Abstract
Long-term outcomes in ischemic heart disease are historically underreported in Eastern Europe. Our aim was to report 10-year survival and to identify outcome predictors in patients with three-vessel coronary artery disease (CAD) treated by either percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) in an Eastern European center. Patients treated for three-vessel CAD with CABG or PCI with drug-eluting stents between August 2013 and February 2025 in a Romanian tertiary hospital were selected for inclusion in this study. All-cause and cardiovascular-cause survival data were available as of May 2025. A total of 7014 patients were included, of which 2759 (39.3%) were treated with CABG and 4255 (60.6%) were treated with PCI. The 10-year all-cause and cardiovascular-cause survival rates were 50.5% and 57.7%, respectively. The CABG group had both higher all-cause and cardiovascular cause mortality than the PCI group (log-rank p = 0.01 and p = 0.03, respectively). CABG was an independent protective predictor, with patients having at least one arterial graft performing better survival than patients having venous grafts only. PCI was an independent predictor of events, with patients undergoing single coronary stenting having better survival than patients undergoing multiple coronary stenting. The 10-year survival after CABG or PCI is lower in Eastern Europe than that reported by other Western registries. However, at the 10-year follow-up, CABG associated with lower incidence of all-cause and cardiovascular-cause death when compared to PCI. These results provide contemporary evidence regarding the long-term benefit of CABG in patients with complex CAD in a real-world scenario.
Similar content being viewed by others
Introduction
Coronary artery disease is the leading cause of death and premature mortality globally, including Europe1. However, long-term outcome reports of coronary artery disease are limited in Eastern Europe. Reports on morbidity and mortality in coronary artery disease from Eastern Europe usually rely on aggregated country-specific data and not individual patient-level details1,2. Moreover, studies examining the influence of comorbidities, pharmacological therapy, and revascularization strategies at the patient level on long-term outcomes in coronary artery disease are largely unavailable in Eastern Europe.
Among patients with CAD, those with three-vessel disease have the worst prognosis3. In such cases, optimal revascularization treatment includes percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG), the latter being usually preferred4. However, the non-inferiority of PCI in multivessel CAD is yet to be established. A recent meta-analysis on 10-year survival outcomes in PCI versus CABG found a relatively low number of available studies and revealed that there was no difference in all-cause death between CABG versus PCI, while cardiovascular cause death was lower in CABG patients5.
The aim of this study was to report 10-year survival in patients with three-vessel disease treated by either percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) in an Eastern European center. Differences in terms of comorbidities, clinical presentation, PCI or CABG procedural characteristics and survival rates were also investigated.
Materials and methods
Patient inclusion
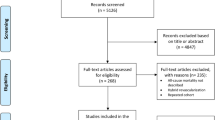
All patients who underwent CABG or PCI procedures for three-vessel disease at the Emergency Institute for Cardiovascular Diseases and Transplantation Târgu Mureș between August 2013 and February 2025 were selected for inclusion in the present study. By our institutional protocol, the Heart Team (cardiologist [clinical and interventional specialist], cardiac surgeon, and anesthetist) decides between PCI or CABG based on anatomical CAD complexity, surgical risk, and other relevant criteria (e.g., patient preference or considerations not captured by the clinical scores). Exclusion criteria consisted of (1) age less than 18 years old, (2) PCI intervention for ST-elevation myocardial infarction, (3) concomitant surgical valvular intervention (e.g., surgical aortic valve replacement), (4) patients who died in-hospital, (5) lack of long-term follow-up (e.g., patients who live abroad), (6) patients who did not have complex coronary artery disease (e.g., single-vessel or two-vessel disease) and (7) plain only balloon angioplasty (POBA) or bare metal stent (BMS) placement. An additional exclusion criterion was considered if patients had treatment crossover (e.g., CABG patients later undergoing PCI).
Research ethics
PCI patients hospitalized between January 2016 and February 2025 were prospectively included in the local PCI registry. All those patients (or their legal representatives) signed the informed consent regarding the PCI procedure and their participation in the study. The prospective PCI registry was approved by the ethical committee of our institution (decision number 8646/22 December 2015). PCI patients hospitalized between August 2013 and December 2015, and all CABG patients were retrospectively included in this registry. An additional ethical committee approval was obtained for the retrospective dataset (decision number 2877/23 May 2025), and informed consent was waived for those patients. The protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by the Declaration of Helsinki, protecting the confidentiality of the personal information of the patients.
