Abstract
The cost-effectiveness of nivolumab plus bevacizumab and chemotherapy for patients with advanced non-squamous non-small-cell lung-cancer (NSCLC) was uncleared yet. The current analysis aimed to evaluate the cost-effectiveness of nivolumab plus bevacizumab and chemotherapy compared with bevacizumab plus chemotherapy for patients with untreated non-squamous NSCLC in Chinese context. A partitioned survival model that simulated 3-week patients transition in 20-year time horizon was conducted to evaluate the economic value. The clinical data were obtained from TASUKI-52 trial, cost and utility values were gathered from the local charges and previously published studies. Sensitivity analyses were conducted to examine the robustness of the model results when parameters changed, subgroup analyses were also conducted to enhance the comprehensiveness of the analysis. Nivolumab plus bevacizumab and chemotherapy yielded an additional 0.90 QALYs with the marginal cost of $231,948.33, resulting in the incremental cost-effectiveness ratio (ICER) of $256,791.53 per additional quality-adjusted life-years (QALYs) gained, which higher than the Chinese willingness-to-pay (WTP) threshold of $39,057/QALY. Sensitivity analyses confirmed the robustness of the model outcomes. Subgroup analyses revealed that nivolumab plus bevacizumab and chemotherapy was unlikely to be the cost-effective option for all subgroups due to the unfavorable ICERs. Nivolumab plus bevacizumab and chemotherapy was unlikely to be the cost-effective first-line therapy for untreated advanced non-squamous NSCLC patients compared with bevacizumab plus chemotherapy from the perspective of Chinese health-care system.
Similar content being viewed by others
Introduction
Lung cancer is one of the leading causes of the global disease burden of non-communicable disease, and has the high incidence and mortality of all malignancy worldwide1. In China, lung cancer caused nearly 22% of cancer-related deaths of all kind of cancers2,3. Approximately 85% of lung cancer were non-small-cell lung cancer (NSCLC) based on the pathology, unfortunately, 61% of NSCLC patients had progressed to an advanced stage at the time of diagnosis due to the asymptomatic nature of early disease4,5. NSCLC could be further divided into squamous NSCLC and non-squamous NSCLC. Platinum-based chemotherapy with bevacizumab was the standard first-line treatment for patients with advanced non-squamous NSCLC without driver mutations, however, the clinical demand was unmet yet and the new anti-cancer treatment needs to be innovated.
Anti-programmed cell death 1 (PD-1) antibodies such as nivolumab demonstrated the potential to improve survival benefit by inhibiting the PD-1 and programmed cell death 1 ligand 1 (PD-L1) pathway that mediated negative regulation, thereby promoting immune-mediated elimination of tumor cells6,7. The phase Ⅲ TASUKI-52 trial, demonstrated nivolumab plus bevacizumab and chemotherapy could significantly reduce the risk of disease progression or death by 41% (hazard ratio (HR), 0.59, 95% confidence interval: 0.47–0.73, P < 0.0001) and the risk of death by 29% (HR, 0.71, 95% confidence interval: 0.57–0.88, P = 0.0015) compared with bevacizumab plus chemotherapy8,9. However, the high price could bring significant marginal costs with the widely used of nivolumab, and it was necessarily to pay attention to the cost-effective profile of high-value anti-cancer drugs in order to clear its economic benefits especially in resource-limited countries such as China10. The results of cost-effectiveness analysis have become the important evidence for whether to include drugs in the reimbursement list. The current analysis aimed to evaluate the cost-effectiveness of adding nivolumab to first-line bevacizumab plus chemotherapy for patients with advanced non-squamous NSCLC from the Chinese health-care system perspective. To the best of our knowledge, the current analysis was the first study to evaluate the cost-effectiveness of nivolumab plus bevacizumab and chemotherapy for advanced non-squamous non-small cell lung cancer patients.
Methods
Analytical overview and model structure
A partitioned survival model (PSM) was conducted to evaluate the clinical and economic value of adding nivolumab to first-line bevacizumab plus chemotherapy for patients with advanced non-squamous NSCLC from the Chinese health-care system. The PSM included three mutually exclusive health states among: PFS, progressed disease (PD), and Death (Fig. 1). The model was constantly evolving, all patients were at PFS state when entered the model, it was assumed that patients could not return to the previous health state, and Death state was the absorbing state. The PSM simulated the patients transition track every 3 weeks in 20-year time horizon, in each cycle, patients would keep in the current state or progressed to the other state.
