Abstract
Heavy metals like lead, cadmium, and mercury can accumulate in meat and organ tissues, posing significant food safety and health risks. In the current study, 315 frozen imported bovine samples (105 each of muscle, liver, and kidney) collected from local markets in Sohag governorate, Egypt, were analyzed by atomic absorption spectrophotometry for the determination of mercury (Hg), lead (Pb), and cadmium (Cd) concentrations. The metals determined were investigated for their potential public health hazards in humans. The mean ± SE concentrations of Hg, Pb, and Cd in beef were 0.312 ± 0.058, 0.684 ± 0.105, and 0.030 ± 0.005, respectively, while the corresponding values in liver samples were 0.273 ± 0.054, 0.763 ± 0.106, and 0.056 ± 0.007, respectively, and in kidney samples were 0.167 ± 0.04, 0.716 ± 0.119, and 0.073 ± 0.014, respectively. Beef samples contained the highest Hg level, while liver samples contained the highest level of Pb, and kidney samples contained the highest level of Cd. More than half of the samples tested exceeded the permissible limits set by International and local food agencies for Hg and Pb; exactly, 62.9, 60, and 54.3% of muscle, liver, and kidney samples, respectively, exceeded the maximum limits of Pb, while 54.3 and 57.1% of muscle and liver samples exceeded the limit of Hg. In contrast, none of the tested liver or kidney samples exceeded the limit of Cd, although only 22.9% of the beef samples exceeded their maximal limit. Although the human health risks estimated in the current study for consuming the contaminated samples were low, high proportions of samples exceeded the permissible limit, and therefore, continuous monitoring of heavy metal residues in food, especially of animal origin, is of great significance.
Similar content being viewed by others
Introduction
Beef is considered one of the most important sources of protein, besides the essential nutrients, including fats, vitamins, and minerals1. In addition, beef has a high proportion of unsaturated fatty acids in addition to being a source of conjugated linoleic acid, which acts on human health as an anti-inflammatory, antithrombotic, and atherosclerotic preventive2. Therefore, the consumption of beef has increased largely worldwide, and it is becoming a preference among consumers, particularly in the Middle East, due to its unique nutritional values with low content of intramuscular fat, saturated fatty acids, and cholesterol when compared to other red meats3,4.
Due to rising demand for beef as a source of animal protein, along with the population increasing to 108 million by 2025 and limited domestic production, Egypt imports frozen beef from Brazil, India, and the USA to meet this need.
Bovine samples, including beef, liver, and kidney, are easily contaminated by different sources of hazards. Heavy metals can contaminate animal tissues through many entry routes, such as grazing behavior in cattle in contaminated soils, which leads to the accumulation of heavy metals in their body via ingestion5. Also, animal feed and water are contaminated by the surrounding environment and constitute an additional source of contamination6. Contamination with heavy metals is a serious threat because of their toxicity, bioaccumulation, and biomagnification in the food chain7. These pollutants often have direct physiological toxic effects because they are stored or incorporated in tissues, sometimes permanently8. Heavy metals can interfere with the functions of enzymes and are responsible for many diseases, especially cardiovascular, renal, and even bone disorders. Some metals are known to be carcinogenic, mutagenic, and teratogenic in experimental animals9. Lead is recognized as a neurotoxic agent, which can impair cognitive performance in children and contribute to cardiovascular diseases, increasing blood pressure in adults10. Cadmium is associated with various carcinogenic conditions11. Mercury exists in nature in different forms, including metallic and inorganic mercury. Acute exposure to elemental mercury vapor can lead to fatal pneumonitis12.
The consumption of toxic metal-contaminated meat over an extended period may cause toxicity; therefore, the main objective of this work is to evaluate mercury, lead, and cadmium concentrations in imported frozen bovine meat, liver, and kidney distributed in Sohag local market, Egypt, as well as to assess the possible hazards of consuming such bovine tissues on the health of the Egyptian general population through calculating the estimated daily intake (EDI), Target Hazard Quotients (THQ), Hazard Index (HI), and Cancer risk (CR).
Materials and methods
Sample collection
A total of 315 imported frozen bovine samples comprising meat, liver, and kidney samples (105 each) were randomly collected from local markets in Sohag City, Egypt. Each sample was separately packed in a clean polyethylene bag, labeled with the sample tissue type and number, and kept frozen until transferred to the Laboratory of Food Hygiene, Safety, and Technology, College of Veterinary Medicine, Mansoura University, for digestion and analysis for heavy metal determination.