Data collection
The PCI and CABG data were collected based on the criteria of Cardiology Audit and Registration Data Standards (CARDS) developed by the Department of Health and Children, European Society of Cardiology, Irish Cardiac Society, and the European Commission6. Briefly, the CARDS recommendations address data regarding demographics, relevant medical history and comorbid conditions, clinical status at hospital admission, PCI/CABG indication, affected and treated coronary artery segments, different invasive diagnostic and therapeutic devices, procedural complications, medical treatment during hospitalization and at discharge, and in-hospital evolution. Patients were selected when first hospitalized (e.g., in the case of staged percutaneous revascularization, only the first hospitalization was considered in order not to overestimate survival). The flowchart of patient selection is illustrated in Fig. 1. The laboratory parameters were the first values determined in the hospital and before the surgical or percutaneous intervention. Additional detailed surgical or interventional parameters not covered by the CARDS recommendation were also collected. Electronic health records provided detailed hospitalization costs.
The clinical endpoint of this study was the incidence of all-cause and cardiovascular-cause mortality. The Romanian National Health Insurance System database supplied the mortality status for all the patients as of May 2025. For patients who died during the follow-up, the Regional Statistics Office of the Romanian National Institute of Statistics supplied the exact date and cause of death according to the tenth revision of the International Classification of Diseases (ICD-10). If the cause of death belonged to diseases of the circulatory system, then death was considered to be of cardiovascular cause.
Statistical analysis
A significance level α of 0.05 and a 95% confidence interval (CI) were considered. All tests were 2-sided. Continuous variables were evaluated for normal distribution using Shapiro-Wilk test. Continuous variables with parametric distributions were reported as mean ± standard deviation and compared using non-paired Student t test, while continuous variables with non-parametric distributions and discrete variables were reported as median (interquartile range) and compared using Mann Whitney U test. Categorical variables were reported as absolute and relative frequencies and were compared using the χ2 or Fisher exact test as appropriate. Missing values were imputed using the MissForest algorithm7. The incidence of all-cause mortality was assessed using the Kaplan-Meier method and compared using the log-rank test. To examine the impact of different predictors on survival, univariable and multivariable Cox proportional hazard models were used to predict the association in the form of hazard ratio (HR) between observed survival and a single or multiple independent variables, respectively. The number of predictors in the multivariable models was chosen so that there would be at least 15 events per covariate8. Multivariable models were constructed in a stepwise fashion, and overfitting was evaluated using Akaike’s Information Criterion (AIC). AIC was calculated at each step to assess if an overfitting problem was incurred by adding more variables to the model. The multivariable model’s performance was assessed using calibration plot, Harrell’s C-index, and integrated Brier score. Statistical analysis was performed using lifelines and scikit-survival libraries in Python version 3.9.13.
Results
A total of 7014 patients were included, of which 5238 (74.7%) were males, and the mean age was 64.2 ± 9.3 years. 2759 (39.9%) were treated with CABG, and 4255 (60.6%) were treated with PCI. The complete clinical characteristics of the included population are reported in Table 1. There were important differences between PCI and CABG groups: (1) PCI patients were significantly older, more frequently females, with a higher incidence of diabetes mellitus, chronic kidney disease and chronic obstructive pulmonary disease, while (2) CABG patients had significantly higher creatinine and a higher incidence of peripheral artery disease and atrial fibrillation. Similarly, PCI patients had significantly lower left ventricular ejection fraction (LVEF) and more frequently presented with non-ST-elevation acute myocardial infarction (NSTEMI).
