inhibition zones by Kirby Bauer’s method up to 346%, they obtained a MIC of SNPs vs. S. aureus of 75 µg/ml, unlike our study, which corresponded to 1.6 µg/ml, suggesting that the controlled size of the synthesized SNPs in our study (10.08 nm) may have been the difference.
Results of the present study suggest that the reported interaction between the synthesized SNPs and the antibiotics involved truly occurred. Mainly based on the performance of the nano paste at the microbiological assessment, because even with a 4% decrease in antibiotics and final concentration of 10%, we were able to observe sensitivity halos of the tested strains and determine their MBC. The synergistic antibactericidal effect between SNPs and antibiotics, like tetracycline and chloramphenicol, results from a merging of complementary mechanisms that boost bacterial susceptibility. The mechanisms of action can be explained if we remember that SNPs disrupt the integrity of bacterial membranes, increasing permeability and facilitating the intracellular uptake of tetracycline and chloramphenicol. At the same time, they generate reactive oxygen species (ROS), which inflict oxidative damage on primordial cellular components such as DNA, proteins, and lipids, weakening the bacterial defenses. Silver ions may also inhibit bacterial efflux pumps, decrease the active expulsion of tetracycline and chloramphenicol, permitting higher intracellular concentrations of the antibiotics. Also, SNPs interfere with the formation of biofilms, causing their disruption, even if the biofilm is already established, improving tetracycline’s and chloramphenicol`s access to protected bacterial communities. The metabolic stress induced by silver exposure further debilitates bacterial function, elevating the vulnerability of cells to antibiotic response. Together, these mechanisms explain the enhanced antimicrobial performance of the SNP-tetracycline-chloramphenicol combination, particularly against resistant and biofilm-forming strains18,20. Also, by adding zinc oxide to the paste in a nano size, we gain greater control and uniformity over the morphology, size, and properties of the compound, an aspect that commercial pastes do not possess, as evidenced by the characterization made of the commercial paste. Therefore, the nano paste provides strict control over its components in terms of quantity, concentration, and morphology.
The difference in concentration between the nano paste (10%) and the commercial paste (13%) could be a limiting factor when making comparisons. However, it is important to emphasize that the manufacturer’s instructions for using the commercial paste were followed. Results obtained in this study by the microdilution method show similar MICs for the commercial paste and the nano paste, except for S. aureus (see Table 2); however, the commercial paste generally showed better bactericidal efficacy than the Nano paste, with lower MBCs. This microbiological evaluation objectively enabled the determination of MBCs that can serve as a basis for obtaining the appropriate in vitro dose for these four tested strains.
For the Kirby Bauer method, 3 concentrations were used (C1 basal, 7.5% and 5%), and both pastes showed sensitive and intermediate halos for the antibiotic components29 . Strains of S. aureus, E. coli, and S. mutans showed sensitive halos at the 3 concentrations for the two antibiotic pastes, while E. faecalis showed halos of sensitivity for C1 and C2, and for C3 showed intermediate halos for both pastes, suggesting that by the Kirby Bauer method, the behavior of both pastes was similar. Regarding statistical significance, this method showed a significant difference between the two pastes: in favor of the nano paste for C1 E. coli (p = 0.000262) and C2 E. coli (p = 0.000388), and for C3 S. mutans (p = 0.00749). And favoring CTZ de México™ paste, for C2 S. aureus (p = 0.0146) and C2 E. faecalis (p = 0.00033).
It can be concluded that it was possible to prepare an antibiotic paste composed of tetracycline and chloramphenicol, supplemented with silver and zinc oxide nanoparticles, by standardizing the process at a known concentration. By Kirby Bauer method, the behavior of both pastes was similar, meanwhile, by the microdilution method, the CTZ de Mexico™ paste (13%) generally demonstrated superior bactericidal efficacy compared to the nano formulation with a 10% concentration and 4% less antibiotic, as indicated by lower minimum bactericidal concentrations (MBCs). This microbiological evaluation established MBC values that may serve as a reference for determining appropriate in vitro dosing against the four bacterial strains tested.
Among the limitations of our study, we can mention that this is an in vitro study using ATCC strains. Following this line, our working group is currently carrying out the same methodology applied to wild E. faecalis strains, collected from necrotic canals of primary teeth, to observe if there is any difference with the ATCC strains.
Data availability
All generated or analyzed data during this study are included in this article and its supplemental information files, titled Supplement 2.
References
Achanta, A., Reche, A., Dakhale, R. & Bharate, R. R. A comprehensive review of lesion sterilization and tissue repair: an alternative for pulpectomy in deciduous teeth. Cureus https://doi.org/10.7759/cureus.48218 (2023).
Portes Zeno, A. P., Marañon-Vásquez, G. A., Guimarães Primo, L. & Pintor, B. A. V. & De Castro Costa, M. Pasta CTZ Para abordaje endodóncico de Dientes primarios: Una revisión narrativa de La literatura. Rev Odontopediatría Latinoam 12, (2022).
Sain, S. et al. Lesion sterilization and tissue repair–current concepts and practices. Int. J. Clin. Pediatr. Dent. 11, 446–450 (2018).
Garrocho-Rangel, A., Jalomo-Ávila, C., Rosales-Berber, M. Á. & Pozos-Guillén, A. Lesion sterilization tissue repair (LSTR) approach of non-vital primary molars with a chloramphenicol-tetracycline-ZOE antibiotic paste: A scoping review. J. Clin. Pediatr. Dent. 45, 369–375 (2021).
Sijini, O. T. et al. Clinical and radiographic evaluation of triple antibiotic paste pulp therapy compared to Vitapex pulpectomy in non-vital primary molars. Clin. Exp. Dent. Res. 7, 819–828 (2021).