Preparation and digestion of samples
Nitric acid (HNO3, ACS reagent ≥ 90.0%; Molecular Weight: 63.01) and Perchloric acid (HClO4, ACS reagent 70%; Molecular Weight: 100.46) were obtained from Merck (Merck KGaA, Darmstadt, Germany). For sample digestion and analysis, 2 g of each sample were taken and dissected using a stainless steel scalpel and forceps, then macerated into small pieces and homogenized in a 20 mL screw-capped tube, containing a mixture of 12 mL concentrated nitric-perchloric acid (2:1), and placed overnight in a water bath adjusted at 55 °C for complete digestion of the samples. The digested samples were cooled to room temperature, diluted with deionized water, and then filtered through Whatman Filter Paper Grade 42 (Merck KGaA, Darmstadt, Germany) into a clean glass beaker. Subsequently, the filtrate was made up to 50 mL with deionized water and analyzed for the selected heavy metals (mercury, lead, and cadmium). Blank solutions of the reagents were digested by the same technique, but without samples to define the background correction of the reagents. The study design chart is shown in Fig. 1.
Heavy metals analysis
All aliquots of the samples sent to be analyzed at the Central Laboratory, Faculty of Veterinary Medicine, Zagazig University, Egypt, for the metals; Hg, Pb, and Cd using “Buck Scientific USA 210 VGP Atomic Absorption Spectrophotometer” provided with an oxidizing air acetylene flame for analysis of Pb and Cd, while mercury hydride system (MHS) “cold vapor technique” was used for determination of Hg. Spectral lamps with wavelengths of 253.65 nm, 283.31 nm, and 228.80 nm were adopted for mercury, lead, and cadmium, respectively. The concentration of metals (mg/kg wet weight) was calculated using the following equation:
where R is the reading of the element concentration (mg/kg) from the digital scale of AAS, D is the final volume of the prepared sample (mL), and W is the wet weight of the sample (g).
Validation of analytical methods
Method validation has been proven as previously described by Sabala et al.13. To create the standard solutions used for the calibration curves, 1000 mg/L of each metal was diluted with acidified ultrapure water (5% v/v HNO3) in accordance with the suggested AOAC Official Method 2015.01. Standard solutions of 0.0, 0.01, 0.05, 0.1, 0.5, 2, and 5 µg/L were used for Hg; 0.0, 0.01, 0.02, 0.1, 0.5, 5, and 10 µg/L were used for Pb; 0.0, 0.005, 0.01, 0.05, 0.25, 2.5, and 5 µg/L were used for Cd. The correlation coefficient (R2) calculated for all calibration curves of the metals analyzed was 0.999.
In order to ensure the accuracy of the results, the analytical method was validated by measuring the linearity of the calibration curve, the detection limit, the quantification limit, the recovery percentage of the certified reference material, and the spiking recoveries of all heavy metal analytes. For the instrumental precision, LOD and LOQ were determined based on the calibration curve as follows:
Where - F: Factor of 3.3 for LOD and 10 for LOQ; SD: Standard deviation of the blank; b: Slope of the regression line. The mean of spike recovery (%) is calculated by dividing the mean of the recovered amount by the mean of the spiked amount. To ensure the accuracy of the results, five consecutive measurements were made for the metals studied in the certified reference material (dogfish liver DOLT-4).
In order to estimate the precision of the digestion method, known concentrations of the metals under analysis were added to the fish tissues. The digestion and analysis were then carried out using the same analytical technique used to measure the metals in the fish samples. Recovery rates of 96.4%, 98.9%, and 95.9%, based on the concentrations of the spiked amount, were calculated for Hg, Pb, and Cd, respectively.
Health risk assessment associated with the consumption of contaminated beef and liver samples
Estimated daily intake
The estimated daily intake (EDI) of heavy metals (µg per day per person) was calculated by multiplying the mean concentration of the metals detected in beef or liver bovine tissue samples by the average daily consumable rates of such samples. The calculation of the EDI of tested heavy metals was done using the following formula established by AOAC14.
MC is the metal concentration (µg/g) in the examined bovine tissues (meat and liver) on a wet weight basis. IR is the average daily ingestion rate of imported bovine meat (7.1 g/day or 0.0071 kg/day) and liver (2.39 g/day or 0.00239 kg/day) for a 70 kg adult Egyptian consumer, calculated according to USDA (2022)15 and USDA (2020)16, respectively.
Target hazard quotient (THQ)
THQ is applied to assess the probable non-carcinogenic health hazards related to heavy metal exposure via adult consumption of imported frozen beef, liver, and kidney samples over a lifetime. The hazard quotient is calculated using the equation established by Chien et al. (2002)17.
The THQ stands for Target Hazard Quotient. EDI refers to the estimated daily intake of tested metals, measured in µg/kg bw/day. The reference doses (RfDs) established for non-carcinogenic effects were 1.0 µg/kg/day for Hg (USEPA, 2019)18, 0.1 µg/kg bw/day for Cd (USEPA, 2019)18, and 0.16 µg/kg bw/day for Pb (FDA, 2018)19.