Following model outputs were estimated among total costs, life-years (LYs), and quality-adjusted life-years (QALYs). Cost and utility values were calculated across the annual discount rate of 5% according to the Chinese guidelines for pharmacoeconomic evaluations11. All costs were shown in 2023 US dollars ($1 = 7.047 CNY). Three times of per capita gross domestic product (GDP) of China in 2023 was set to be the willingness-to-pay (WTP) threshold ($39,057/QALY) to judge the cost-effective by comparing to the incremental cost-effectiveness ratio (ICER) of the two competing strategies12,13, ICER could be calculated by the formula: \(({C}_{1}-{C}_{2})/({E}_{1}-{E}_{2})\). All methods were carried out in accordance with relevant guidelines and regulations. The experimental protocols of the TASUKI-52 trial were approved by the licensing committee, and the informed consent of the cohort patients have been obtained in the TASUKI-52 trial. We equally accessed the potential economic benefits in terms of the cost-effective of the two competing treatments in subgroup patients to achieve the precise decision making, the subgroup PFS rates in nivolumab plus bevacizumab and chemotherapy group were calculated by multiplying the PFS rates of intention-to-treat (ITT) patients in bevacizumab plus chemotherapy group and the reported subgroup-specific HR.
Clinical data
The model clinical and safety data were obtained from the TASUKI-52 trial, first, we gathered the time and survival rate from Kaplan–Meier (K-M) curves in TASUKI-52 trial by using the GetData Graph Digitizer software (version 2.26), individual time-to-event data were reconstructed by the algorithm developed by Guyot et al.14. Then the individual time-to-event data were used to fit the following parametric survival model included Exponential, Gamma, generalized gamma, Weibull, Log-normal, Log-logistic, Gompertz, and Royston/Parmar spline model15, the mathematical methods were conducted in the R software (version 4.5.0) by “flexsurv” package. The goodness between fitting model and the reported Kaplan–Meier (K-M) curves in TASUKI-52 trial was based on the Akaike information criterion (AIC), Bayesian information criterion (BIC) and visual inspection, the best fitting parametric survival model was used to extrapolate long-term survival data. AIC and BIC values of the fitting model were shown in Supplementary Table 1, the comparison of original K-M curves and selected fitting model of the two therapies were performed in Supplementary Fig. 1, the survival parameter of the adopt fitting model were shown in Table 1. After the disease progressed, no re-challenge was happened in two groups, patients would receive subsequence anti-cancer treatment or supportive care, the proportion of patients received subsequence anti-cancer treatments of the two competing regimens were obtained from the TASUKI-52 trial.
Cost and utility values
The current analysis was conducted from the perspective of Chinese health-care system, only direct medical costs were included in the model, included the cost of anti-cancer treatments, routine follow-up, best supportive care, end-of-life care, and management of treatment-related serious adverse events (SAEs) which grade ≥ 3. Drug administration schedules in the PSM were consistent with the TASUKI-52 trial, body surface area (BSA) of 1.72m2 (a typical patient with weight of 65 kg and height of 1.64 m) was used to calculate the dosage of anti-cancer drugs16, the cost inputs were gathered from the local bid-winning price and published literatures, all costs values were selected from similar studies of the same disease or adverse events, and sensitivity analyses was conducted based on their possible ranges.
Utility values on the scale of 0 to 1 which influenced by race and religion was assigned to the PFS, PD, and Death state as 0.804, 0.321, and 0, respectively, the utility values were gathered from Chinese population data from an international study. The disutility values due to the SAEs were also considered in the model, costs and utility inputs were shown in Table 1.
Sensitivity analyses
Sensitivity analyses among one-way and probabilistic sensitivity analyses (PSA) were typically conducted to evaluate the robustness of the model outcomes when the model parameters were changed. In one-way sensitivity analyses, parameters were changed one-by-one according to the lower and upper boundaries, record changes of the ICER to judge which parameter was the most influence factor of the model. The range of each parameter were obtained from the 95% confidence intervals or assuming ± 25% of the base-case value when the 95% confidence intervals was not available (Table 1), the results of one-way sensitivity analyses were shown in Tornado diagram. In PSA, 1,000 iterations of Monte Carlo simulation were conducted by repeatedly sampling the model parameters based on the statistical distribution, Gamma distribution was used for cost values, Beta distribution was used for proportions, probability values, rate values, utility and disutility values23, the results of PSA were shown in the cost-effectiveness acceptability curves (CEACs).