Chouchene, F., Masmoudi, F., Baaziz, A., Maatouk, F. & Ghedira, H. Antibiotic mixtures in noninstrumental endodontic treatment of primary teeth with necrotic pulps: a systematic review. Int. J. Dent. 2021, 1–12 (2021).
Dhakshinamoorthy, S., Jayakaran, T. G. & Bommareddy, C. S. Comparison of modified triple antibiotic paste in two concentrations for lesion sterilization and tissue repair in primary molars: an in vivo interventional randomized clinical trial. Int. J. Clin. Pediatr. Dent. 14, 388–392 (2021).
Parakh, K. & Shetty, R. M. Evaluation of paste containing gentamicin, amoxicillin and metronidazole in endodontic treatment of primary molars in vivo. Chin. J. Dent. Res. 22, 57–64 (2019).
Luengo Fereira, J. et al. Efectividad Clínica y Radiográfica de La pasta Antibiótica CTZ En Pulpotomías de molares primarios: Ensayo Clínico aleatorio controlado. Int. J. Odontostomatol. 10, 425–431 (2016).
Santos, P. S. et al. Efficacy of the non-instrumentation endodontic treatment with CTZ paste in primary molars: protocol of a multicenter randomized clinical trial with two years of follow-up. Res. Soc. Dev. 11, e111111637140 (2022).
Almarji, W., Laflouf, M. & Tolibah, Y. A. Evaluation of the modified 3Mix-Simvastatin combination in non‐instrumental endodontic therapy of necrotic primary molars: A two‐arm randomized controlled trial. Clin. Exp. Dent. Res. 10, e860 (2024).
Lövestam, G. et al. Considerations on a Definition of Nanomaterial for Regulatory Purposes (Publications Office, 2010).
Song, W. & Ge, S. Application of antimicrobial nanoparticles in dentistry. Molecules 24, 1033 (2019).
Bhandi, S. et al. Antimicrobial efficacy of silver nanoparticles as root canal irrigant’s: A systematic review. J. Clin. Med. 10, 1152 (2021).
De Almeida, J., Cechella, B., Bernardi, A., De Lima Pimenta, A. & Felippe, W. Effectiveness of nanoparticles solutions and conventional endodontic irrigants against Enterococcus faecalis biofilm. Indian J. Dent. Res. 29, 347 (2018).
Raura, N., Garg, A., Arora, A. & Roma, M. Nanoparticle technology and its implications in endodontics: a review. Biomater. Res. 24, 21 (2020).
Carrouel, F., Viennot, S., Ottolenghi, L., Gaillard, C. & Bourgeois, D. Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: A review of the current situation. Nanomaterials 10, 140 (2020).
Casals, E., Gusta, M. F., Bastus, N., Rello, J. & Puntes, V. Silver nanoparticles and antibiotics: A promising synergistic approach to multidrug-resistant infections. Microorganisms 13, 952 (2025).
Filgueiras, A. L., Paschoal, D., Dos Santos, H. F. & Sant’Ana, A. C. Adsorption study of antibiotics on silver nanoparticle surfaces by surface-enhanced Raman scattering spectroscopy. Spectrochim Acta Mol. Biomol. Spectrosc. 136, 979–985 (2015).
Khurana, C., Vala, A. K., Andhariya, N., Pandey, O. P. & Chudasama, B. Influence of antibiotic adsorption on biocidal activities of silver nanoparticles. IET Nanobiotechnol. 10, 69–74 (2016).
Bruniera, J. F. B. et al. Green synthesis, characterization and antimicrobial evaluation of silver nanoparticles for an intracanal dressing. Braz Dent. J. 31, 485–492 (2020).
Martínez-Castañon, G. A., Nino, N., Martínez-Gutiérrez, F., Martinez, J. R. & Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 10, 1343–1348 (2008).
Ranoszek-Soliwoda, K. et al. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanoparticle Res. Interdiscip Forum Nanoscale Sci. Technol. 19, 273 (2017).
M100. Performance Standards for Antimicrobial Susceptibility Testing.
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2024).
Chakraborty, B., Nayak, A. & Rao, A. Efficacy of lesion sterilization and tissue repair in primary tooth with internal resorption: A case series. Contemp. Clin. Dent. 9, 361 (2018).
Sobral, A. P. T. et al. Efficacy of antibiotic and iodoform pastes in non-instrumental endodontic treatment of anterior primary teeth—Protocol for a randomized controlled clinical. PLOS ONE. 18, e0291133 (2023).
Pardi, G., Guilarte, C., Cardozo, E. I. & Briceño, E. N. Detección de Enterococcus faecalis En Dientes Con Fracaso En El Tratamiento Endodóntico. Acta Odontológica Venez. 47, 110–121 (2009).
Yelle, S. & Mascarenhas, J. Detection of ESBL Producing Gram-Negative Bacteria in Public Drinking Water Sources from South Mumbai. 288–300 (2020).
Funding
Resources and funding were provided by Universidad Autonoma de San Luis Potosí.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis, and writing of the main paper were performed by Daniela Guzmán Uribe and Gabriel Martínez Castañón. Supervision of the laboratory process was performed by Idania de Alba Montero. Funding acquisition and general supervision were performed by Facundo Ruiz and Nereyda Niño Martínez. The first draft of the manuscript was revised and accepted by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guzmán Uribe, D., De Alba Montero, I., Martínez Castañón, G.A. et al. Development of a nano antibiotic paste for lesion sterilization and tissue repair technique in pediatric dentistry. Sci Rep 15, 44880 (2025). https://doi.org/10.1038/s41598-025-28545-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28545-x