Abstract
The terminology and diagnostic criteria for fatty liver disease have undergone recent evolution, from non-alcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated fatty liver disease (MAFLD), and more recently to metabolic dysfunction-associated steatotic liver disease (MASLD). We aimed to compare the estimated prevalence of FLD with that of NAFLD, MAFLD, and MASLD using different definitions. Data were analyzed from 10,520 participants in the first phase of the Guilan Persian Cohort Study, part of the nationwide PERSIAN Cohort. Participants were classified based on NAFLD, MAFLD, and MASLD. Of 10,520 participants, 5,025 (47.8%) had a fatty liver index (FLI) ≥ 60 and were diagnosed with SLD. The prevalence of MASLD, MAFLD, and NAFLD was 33.7%, 47.6%, and 33.7%, respectively, while 4.5% (n = 471) were classified as metabolic dysfunction-associated alcohol-related liver disease (MetALD). Among those with NAFLD, 99.7% also met MAFLD criteria, and all met MASLD criteria. MAFLD was associated with the highest adjusted odds of elevated FIB-4 (OR: 1.27; 95% CI: 1.24–1.30) and hepatic steatosis index (OR: 2.01; 95% CI: 1.82–2.21). NAFLD and MASLD had a similar, lower prevalence compared to MAFLD. MetALD was not included under MASLD but was encompassed by MAFLD.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD), characterized by excessive fat accumulation in hepatocytes in the absence of significant alcohol intake, is one of the most common liver disorders worldwide, with a global prevalence estimated at 38% among adults. Globally, the prevalence of NAFLD has increased by more than 50% over the last three decades, from 25.3% in 1990–2006 to 38.0% in 2016–20191.
A major limitation of the NAFLD definition is its exclusionary nature, which requires the absence of significant alcohol consumption or other chronic liver diseases. This has created diagnostic uncertainty and sometimes stigmatizing terminology. To address these issues, in 2020 experts proposed the term metabolic dysfunction-associated fatty liver disease (MAFLD)2. Unlike NAFLD, MAFLD emphasizes inclusion and diagnosis based on the presence of metabolic risk factors such as obesity, type 2 diabetes mellitus (T2DM), and dyslipidemia in concordance with hepatic steatosis2.
However, in MAFLD, the term ‘fatty’ and the overlap with other liver diseases such as viral hepatitis led to further concerns. In 2023, a multisociety Delphi consensus introduced the term metabolic dysfunction-associated steatotic liver disease (MASLD)3. This nomenclature removes the word ‘fatty,’ better reflects the pathophysiology, and introduces MetALD as a subgroup for individuals with both metabolic dysfunction and alcohol intake at potentially hepatotoxic levels3. These terminologies affect epidemiology, patient selection, and public health policies. Previous studies in Western and Asian populations have shown that MAFLD criteria can capture a broader range of at-risk individuals compared with NAFLD or MASLD4,5.
In Iran, rapid lifestyle transitions have contributed to rising the prevalence of obesity and metabolic disorders, major risk factors for liver disease. A recent national meta-analysis estimated the prevalence of NAFLD at approximately 33%6. The PolyIranLiver study reported a MAFLD prevalence of 39.8% among adults over 50, with notable variation across sex and ethnic groups7. To our knowledge, no study has yet comprehensively compared NAFLD, MAFLD, and MASLD in an Iranian population. Given Iran’s cultural and legal restrictions on alcohol, evaluating these diagnostic transitions offers a unique opportunity to assess their implications for public health. Our study used baseline data from the Guilan PERSIAN Cohort, including more than 10,500 adults, to compare the prevalence and overlap of NAFLD, MAFLD, and MASLD8. The findings may guide future diagnostic strategies and public health decisions in Iran and similar settings.