During a median follow-up of 5.50 (2.16–8.06) years, a total of 1642 and 1235 all-cause and cardiovascular-cause events occurred, respectively. Survival curves for PCI and CABG groups are reported in Fig. 2, with the CABG group having better survival than the PCI group in both all-cause death (log-rank p = 0.01) and cardiovascular-cause death (log-rank p = 0.03). Out-of-hospital death is reported in Supplemental Fig. 1. All-cause deaths occurred in 4.49 cases per 100 patient-years in the entire population of the study with three-vessel disease, with CABG having 4.17 deaths per 100 patient-years and PCI having 4.70 deaths per 100 patient-years. Cardiovascular cause deaths occurred in 3.38 cases per 100 patient-years in the entire population of the study with three-vessel disease, with CABG having 3.16 deaths per 100 patient-years and PCI having 3.51 deaths per 100 patient-years. In-hospital death occurred in 112 (3.9%) patients in the CABG group and 80 (1.8%) patients in the PCI group (p < 0.0001). The overall 10-year all-cause and cardiovascular-cause survival rates were 50.5% and 57.7%, respectively. The 10-year all-cause survival rate was 58.0% in the CABG group and 42.4% in the PCI group (p < 0.0001). The 10-year cardiovascular-cause survival rate was 64.5% in the CABG group and 50.2% in the PCI group (p < 0.0001). The geographical distribution of the included patients is illustrated in Fig. 3, revealing that the studied patients originated from all the main counties of Romania. Patients who died during the follow-up were significantly older and were more frequently treated by PCI, while sex did not impact survival. All major comorbidities such as diabetes mellitus, atrial fibrillation, chronic kidney disease, history of stroke or myocardial infarction, peripheral artery disease, or chronic obstructive pulmonary disease were significant predictors of impaired survival (Table 2 and Supplemental Table 1). Similarly, higher creatinine and lower left ventricular ejection fraction were significant predictors of events.
Among patients treated with CABG, operative technique significantly influenced survival (Table 3 and Supplemental Table 2). Patients who received venous-only grafts had lower survival than those with combined (venous and arterial) grafts and those with arterial-only grafts, the latter having the highest survival. Similarly, patients with at least one arterial graft had better survival, and patients with three saphenous grafts had the worst survival among patients treated with CABG. Among patients treated with PCI, survival was also significantly impacted by operative technique (Table 3). Patients with multiple stentings on multiple coronary arteries had worse survival than patients with stenting on a single coronary artery. A higher number of stents was associated with inferior outcomes in PCI patients, while stent type did not affect survival, although there was a low number of non-drug-eluting stents used.
In the multivariable Cox analysis, survival was best predicted by left ventricular ejection fraction, age, creatinine and hemoglobin (Fig. 4 and Supplemental Fig. 2). CABG remained an independent predictor of events and was superior to PCI (Fig. 4 and Supplemental Fig. 2). Additionally, technical procedural details also had predictive value – arterial grafting and single coronary angioplasty were protective of events, while exclusive venous grafting and multiple coronary angioplasties were predictive of events. Performance metrics for the multivariable model are reported in Fig. 5. Performance metrics for the out-of-hospital mortality prediction model are reported in Supplemental Fig. 3.
Multivariate Cox regression analysis. COPD – chronic obstructive pulmonary disease; DCM – dilated cardiomyopathy; DES – drug-eluting stent; DM – diabetes mellitus; HDL – high-density lipoprotein; LDL – low-density lipoprotein; LVEF – left ventricular ejection fraction; NSTEMI – non-ST-elevation myocardial infarction at presentation; PAD – peripheral artery disease.
Discussions
The findings of our study can be summarized as follows: (1) the overall 10-year all-cause and cardiovascular-cause survival rates were 50.5% and 57.7%, respectively, which is lower than that reported by other clinical studies, (2) all-cause and cardiovascular-cause survival was higher in the CABG group than in the PCI group and (3) procedural details had additional impact on survival, patients with arterial grafts having better outcomes than patients with venous grafts and patients with single coronary stenting had better outcomes than patients with multiple coronary stenting.
Our study aligns with prior evidence while highlighting important differences. The overall 10-year mortality in our three-vessel disease cohort (≈ 50–57%) is higher than rates reported in major trials like MASS II, which observed 10-year survival ~ 75% with revascularization (CABG 74.9%, PCI 75.1%)5. However, consistent with these studies, we found that CABG was associated with significantly better long-term survival than PCI. This mirrors the 10-year SYNTAX trial findings: in patients with three-vessel CAD (without left main involvement), CABG conferred higher all-cause survival than PCI9. By contrast, in isolated left main disease (usually excluded from our registry), multiple trials have shown equivalent 5–10 year survival between PCI and surgery9,10, especially when anatomic complexity is low. These observations underscore that the survival advantage of CABG emerges most clearly in extensive multivessel disease. Previous studies reported lower survival in patients with CAD from Romania11,12.