Results
Base-case results
From the Chinese health-care system perspective, in 20-year time horizon, adding nivolumab to first-line bevacizumab plus chemotherapy could bring additional 1.28 LYs and 0.90 QALYs with the incremental cost of $231,948.33, resulting in an ICER of $256,791.53 per QALY gained for nivolumab plus bevacizumab and chemotherapy versus bevacizumab plus chemotherapy, which higher than the Chinese cost-effectiveness WTP threshold of $39,057/QALY, the base-case results were shown in Table 2. Subgroup analyses showed that the ICERs of nivolumab plus bevacizumab and chemotherapy versus bevacizumab plus chemotherapy were higher than WTP threshold in all subgroup populations, the results of subgroup analyses were shown in Supplementary Table 2.
Sensitivity analyses
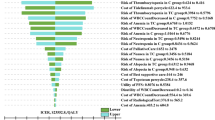
One-way sensitivity analyses revealed that the utility of PFS and the cost of nivolumab had the substantial impact of the model results, other model inputs such as utility of PD, discount rate, cost of bevacizumab, and cost of subsequent anti-cancer therapy had medium or minimal impact of the model outcomes, however, none of the parameters changed could lead the ICER nearly or lower than the Chinese WTP threshold with $39,057 per additional QALY gained (Fig. 2), and the reported absolute ICER values of the key parameter were showed in the Supplementary Table 3.
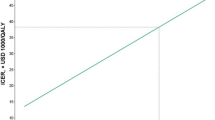
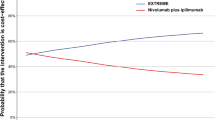
In PSA, the CEACs showed the probability of nivolumab plus bevacizumab and chemotherapy could be considered cost-effective was 0% compared with bevacizumab plus chemotherapy at the WTP threshold of $39,057/QALY in China. And subgroup analyses showed that the cost-effective probability of nivolumab plus bevacizumab and chemotherapy was 0% for all subgroup patients (Supplementary Table 2). The PSA showed the mean ICER was $328,223.20/QALY, and 95% confidence interval was $306,460.14/QALY to $349,986.25/QALY. When the price of nivolumab reduced 30%, 50%, 70%, and 90%, respectively, the probability of nivolumab plus bevacizumab and chemotherapy could be considered cost-effective was 0%, 0%, 0%, and 27%, respectively (Fig. 3).
Discussion
Health-care decision makers and oncologists were interested by the TASUKI-52 trial reported clinical benefits of adding nivolumab to first-line bevacizumab plus chemotherapy for patients with untreated advanced non-squamous NSCLC. However, the marginal healthcare cost and disease economic burden would dramatically increase with the widespread use of the immunotherapy due to its high price, and we need pay more attention to “economic toxicity” of the high-value innovative drugs for the resource-limited countries such as China. Cost-effectiveness analysis was the important method to solve the issue. And cost-effectiveness analysis results constitute essential evidence for the Chinese government’s decisions whether regarding innovative drug inclusion in the national reimbursement drug list. In current analysis, we conducted the PSM to evaluate the economic benefit of adding nivolumab to first-line bevacizumab plus chemotherapy for patients with untreated advanced non-squamous NSCLC from the Chinese health-care system perspective, to the best of our knowledge, this is the first study to evaluate the cost-effectiveness of nivolumab plus bevacizumab and chemotherapy as first-line treatment for patients with untreated advanced non-squamous NSCLC in Chinese context based on the TASUKI-52 trial. Base-case analysis suggested that adding nivolumab to first-line bevacizumab plus chemotherapy could bring additional 1.28LYs and 0.90QALYs with the incremental costs of $231,948.33, resulting in an ICER of $256,791.53/QALY, which far above the WTP threshold in China of $39,057/QALY. One-way sensitivity analyses showed the utility value of PFS and the cost of nivolumab was the main driver of the ICER, PSA demonstrated the model results were robustness, the cost-effective probability of nivolumab plus bevacizumab and chemotherapy was 0% at the WTP threshold of $39,057 per additional QALY gained. Subgroup analyses revealed that nivolumab plus bevacizumab and chemotherapy could not be considered the cost-effectiveness option due to the unfavorable ICERs for all subgroup patients. The model results suggested that nivolumab was not appropriate to enter the drug reimbursement list and in clinical practice at current price in the context of healthcare payment reform in China. Based on the innovative drug medical insurance admission policy implemented by the Chinese government, we also explored the effect of price reducing on the model outcomes, based on the national price negotiation experience and reasonable hypothetical, we have set four price gradients, when the price of nivolumab decreased by 30%, 50%, 70%, and 90%, respectively, the cost-effective probability of nivolumab plus bevacizumab and chemotherapy was 0%, 0%, 0%, and 27%, respectively at the WTP threshold of $39,057/QALY.