The Total Target Hazard Quotients (TTHQs) were calculated by summing the THQs according to USEPA (2011)20 for each metal as follows:
Cancer risk (CR) assessment
The CR can be evaluated to assess the creation of cancer over a lifespan from the consumption of metal-contaminated food according to the formula set by USEPA (2011)20.
Where CR refers to the cancer risk, EDI (mg/kg/day) is the estimated daily ingestion of each metal. The cancer slope factor (CSF) was 0.0085 and 0.38 mg/kg/day for lead and cadmium, respectively, while the CSF is not available for Hg20.
Statistical analysis
The data obtained were subjected to the one-way analysis of variance to determine the differences in the heavy metal levels among the various tissue samples (beef, liver, and kidney) tested. Differences among the means of heavy metals were detected using Tukey’s Honestly Significant Difference (HSD) test (P < 0.05). The data were analyzed using GraphPad PRISM® 9.1.2. (Graph Pad Software Incorporated, San Diego, USA).
Results and discussion
Heavy metal concentrations (mg/kg wet weight) in various tissues of imported frozen cattle samples
The mean concentrations of Hg in the examined bovine tissue samples ranged from 0.013 to 1.228 mg/kg in the meat, 0.001 to 1.190 mg/kg in the liver, and 0.001 to 0.790 mg/kg in the kidney, with mean levels of 0.312 ± 0.058, 0.273 ± 0.054, and 0.167 ± 0.04 mg/kg, respectively (Table 1). Beef samples had the highest mean concentrations of Hg compared to liver and kidney samples, with significant differences between the Hg in kidney and each of liver (P-value < 0.05) and muscle (P-value < 0.01) (Table 1). The Hg levels in the imported frozen bovine samples tested in this study are about 2.5 times higher than those reported in a previous study in Iraq, where the Hg concentration in imported frozen boneless beef of Brazilian origin tested in Iraq was 0.122 ± 0.006 mg/kg21; However much lower Hg levels of 0.003, 0.002, and 0.003 mg/kg were reported in bovine muscle, liver, and kidney, respectively, from Iran22. Additionally, a lower Hg level of 0.11 ± 0.02 mg/kg was detected in imported frozen bovine liver in Egypt23. Conversely, high Hg levels of 1.15 ± 0.27 and 1.18 ± 0.32 mg/kg were detected in bovine liver and kidney tested in Sharkia governorate, Egypt24.
The levels of Pb in the current study varied from 0.006 to 1.841 mg/kg in liver samples, 0.020 to 1.999 mg/kg in meat samples, and 0.003 to 2.050 mg/kg in kidney samples, with mean values of 0.763 ± 0.106, 0.684 ± 0.105, and 0.716 ± 0.119 mg/kg, respectively (Table 1). There was no significance among the mean levels of Pb in all the tested samples. However, high levels were detected in the liver and kidney samples. Such results are in accordance with the fact that heavy metals are accumulated more gradually in edible organs such as the liver and kidneys that have detoxification functions25.
The mean ± SE values of Pb determined in bovine tissue samples in this study were nearly similar to the contents of 0.69 ± 0.05 mg/kg in imported frozen bovine liver from Egypt23 and 0.672 ± 0.473 mg/kg detected in bovine liver collected from Slovakia26. Conversely, lower Pb levels of 0.221, 0.273, and 0.244 mg/kg were detected in bovine muscle, liver, and kidney, respectively, from Iran22. Furthermore, other lower Pb concentrations of 0.19 ± 0.02, 0.38 ± 0.08, and 0.32 ± 0.04 mg/kg were recorded in muscle, liver, and kidney from cattle carcasses in Egypt24. In contrast, higher Pb levels of 1.05 ± 0.12, 2.4 ± 0.19, and 1.33 ± 0.14 mg/kg were found in muscle, liver, and kidney from cattle carcasses in Egypt27.
Cadmium levels in the current study were ranged found from 0.010 to 0.390 mg/kg in liver samples, 0.007 to 0.100 mg/kg in meat samples, and 0.011 to 0.211 mg/kg in kidney samples, with a higher mean concentration of 0.073 ± 0.014 mg/kg in the Kidney than in Liver (0.056 ± 0.007 mg/kg) and meat samples (0.030 ± 0.005 mg/kg), as noticed in Table 1. This finding claimed that the kidney is considered a vital organ that plays a role in metabolic processes and mineral storage, making it one of the most expressive indicators of any biological issue, and normally contains the highest levels of toxicants because they are responsible for excretion22.