Our results confirm prior randomized data on PCI vs. CABG. Notably, the survival benefit we observed with surgery accords with the FREEDOM trial in diabetics: in that cohort, CABG halved long-term mortality compared to first-generation DES-PCI (≈ 19% vs. 24% at ~ 7.5 years)13. A recent meta-analysis of 5 RCTs and 4 observational studies reported no significant difference in 10-year all-cause mortality overall5, but it did find higher cardiac mortality and repeat revascularization with PCI5, trends consistent with our registry (more late deaths in the PCI group and likely more re-interventions, though non-fatal events were not tracked). Conversely, contemporary trials suggest the outcome gap may be narrowing. The FAME 3 trial, which compared FFR-guided PCI with second-generation DES vs. CABG in multivessel disease, initially failed to show noninferiority of PCI at 1 year, but by 5 years the rates of death, stroke, or MI were statistically non-significant between PCI and CABG14. PCI patients in FAME 3 did experience more MI and repeat revascularization, reflecting the increased incidence of late adverse events despite similar hard endpoints14. Thus, while our findings underscore CABG’s long-term advantages, they are also tempered by emerging evidence that improved PCI techniques and medical therapy can achieve comparable intermediate outcomes in selected patients. Differences in study designs and patient profiles (e.g., the EXCEL vs. NOBLE trials in left main disease) further illustrate how outcomes can vary15. Overall, our Eastern European registry adds a real-world perspective: even in an era of advanced stents and adjunctive therapies, surgical revascularization offered a durable survival benefit in patients with complex three-vessel CAD. This advantage aligns with the SYNTAX 10-year analysis and other long-term datasets, confirming that when coronary disease is diffuse or severe, CABG tends to yield better survival9.
Our study’s findings support current revascularization guidelines and provide specific insights for patient management, particularly in underrepresented populations. The 2019 European Society of Cardiology guidelines for the diagnosis and management of chronic coronary syndromes guidelines and the 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guideline for coronary artery revascularization both emphasize the preference for CABG in patients with multivessel CAD who have high-risk features4,16. For example, CABG carries a Class I recommendation for patients with diabetes and multivessel disease and for those with anatomically complex CAD (e.g. SYNTAX > 33 or concomitant left main), owing to its proven survival benefit. Our observation that CABG was an independent predictor of improved survival aligns with these recommendations, reinforcing that adhering to guideline-directed strategy can translate into tangible long-term benefits. Importantly, the heart team approach should be stressed: in our cohort, PCI was often chosen for older, comorbid patients (reflecting real-world practice), highlighting that clinical decisions are nuanced. Guidelines call for individualized decision-making and, indeed, in patients with less complex disease (SYNTAX < 22–33) or those at high surgical risk, modern PCI can be a reasonable alternative (Class IIa). Our data suggest that in Eastern European settings careful patient selection is crucial: those with extensive disease burden stand to gain a survival advantage from upfront surgery, whereas patients with milder disease or prohibitive surgical risk may do just as well with PCI. The early hazard of surgery (we noted higher perioperative mortality in CABG patients) must be weighed against its long-term payoff. It is known that a main limitation of CABG is the higher in-hospital mortality due to its invasive nature, in-hospital death diminishing the benefit of CABG versus PCI17. Similarly, in the present study, the survival benefit of CABG over PCI in patients with three-vessel disease was more pronounced when considering out-of-hospital death by excluding in-hospital death (Supplemental Material). Ultimately, our findings encourage clinicians in similar populations to follow evidence-based revascularization strategies, using CABG for appropriate candidates to improve longevity, while ensuring aggressive medical therapy and risk factor control for all. This is particularly impactful for Eastern Europe, where historically, long-term outcomes data have been scarce. Providing region-specific evidence that confirms international trials can improve confidence in guideline adoption and optimize care for complex CAD patients who might otherwise be managed conservatively or with PCI due to limited local data.