There was no relevant cost-effectiveness analysis based on the TASUKI-52 trial. Several studies evaluated the economic values of adding immunotherapy to first-line chemotherapy24,25,26,27,28,29,30, the immunotherapy included domestic immune checkpoint inhibitors such as camrelizumab, sintilimab, toripalimab, tislelizumab, and sugemalimab. The seven analyses were conducted under the consistent methodological framework, analyses revealed that adding camrelizumab, sintilimab, toripalimab, and tislelizumab to first-line chemotherapy could be considered the cost-effective strategy for patients with previously untreated non-squamous NSCLC, the unfavorable ICER of sugemalimab plus chemotherapy versus chemotherapy alone due to the high cost made the combination therapy was unlikely to be the cost-effective treatment. The price of the above drugs has significantly decreased compared with the initial stage of market launch except sugemalimab, the influence of the price level factors of sugemalimab and nivolumab to the model results displayed consistent and comparable with the expected model results of our analysis, it demonstrated the external stability of our model results.
Several limitations must be considered when the study results were used for health-care decision making. First, survival data beyond follow-up period of TASUKI-52 trial was extrapolated by fitting parametric survival model not the long-term follow-up data due to the time constraints and data accuracy, it was an inevitable limitation of the analysis that may cause the bias from the model outcomes to real-world evidences. Second, cost inputs among cost of routine follow-up, supportive care, terminal care, subsequent anti-cancer therapy, and management of SAEs in both nivolumab plus bevacizumab and chemotherapy and bevacizumab plus chemotherapy group were obtained from the published literatures rather than real-world data, however, one-way sensitivity analyses demonstrated the above model parameters only had minimal impact of the model results. Third, because of the absence of head-to-head clinical trial, our model was not included other potential competing first-line treatments such as camrelizumab plus chemotherapy, tislelizumab plus chemotherapy, and sintilimab plus chemotherapy, however, chosen bevacizumab plus chemotherapy as the control group for economic evaluation was the reasonable practice, as we could use the efficacy data demonstrated by the randomized controlled trial, which served as the “gold standard” for effective evaluation, to conduct the cost-effectiveness analysis. Fourth, SAEs related disutility values were gathered from the analysis about breast cancer, which different from the focus disease in our analysis, however, it only has minimal influence of the model results. Despite these limitations, we are confident that the study accurately reflected the clinical conditions and potential economic value of advanced non-squamous NSCLC in China because the study was based on the standard methodology about cost-effectiveness analysis of anti-cancer drug, and the model results were valuable for Chinese health-care decision makers. And we suggested that the future studies which compared the cost-effectiveness across these treatments should under the consistent methodology framework, which could provide the reimbursement policy for Chinese health-care decision makers.
Conclusion
In conclusion, nivolumab plus bevacizumab and chemotherapy was unlikely to be the cost-effective first-line treatment compared with bevacizumab and chemotherapy for patients with untreated advanced non-squamous NSCLC from the Chinese health care perspective due to the unfavorable ICER, and it was not appropriate to enter the drug reimbursement list at current price level in China.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. And the Tasuki 52 trial dataset was available at https://www.lungcancerjournal.info/article/S0169-5002(25)00030-3/fulltext.
References
GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392 (10159): 1859–1922.
Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 4, 1553–1568 (2018).
Zheng, R. S. et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi 41, 19–28 (2019).
Feng, R. M. et al. Current cancer situation in China: good or bad news from the 2018 global cancer statistics?. Cancer Commun. 39, 22–33 (2019).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 69, 363–385 (2019).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359(6382), 1350–1355 (2018).
Luke, J. J. & Ott, P. A. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget 6, 3479–3492 (2015).
Sugawara, S. et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 32(9), 1137–1147 (2021).
Lee, K. H. et al. First-line nivolumab plus platinum chemotherapy and bevacizumab for advanced nonsquamous non-small cell lung cancer: A 3-year follow-up of the phase 3 randomized TASUKI-52 trial. Lung Cancer 201, 108109 (2025).
Saltz, L. B. Perspectives on cost and value in cancer care. JAMA Oncol. 2(1), 19–21 (2016).
Liu, G. et al. China Guidelines for Pharmacoeconomic Evaluations (China Market Press, 2020).