The mean values of Cd detected in bovine tissue samples were comparable with those recorded in Egypt by Morshdy et al. (2018)24 at 0.03 ± 0.02, 0.06 ± 0.01 mg/kg in beef muscle and kidney samples, and by El-Ghareb et al. (2025)23 at 0.03 ± 0.001 mg/kg in imported frozen bovine liver. Similar to the Cd levels detected in the current study, Cd levels of 0.04 ± 0.01 mg/kg were reported in liver samples of female cattle carcasses from Nigeria31, as well as at concentrations of 0.05 ± 0.01, 0.17 ± 0.01 mg/kg, in liver and kidney samples from Bulgaria, respectively32. Nonetheless, lower Cd levels of 0.50 ± 0.00 µg/kg were reported in bovine liver samples collected from Nigeria33, as well as at a concentration of 0.023 ± 0.025 mg/kg in cattle meat samples collected from Bangladesh34. In contrast, a high Cd level of 4.31 mg/kg was found in beef samples collected from Iran35. Furthermore, high Cd levels of 0.079 ± 0.01, 0.11 ± 0.01, and 0.16 ± 0.01 mg/kg were detected in muscle, liver, and kidney from cattle carcasses in Egypt27.
The variations in heavy metal levels in this study and other studies worldwide are attributable to various factors, including feed sources, environmental contamination, animal age, and meat origin36. In addition, the different degrees of freezing, food packaging, and poor storage conditions available for the bovine sample storage can affect the levels of heavy metal residues37.
The summary of the analysis of variance (ANOVA) and Tukey HSD Test data for the heavy metals determined in the tissue analyzed is shown as supplementary data in Table S1.
Heavy metals analyzed in bovine tissue samples tested in comparison to the legal limits
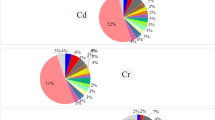
Our results indicated that 54.3% (57/105), 57.1% (60/105), and 40% (42/105) of the examined bovine meat, liver, and kidney samples, respectively, had Hg concentrations (Fig. 2) above the allowable limits set by the USDA (2023)28. Similarly, 75.3, 100, and 92.7% of bovine muscle, liver, and kidney samples tested previously in Egypt exceeded the allowable limits24. However, the result of the current study was in contrast with those reported by Hashemi in Iran22 and by Unguryanu et al. in Russia, who found that the Hg levels in all bovine muscle, liver, and kidney samples did not exceed the maximum recommended limits38.
Lead values in 62.9% (66/105), 60% (63/105), and 54.3% (57/105) of the examined bovine meat, liver, and kidney samples, respectively (Fig. 2), exceeded the MPL set by EC (2015)29. Conversely, 75%, 15.3%, and 13.9% of muscle, liver, and kidney collected from cattle carcasses, respectively, had Pb levels above the MPLs22. On the other hand, 100% of bovine tissue samples collected from Russia contained Pb levels above the maximum legal limits38. Furthermore, 81.3%, 88.7%, and 71.3% of meat, liver, and kidney samples collected from buffalo carcasses in Egypt, respectively, had Pb levels above the MPLs27.
In this study, 22.9% of the examined samples had Cd levels higher than MPLs, set by EC (2014)30, and recovered only from the beef samples, while the liver and kidney samples had Cd residue levels within the permissible limits (Fig. 2). Nearly similar results were recovered in bovines slaughtered in Iran, where the Cd concentrations in all liver and kidney samples did not exceed the maximum recommended limits, while 8.3% of bovine muscle samples were above the MPLs in Iran22. However, in another study, the Cd levels in all samples examined in Ethiopia, including beef muscle, were within permissible levels39. In contrast, Cd contents in 68% of bovine muscle samples collected from Egypt were above the maximum legal limits27.
Health risks assessment associated with the intake of the tested tissues of imported frozen beef and liver
The process of identifying potential health effects of a specific toxicant on individuals through one or more exposure routes is called risk assessment. Concerns are rising regarding public health risks from heavy metal contamination in food. Heavy metals have been shown to impact cellular organelles and components, including the cell membrane, nuclei, lysosomes, enzymes, and mitochondria. Metal ions can bind to DNA and nuclear proteins, causing DNA damage that may lead to apoptosis or cancer40. The health risks associated with consuming bovine tissues, as examined in this study, were evaluated using the EDI, THQ, and TTHQ. The hazard health risk assessment was not conducted on kidney tissues because fewer samples exceeded the maximum permissible metal levels compared to meat and liver. Additionally, the per capita consumption of kidney is significantly lower than that of beef and liver.
Comparison of EDI of heavy metals detected in the examined beef and liver with their PTDI/BMDL for non-carcinogenic risk assessment
The calculated EDIs of Hg, Pb, and Cd from the consumption of examined bovine samples were lower than the PTDI/BMDL by 13.75%, 11.01%, and 0.366% in beef and 4.05%, 4.135%, and 0.3% in liver, respectively (Table 2). These findings suggested the absence of non-carcinogenic health risks from the consumption of imported frozen bovine meat and liver for Egyptian consumers. The results of the current study were in accordance with previous studies that estimated the EDI values for the Levels of Hg, Pb, and Cd contaminating meat and offal in Russia38, Egypt41, Nigeria42, and Bangladesh43. The current results suggest that the potential cancer risk from the consumption of such contaminated imported frozen food is very low or negligible. However, the periodic studies on the heavy metal residue levels in food are of great importance.