Beyond the choice of revascularization modality, our study highlights that procedural details can significantly influence outcomes. In the surgical arm, the use of arterial grafts was associated with markedly better survival than venous grafting alone. Patients who received at least one internal mammary artery (IMA) graft had superior long-term outcomes, whereas those with only saphenous vein grafts had the lowest survival. This finding is in line with established surgical practice, as arterial conduits (especially IMA to the LAD) are known to confer greater long-term patency and survival, and current guidelines uniformly recommend their use in CABG18. It is known that survival after CABG is linked to graft patency, and arterial grafts have higher long-term patency19,20. The arterial endothelium, compared to the venous endothelium, is more stable in high blood flow conditions and is protective against platelet activation21. The arterial endothelium appears to be particularly important in long-term patency of grafts with small diameters, under 5 mm, such as CABG grafts21. Likewise, in the PCI arm, we found that achieving complete revascularization with fewer stents was associated with better survival. Patients who required multiple stents across multiple arteries had inferior outcomes than those treated with a single-stent PCI, suggesting that a higher burden of residual disease or a more diffuse stenting strategy diminishes long-term efficacy. A residual pro-atherosclerotic effect of stenting per se was suggested by a study in which CABG graft patency was lower in vessels with previous PCI22. This underscores the importance of careful lesion selection and the limits of PCI in very diffuse three-vessel disease, as when the disease extent mandates many stents, the benefits of surgery in bypassing all lesions become more pronounced9. Diffuse CAD (which often necessitates multiple stents) remains a limiting factor for PCI9. These procedural insights reinforce that quality of revascularization matters: surgeons should prioritize arterial grafting and complete revascularization, while interventionalists should aim for complete anatomical revascularization with the minimum number of stents. Our data also indirectly reflect the known trade-offs in periprocedural risk: for instance, although not the focus of our study, prior analyses have shown PCI tends to carry a lower immediate risk of stroke compared to CABG, whereas CABG offers more durable freedom from angina and repeat procedures5. Improving PCI outcomes might involve advanced techniques (e.g., physiologic guidance, imaging, hybrid approaches) to mitigate the disadvantage of incomplete revascularization. Meanwhile, optimizing surgical techniques (multi-arterial grafting, etc.) can further widen the long-term benefit. Our real-world findings, therefore, provide practical confirmation that procedural details, not just the choice between PCI vs. CABG, critically influence patient prognosis over a decade of follow-up.
The strengths of this study include its large sample size and long follow-up in an understudied population. We provide one of the first 10-year, patient-level outcome reports for an Eastern European cohort with complex CAD, filling a notable gap in the literature. This all-comers analysis allows generalizability, reflecting real-world practice. However, the study has important limitations inherent to observational research. CABG patients were retrospectively included. Treatment allocation was not randomized, so despite multivariable adjustments, unmeasured confounding factors can influence the results. For example, the PCI group’s higher comorbidity burden could exaggerate the apparent benefit of CABG. Given the large number of events per covariate, standard multivariable methods such as Cox proportional hazards analysis were the preferred approach and perform well compared with propensity score matching23. Nonetheless, residual confounding cannot be excluded.
Conclusions
The 10-year survival after CABG or PCI is lower in Eastern Europe than that reported by other Western registries. However, at the 10-year follow-up, CABG was associated with a lower incidence of all-cause and cardiovascular-cause death when compared to PCI. Procedural details had an additional impact on survival, patients with arterial grafts having better outcomes than patients with venous grafts, and patients with single coronary stenting had better outcomes than patients with multiple coronary stenting. These findings offer contemporary evidence regarding the long-term benefit of CABG in patients with complex CAD in a real-world scenario.
Data availability
Due to national and EU regulations, particularly the General Data Protection Regulation (GDPR), the data used in this study cannot be made publicly available and shared with the wider research community. However, the data can be shared by the corresponding author for use in secure environments upon reasonable request.
References
Luengo-Fernandez, R. et al. Cardiovascular disease burden due to productivity losses in European society of cardiology countries. Eur. Heart J. Qual. Care Clin. Outcomes. 10, 36–44 (2024).
Luengo-Fernandez, R. et al. Economic burden of cardiovascular diseases in the European union: a population-based cost study. Eur. Heart J. 44, 4752–4767 (2023).
Ikeno, F. et al. SYNTAX score and Long-Term outcomes. JACC 69, 395–403 (2017).
Knuuti, J. et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European society of cardiology (ESC). Eur. Heart J. 41, 407–477 (2020).