Thokala, P. et al. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics 36(5), 509–522 (2018).
Tzanetakos, C. & Gourzoulidis, G. Does a standard cost-effectiveness threshold exist? The case of Greece. Value Health Reg Issues. 36, 18–26 (2023).
Guyot, P. et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 12, 9 (2012).
Jackson, C. H. flexsurv: A platform for parametric survival modeling in R. J Stat Softw. 70, i08 (2016).
Gu, X. et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer 127, 84–89 (2019).
Luo, X. et al. Cost-effectiveness of bevacizumab biosimilar LY01008 combine with chemotherapy as first-line treatment for Chinese patients with advanced or recurrent nonsquamous non-small cell lung cancer. Front Pharmacol. 13, 832215 (2022).
Qiao, L. et al. Cost-effectiveness of domestic PD-1 inhibitor camrelizumab combined with chemotherapy in the first-line treatment of advanced nonsquamous non-small-cell lung cancer in China. Front Pharmacol. 12, 728440 (2021).
Rui, M. & Li, H. Cost-effectiveness of osimertinib vs docetaxel-bevacizumab in third-line treatment in EGFR T790M resistance mutation advanced non-small cell lung cancer in China. Clin Ther. 42(11), 2159–2170 (2020).
Wu, B. & Lu, S. The effect of PD-L1 categories-directed pembrolizumab plus chemotherapy for newly diagnosed metastatic non-small-cell lung cancer: A cost-effectiveness analysis. Transl Lung Cancer Res. 9, 1770–1784 (2020).
Nafees, B. et al. Health state utilities in non-small cell lung cancer: An international study. Asia Pac J Clin Oncol. 13, e195–e203 (2017).
Wu, B. & Ma, F. Cost-effectiveness of adding atezolizumab to first-line chemotherapy in patients with advanced triple-negative breast cancer. Ther Adv Med Oncol. 12, 1758835920916000 (2020).
Briggs, A. H. et al. Model parameter estimation and uncertainty analysis: A report of the ISPOR-SMDM Modeling good research practices task force working group-6. Med Decis Making. 32, 722–732 (2012).
Zhu, C. et al. Cost-effectiveness analysis of camrelizumab plus chemotherapy vs. chemotherapy alone as the first-line treatment in patients with IIIB-IV non-squamous non-small cell lung cancer (NSCLC) without EGFR and ALK alteration from a perspective of health-care system in China. Front Pharmacol. 12, 735536 (2021).
Shi, Y. et al. Comparing the cost-effectiveness of sintilimab + pemetrexed plus platinum and pemetrexed plus platinum alone as a first-line therapy for Chinese patients with nonsquamous non-small cell lung cancer. Transl Cancer Res. 12(4), 928–938 (2023).
Liu, H., Wang, Y. & He, Q. Cost-effectiveness analysis of sintilimab plus pemetrexed and platinum versus chemotherapy alone as first-line treatment in metastatic non-squamous non-small cell lung cancer in China. Health Econ Rev. 12(1), 66 (2022).
Zheng, Z. et al. A cost-effectiveness analysis of first-line toripalimab plus chemotherapy in advanced nonsquamous non-small cell lung cancer in China. Expert Rev Clin Pharmacol. 16(3), 267–273 (2023).
Luo, X. et al. The cost-effectiveness of tislelizumab plus chemotherapy for locally advanced or metastatic nonsquamous non-small cell lung cancer. Front Pharmacol. 13, 935581 (2022).
Wang, L. et al. Economics of first-line treatment with tislelizumab in patients with nonsquamous non-small cell lung cancer. Immunotherapy 16(20–22), 1217–1226 (2024).
Zheng, Z. et al. Cost-effectiveness analysis of sugemalimab vs. chemotherapy as first-line treatment of metastatic nonsquamous non-small cell lung cancer. Front Pharmacol. 13, 996914 (2022).
Acknowledgements
Not Applicable.
Funding
This paper was not funded.
Author information
Authors and Affiliations
Contributions
SZ, and SK were involved in the design of the study, SZ and SK were collected the data and performed the economic analysis. SZ and SK drafted and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The experimental protocols of the TASUKI-52 trial were approved by the TASUKI-52 trial institutional review board.
Consent for publication
Not Applicable.
Accordance statement
All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, S., Kang, S. Cost effectiveness of first line nivolumab plus bevacizumab and chemotherapy for advanced non squamous non small cell lung cancer. Sci Rep 16, 288 (2026). https://doi.org/10.1038/s41598-025-29534-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29534-w