THQ and TTHQ of metals detected in the examined imported frozen beef and liver samples for non-carcinogenic risks assessment
THQ and TTHQ were applied to measure the health risks associated with beef and liver consumption. Consuming imported frozen beef contaminated with heavy metals would not have any negative non-carcinogenic health effects if the THQ or TTHQ is estimated to be less than 1; if the THQ or TTHQ is greater than 1, there would be a greater likelihood of non-carcinogenic chronic public health effects47. Although the THQ estimation methodology does not yield a quantitative estimate of the likelihood that an exposed population will have a reverse health consequence, it does provide an indicator of the risk level associated with pollutant exposure48.
The THQ and TTHQ values of metals tested in imported frozen beef and liver estimated in this study are shown in Table 3. The THQ index for Pb was 0.433 and 0.1625 in beef and liver, respectively. The THQ values for Cd in both beef and liver samples were 0.00304 and 0.00248, respectively. The THQ values for Hg were 0.316 and 0.0928 in beef and liver, respectively. In the current study, the total hazard (TTHQ) of Hg, Pb, and Cd was 0.752 from the consumption of contaminated beef, while the TTHQ value for Hg, Pb, and Cd collectively was 0.257 in liver (Table 3). The calculated THQ and TTHQ values for Pb and Cd were less than 1 in all examined beef and liver samples, reflecting that there were no potential risks to the Egyptian community associated with the intake of imported frozen bovine meat and liver contaminated with such metals. The THQ findings in this study are similar to the results of previous studies, where the THQ values of Pb and Cd were less than 1 from the consumption of beef in Iran49 and the analyzed bovine liver in Egypt41. Similarly, THQ values for Hg, Pb, and Cd were < 1.0 from the consumption of buffalo meat and liver in Egypt27. Contrastingly, previous studies revealed higher THQ values than the standard limits for Pb and Cd in beef samples examined in Uganda50,51,52. Furthermore, higher TTHQ values were recorded in beef liver samples collected from Bangladesh43 and Nigeria53, indicating the serious health effects associated with the consumption of such an organ.
Several studies stated that health risk assessments linked with toxic metals based on overall metal values in raw foods did not seem to be reasonable and resulted in overestimating the risk evaluation, since different preparation methods, which were not considered, such as soaking, washing, and heating foods before consumption, may decrease the heavy metal levels13,54.
Cancer risks (CR) for assessment of carcinogenic risk from consumption of imported frozen beef and liver
The CR describes the potential cancer risks posed to an individual after ingesting the contaminated meat over a lifetime. Generally, the CR values above E-4 are regarded as unacceptable, below E-6 are negligible, and between E-6 and E-4 are acceptable. In the current study, the CR values of Cd and Pb detected in imported frozen bovine meat and liver were less than E-04 (Table 4). This suggested the lack of carcinogenic risks for Egyptian consumers from the consumption of such tested imported frozen bovine tissues; therefore, there are no reasons to be concerned about the continuous intake of such tested bovine tissues. these results are in accordance with those detected in Russia, where CR of Pb and Cd were less than E-04, indicating no risk associated with the consumption of meat and offal of cow38. Similar results were estimated in previous studies in Iran49 and in Bangladesh43, where the CR determined for Pb was within the acceptable value due to the consumption of cow meat and liver.
Additionally, in Egypt, the CR values for both Pb and Cd were also found to be within acceptable limits due to the consumption of buffalo meat and liver24 and imported frozen bovine liver23. In contrast, the CR of Cd was higher than the acceptable limits due to the intake of meat and liver of the cow49.
Although the present health risk assessment was based on raw tissue concentrations, it is important to consider that food processing and cooking (e.g., hamburger production, boiling, frying) may alter heavy metal levels, potentially reducing the actual exposure of consumers. Future studies should investigate the influence of these processing steps on heavy metal residues in beef-based products.
Conclusion
The current study focuses on the toxic heavy metal contents in imported frozen beef, liver, and kidney samples. The analysis illustrated that all beef and offal samples were contaminated with high levels of mercury, lead, and cadmium, with more than half of the samples recording levels that exceeded the MPLs for Hg and Pb in beef. The estimation of the human health risk was detected. Despite low calculated health risk indices (THQ, CR < 1), the frequent exceedance of legal limits (MPLs) indicates potential long-term hazards and the need for stronger monitoring of imports, including regulatory actions by local and international authorities, along with consumer awareness and veterinary interventions, should be taken to ensure the toxic metal concentrations in imported frozen bovine samples do not exceed the recommended limits.
Data availability
The data sets generated in this study are included within the article and its supplementary information files. Any additional information is available from the corresponding author upon request.
References
Ahmad, R. S., Imran, A. & Hussain, M. B. Nutritional composition of meat. Meat Sci. Nutr. 61 (10.5772), 61–75. https://doi.org/10.5772/intechopen.77045 (2018).