Feng, S. et al. Ten-year outcomes after percutaneous coronary intervention versus coronary artery bypass grafting for multivessel or left main coronary artery disease: a systematic review and meta-analysis. J. Cardiothorac. Surg. 18, 54 (2023).
Flynn, M. R. et al. The cardiology audit and registration data standards (CARDS), European data standards for clinical cardiology practice. Eur. Heart J. 26, 308–313 (2005).
Stekhoven, D. J. & Bühlmann, P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics 28, 112–118 (2012).
Harrell, F. E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. (2015). https://doi.org/10.1007/978-3-319-19425-7
Yu, X-P., Li, Y., He, J-Q. & Jin, Z-N. Twelve-year outcomes after revascularization for ostial/shaft lesions in unprotected left main coronary artery. J. Geriatr. Cardiol. 17, 338–343 (2020).
Buszman, P. E. et al. Left main stenting in comparison with surgical revascularization. JACC Cardiovasc. Interv. 9, 318–327 (2016).
Călburean, P-A. et al. Addition of Eptifibatide and manual thrombus aspiration to Ticagrelor does not improve long-term survival after STEMI treated with primary PCI. Front. Pharmacol. https://doi.org/10.3389/fphar.2024.1415025 (2024).
Călburean, P-A. et al. High long-term mortality in ischaemic heart disease accentuated among ethnic minorities in Eastern europe: findings from a prospective all-comers percutaneous coronary intervention registry in Romania. J. Epidemiol. Community Health. 79, 272–279 (2025).
Farkouh, M. E. et al. Long-Term survival following multivessel revascularization in patients with diabetes. JACC 73, 629–638 (2019).
Fearon, W. F. et al. Outcomes after fractional flow reserve-guided percutaneous coronary intervention versus coronary artery bypass grafting (FAME 3): 5-year follow-up of a multicentre, open-label, randomised trial. Lancet https://doi.org/10.1016/S0140-6736(25)00505-7 (2025).
Shaik, T. A. et al. Comparative effectiveness of coronary artery bypass graft surgery and percutaneous coronary intervention for patients with coronary artery disease: A Meta-Analysis of randomized clinical trials. Cureus 14, e29505 (2022)
Lawton, J. S. et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: A report of the American college of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation 145, e18–e114 (2022).
Jain, S. S. et al. Impact of periprocedural adverse events after PCI and CABG on 5-Year mortality. JACC: Cardiovasc. Interventions. 16, 303–313 (2023).
Caliskan, E. et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 17, 155–169 (2020).
Fukui, T., Tabata, M., Manabe, S., Shimokawa, T. & Takanashi, S. Graft selection and One-Year patency rates in patients undergoing coronary artery bypass grafting. Ann. Thorac. Surg. 89, 1901–1905 (2010).
Gaudino, M. et al. The association between coronary graft patency and clinical status in patients with coronary artery disease. Eur. Heart J. 42, 1433–1441 (2021).
Sánchez, P. F., Brey, E. M. & Briceño, J. C. Endothelialization mechanisms in vascular grafts. J. Tissue Eng. Regen. Med. 12, 2164–2178 (2018).
Songur, M. Ç. et al. Does really previous stenting affect graft patency following CABG? A 5-year follow-up. Heart Vessels. 31, 457–464 (2016).
Biondi-Zoccai, G. et al. Are propensity scores really superior to standard multivariable analysis? Contemp. Clin. Trials. 32, 731–740 (2011).
Acknowledgements
The authors would like to thank Dr. Cristian Alexandru Udroiu for his help in acquiring long-term patient follow-up.
Funding
This work was supported by the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureș Research Grant number 792/1/22.01.2025.
Author information
Authors and Affiliations
Contributions
Conceptualization - H.S. and M.H.; methodology - P.A.C.; formal analysis - P.A.C.; investigation - H.S., M.H., A.S., A.C.S., H.A.H., D.E.A., L.H.; resources - H.S., M.H., L.H.; data curation - P.A.C.; writing—original draft preparation - H.S., M.H., P.A.C.; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Suciu, H., Harpa, M.M., Călburean, PA. et al. Ten year outcomes in three vessel disease treated by CABG versus PCI in an Eastern European registry. Sci Rep 15, 43284 (2025). https://doi.org/10.1038/s41598-025-29361-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29361-z