Sokoła-Wysoczańska, E. et al. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—a review. Nutrients 10 (10), 1561. https://doi.org/10.3390/nu10101561 (2018).
Pighin, D. et al. A contribution of beef to human health: A review of the role of the animal production systems. TSWJ (1), 8681491. https://doi.org/10.1155/2016/8681491(2016) (2016).
Troy, D. J., Ojha, K. S., Kerry, J. P. & Tiwari, B. K. Sustainable and consumer-friendly emerging technologies for application within the meat industry: an overview. Meat Sci. 120:2–9. https://doi.org/10.1016/j.meatsci.2016.04.002(2016).
Wilkinson, J. M., Hill, J. & Phillips, C. J. C. The accumulation of potentially toxic metals by grazing ruminants. Proc. Nutri Soc. 62 (2), 267–277. https://doi.org/10.1079/PNS2003209 (2003).
Kan, C. A. & Meijer, G. A. L. The risk of contamination of food with toxic substances present in animal feed. Anim. Feed Sci. Technol. 133 (1–2), 84–108. https://doi.org/10.1016/j.anifeedsci.2006.08.005 (2007).
Zwolak, A., Sarzy´nska, M., Szpyrka, E. & Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: a review. Water Air Soil. Pollut. 230 (7), 164. https://doi.org/10.1007/s11270-019-4221-y (2019).
Abdel-Salam, N. M. et al. Distribution of heavy metals in the liver, kidney, heart, pancreas and meat of cow, buffalo, goat, sheep and chicken from Kohat market Pakistan. Life Sci. J. 10 (7s), 937–940 (2013).
Abou-Arab, A. A. K. Heavy metal contents in Egyptian meat and the role of detergent washing on their levels. FCT 39 (6), 593–599. https://doi.org/10.1016/S0278-6915(00)00176-9 (2001).
Yakupa, N. Y., Sabowa, A. B., Saleha, S. J. & Mohammed, G. R. Assessment of heavy metal in imported red meat available in the markets of Erbil City. JUBPAS 26 (6), 177–183 (2018). https://www.journalofbabylon.com/index.php/JUBPAS/article/view/1469
Rakib, M. R. J. et al. Levels and health risk assessment of heavy metals in dried fish consumed in Bangladesh. Sci. Rep. 11 (1), 14642 (2021). https://www.nature.com/articles/s41598-021-93989-w
Bernhoft, R. A. Mercury toxicity and treatment: a review of the literature. J. Environ. Public Health. 460508. (2012). (1) https://doi.org/10.1155/2012/460508 (2012).
Sabala, R. F. et al. I. Potential cancer risks associated with the consumption of raw, salted, and canned sardine contaminated by mercury, arsenic, lead, and cadmium in Egypt. J. Food Compos. Anal. 134, 106516. https://doi.org/10.1016/j.jfca.2024.106516 (2024).
AOAC International. AOAC official method (2015.01) Heavy metals in food. J AOAC Int. 96:704. (2012). https://doi.org/10.5740/jaoac.int.2012.007 (2013).
USDA. United States Department of Agriculture-Foreign Agriculture Service. Livestock and Products Annual. Report Number: EG2022-0032. Cairo, Egypt. (2022). https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Livestock%20and%20Products%20Annual_Cairo_Egypt_EG2022-0032
USDA. United States Department of Agriculture-Foreign Agriculture Service. Food and Agricultural Import Regulations and Standards. Report Number: EG2020-0063, FAIRS Export Certificate Report, Cairo, Egypt. (2020). https://agriexchange.apeda.gov.in/ImportRegulations/FoodandAgriculturalImportRegulationsandStandardsExportCertificateReportCairoEgypt12312020.pdf
Chien, L. C. et al. Daily intake of TBT, Cu, Zn, cd and as for fishermen in Taiwan. Sci. Total Environ. 285 (1–3), 177–185. https://doi.org/10.1016/s0048-9697(01)00916-0 (2002).
USEPA. United States Environmental Protection Agency Regional Screening Level (RSL) Summary Table (TR = 1E-06, HQ = 1). (2019). Available from: https://semspub.epa.gov/work/HQ/199626.pdf (2019).
FDA. Food and Agriculture Organization of the United Nations. Lead in Food, Foodwares, dietary supplements | FDA. Retrieved August 27, (2019).
USEPA. United States Environmental Protection Agency. USEPA Regional Screening Level (RSL) summary table: November (2011). Available from: http://www.epa.gov/regshwmd/risk/human/Index.htm (2011).
Sabow, A. B., Qadir, S. W., Majed, Z. J. & Mahmwd, A. A. Chemical compositions and heavy metal contents of local fresh and imported frozen beef cattle meat available in Ranya markets. AJDFR 39 (4). https://doi.org/10.18805/ajdfr.DR-194 (2020).
Hashemi, M. Heavy metal concentrations in bovine tissues (muscle, liver and kidney) and their relationship with heavy metal contents in consumed feed. Ecotoxicol. Environ. Saf. 154, 263–267. https://doi.org/10.1016/j.ecoenv.2018.02.058 (2018).
El-Ghareb, H. M., Sayed-Ahmed, M. Z., Abd-Elghany, S. M., Zakaria, A. I. & Sallam, K. I. Health risk assessment of toxic elements in imported beef livers in egypt: influence of cooking. J. Food Compos. Anal. 148, 108131. https://doi.org/10.1016/j.jfca.2025.108131 (2025).
Morshdy, A. E. M. A., Bayomi, E., Galil, R. M. A. E., Mahmoud, A. F. & G. M. & Heavy metal concentrations and their risk assessment in marketed slaughtered animals in Sharkia Governorate. Egypt. Slov. Vet. Res. 55 (20), 103–112. https://doi.org/10.26873/SVR-635-2018 (2018).
López-Alonso, M. et al. Toxic and essential metals in liver, kidney and muscle of pigs at slaughter in Galicia, north-west Spain. Food Addit. Contam. 24 (9), 943–954. https://doi.org/10.1080/02652030701216719 (2007).
Skalicka, M., Korenekova, B. & Nad, P. Concentrations of selected trace elements in organs and tissues of livestock from a polluted area. J. Environ. Sci. Health Part. A. 47 (9), 1207–1211. https://doi.org/10.1080/10934529.2012.672064 (2012).
Samy, R. A., Zaher, H. A. & Sallam, K. I. Health risk assessment of heavy metals in Buffalo carcasses marketed in Mansoura. Egypt. J. Food Compos. Anal. 146, 107965. https://doi.org/10.1016/j.jfca.2025.107965 (2025).
USDA. United States Department of Agriculture, Foreign Agricultural Service. China Releases the Standard for Maximum Levels of Contaminants in Foods. Report Number: CH2023-0040. November (2025). Available at: https://www.fas.usda.gov/data/china-china-releases-standard-maximum-levels-contaminants-foods-0. Accessed 1 (2023).
EC. European Commission. Commission Regulation (EU). / 1005 of 25 June 2015. Amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs. Off. J. Eur. Union L. 161, 9–13. (2015). http://data.europa.eu/eli/reg/2015/1005/oj. Accessed on 1 November 2025.
EC. European Commission. Commission Regulation (EU) No 488/ 2014 of 12 May 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off. J. Eur. Union L. 138, 75–79. November (2025). http://data.europa.eu/eli/reg/2014/488/oj. Accessed on 1.
Milam, C., Dimas, B. J., Jang, A. L. & Eneche, J. E. Determination of some heavy metals in vital organs of cows and bulls at Jimeta Abattoir, Yola, Adamawa state. Nigeria Am. Chem. Sci. J. 8 (4), 1–7. https://doi.org/10.9734/ACSJ/2015/17012 (2015).
Jukna, C., Jukna, V. & Siugzdaite, J. Determination of heavy metals in viscera and muscles of cattle. Bulg. J. Vet. Med. 9 (1), 35–41 (2006).
Oloruntoba, A. & Nathaniel, I. A. Assessment of heavy metal levels in offal meats (kidney and liver) of beef sold at Gwagwalada market, Abuja, Nigeria. Asian J. Phys. Chem. Sci. 7 (2), 1–8. https://doi.org/10.9734/ajopacs/2019/v7i230090 (2019).
Islam, M. S., Ahmed, M. K., Habibullah-Al-Mamun, M. & Raknuzzaman, M. The concentration, source and potential human health risk of heavy metals in the commonly consumed foods in Bangladesh. Ecotoxicol. Environ. Saf. 122, 462–469. https://doi.org/10.1016/j.ecoenv.2015.09.022 (2015).
Raeeszadeh, M., Gravandi, H. & Akbari, A. Determination of some heavy metals concentration in species animal meat (sheep, beef, turkey, and ostrich) and carcinogenic health risk assessment in Kurdistan province, Western Iran. Environ. Sci. Pollut Res. 29 (41), 62248–62258. https://doi.org/10.1007/s11356-022-19589-x (2021).
Kim, I. et al. Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of daphnia magna. Chemosphere 143, 99–105. https://doi.org/10.1016/j.chemosphere.2015.06.046 (2016).
Raikwar, M. K., Kumar, P., Singh, M. & Singh, A. Toxic effect of heavy metals in livestock health. Vet. World. 1 (1), 28. https://doi.org/10.5455/vetworld.2008.28-30 (2008).
Unguryanu, T. N., Lyzhina, A. V., Mitrokhin, O. V. & Polibin, R. V. Human health risk assessment of heavy metals from meat and offal of reindeer and cow in the Far North of European Russia. Scand. J. Public. Health. 51 (7), 1009–1015. https://doi.org/10.1177/14034948221096243 (2023).
Akele, M. L. et al. Heavy metal contents in bovine tissues (kidney, liver and muscle) from central gonder zone. Ethiopia Heliyon. 8 (12). https://doi.org/10.1016/j.heliyon.2022.e12416 (2022).
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K. & Sutton, D. J. Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology. Environ. Toxicol. 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6 (2012).
Mohamed, N. H. et al. Detection and health risk assessment of toxic heavy metals in chilled and frozen meat collected from Sharkia Province in Egypt. Open. Vet. J. 13 (12), 1729. https://doi.org/10.5455/OVJ.2023.v13.i12.21 (2023).
Ogbomida, E. T. et al. Accumulation patterns and risk assessment of metals and metalloid in muscle and offal of free-range chickens, cattle and goat in Benin City, Nigeria. Ecotoxicol. Environ. Saf. 151, 98–108. https://doi.org/10.1016/j.ecoenv.2017.12.069 (2018).
Chowdhury, A. I. et al. Human health risk assessment of heavy metals in vegetables of Bangladesh. Sci. Rep. 14 (1), 15616. https://doi.org/10.1038/s41598-024-65734-6 (2024).
the Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: seventy-second report of. WHO TRS. 959, 21–27 (2010). https://iris.who.int/bitstream/handle/10665/44514/WHOTRS959eng.pdf;jsessionid=575576494C3DF2FB099AE92911A72A91?sequence=.
EFSA. Panel on contaminants in the food chain (CONTAM); scientific opinion on lead in food (European food safety Authority). EFSA J. 8 (4), 1570. https://doi.org/10.2903/j.efsa.2010.1570 (2010).
JECFA & Joint FAO/WHO Expert Committee on Food Additives. Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: seventy-third report of the WHO TRS 960, 149–177. https://iris.who.int/bitstream/handle/10665/44515/WHO_TRS_960_eng.pdf?sequence=%201&isAllowed=y (2010).
Wang, X., Sato, T., Xing, B. & Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 350 (1–3), 28–37. https://doi.org/10.1016/j.scitotenv.2004.09.044 (2005).
Ihedioha, J. N. & Okoye, C. O. B. Dietary intake and health risk assessment of lead and cadmium via consumption of cow meat for an urban population in Enugu state. Nigeria Ecotoxicol. Environ. Saf. 93, 101–106. https://doi.org/10.1016/j.ecoenv.2013.04.010 (2013).
Zeinali, T., Salmani, F. & Naseri, K. Dietary intake of cadmium, chromium, copper, nickel, and lead through the consumption of meat, liver, and kidney and assessment of human health risk in Birjand, Southeast of Iran. Biol. Trace Elem. Res. 191, 338–347. https://doi.org/10.1007/s12011-019-1637-6 (2019).
Bamuwamye, M., Ogwok, P. & Tumuhairwe, V. Cancer and non-cancer risks associated with heavy metal exposures from street foods: evaluation of roasted meats in an urban setting. JEPHH 3 (2), 24–30. https://doi.org/10.12691/jephh-3-2-1 (2015).
Kasozi, K. I. et al. Descriptive analysis of heavy metals content of beef from Eastern Uganda and their safety for public consumption. Front. Nutr. 8, 592340. https://doi.org/10.3389/fnut.2021.592340 (2021).
Ogwok, P., Bamuwamye, M., Apili, G. & Musalima, J. H. Health risk posed by lead, copper and iron via consumption of organ meats in Kampala City (Uganda). JEPHH 2 (3), 69–73. https://doi.org/10.12691/jephh-2-3-3 (2014).
Emurotu, J. E. et al. Carcinogenic and non-carcinogenic health risk assessment of heavy metals in the offal of animals from felele Abattoir, Lokoja, Nigeria. Toxicol. Rep. 13, 101701. https://doi.org/10.1016/j.toxrep.2024.101701 (2024).
Sharafi, K. et al. Bioaccessibility analysis of toxic metals in consumed rice through an in vitro human digestion model–Comparison of calculated human health risk from raw, cooked and digested rice. Food Chem. 299, 125126. https://doi.org/10.1016/j.foodchem.2019.125126 (2019).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohammed Ashry Rabeey Formal analysis and Methodology. Rana Fahmi Sabala Supervision, Formal analysis, and Writing – review & editing. Amira Ibrahim Zakaria Conceptualization, Supervision, and Writing–original draft. Khalid Ibrahim Sallam Conceptualization, Supervision, and Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rabeey, M.A., Sabala, R.F., Zakaria, A.I. et al. Health risk assessment of heavy metals in imported frozen bovine meat and organs marketed in Sohag, Egypt. Sci Rep 15, 43828 (2025). https://doi.org/10.1038/s41598-025-29927-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29927-x