Results
Baseline characteristics and prevalence of NAFLD, MAFLD, and MASLD
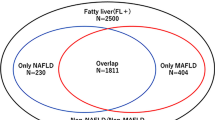
Of the 10,520 participants in the first phase of the PGCS survey, 5,025 (47.8%) had FLI ≥ 60, indicating steatotic liver disease (SLD). The mean age of SLD patients was 51.1 ± 9.1 years, and 65.7% (n = 3,299) were female. The prevalence was 33.7% for NAFLD, 47.6% (for MAFLD, 33.7% for MASLD, and 4.5% for MetALD (Table 1). Among lean participants, the prevalence was 1.8% for NAFLD, 2.5% for MAFLD, 1.8% for MASLD, and 0.7% for MetALD. Twenty-seven patients had hepatitis B (HBV) and 19 had hepatitis C (HCV) infection; of these, 10 patients with HBV and 6 patients with HCV had SLD. As illustrated in the Venn diagram, MAFLD identified more patients than NAFLD, MASLD, or MetALD. The discrepancy between NAFLD and MASLD was minimal, with most patients in both groups also meeting MAFLD criteria. MetALD was entirely encompassed within the MAFLD definition (Fig. 1A).
Overlap of NAFLD, MAFLD, and MASLD
Among the 5,025 individuals with SLD (FLI ≥ 60), the overlap between the definitions is detailed in Supplementary Table 1. The vast majority (70.3%, n = 3,535) simultaneously met the criteria for all three definitions (NAFLD+/MAFLD+/MASLD+). A substantial proportion (29.4%, n = 1,475) were identified only by the MAFLD criteria (NAFLD-/MAFLD+/MASLD-). A very small group (0.2%, n = 9) were positive for both NAFLD and MASLD but negative for MAFLD. Overlap analysis confirmed that all patients with NAFLD (100%) also met the MASLD criteria, and nearly all (99.7%) met the MAFLD criteria (Fig. 1B).
In general, MASLD patients were older, more often male, and had higher BMI, waist circumference (WC), fasting blood glucose (FBG), triglycerides (TG), low-density lipoprotein cholesterol (LDL-c), liver enzymes (ALT, AST, GGT), compared with non-MASLD or non-MAFLD groups. These patients also had a higher prevalence of type 2 diabetes, hypertension, and metabolic syndrome (Table 1). More details regarding the overlaps of NAFLD, MAFLD, and MASLD in present population were shown in Supplementary Table 1.
Associations of NAFLD, MAFLD, and MASLD with FIB-4 and HSI indices
The age distribution of SLD was similar across definitions, with peak prevalence in the 40–49-year age group (Fig. 2). The prevalence of elevated noninvasive indices — including FIB-4, HSI, K-NAFLD, and the AST/ALT ratio — showed consistent trends, with the highest values in MAFLD patients, followed by MASLD and NAFLD (Fig. 3). Logistic regression analysis indicated that the odds of an elevated FIB-4 score were significantly higher in NAFLD, MAFLD, and MASLD groups compared with controls, and these associations remained robust after full adjustment. As shown in Table 2, the strongest adjusted odds ratio (OR) for FIB-4 was observed in the MAFLD group compared with non-MAFLD participants (OR 2.01, 95% CI: 1.82–2.21). NAFLD and MASLD showed statistically significant association with FIB-4 scores (OR 1.45, 95% CI: 1.25–1.68).
Similarly, the MAFLD group had a significantly increased risk for HSI (OR 1.55, 95% CI: 1.52–1.57) compared with non-MAFLD.
Across all models, MAFLD consistently demonstrated the strongest association with both HSI and FIB-4 scores. Even after adjusting for age, gender, smoking status, physical activity, and BMI, MAFLD remained significantly associated with advanced fibrosis (OR 2.01, 95% CI: 1.82–2.21) and HSI (OR 1.27, 95% CI: 1.24–1.30). NAFLD and MASLD showed highly overlapping odds ratios, underscoring their redundancy in risk stratification. These findings suggest that MAFLD may provide a more clinically meaningful framework for identifying high-risk patients.
Discussion
In this large-scale cohort, we observed that nearly half (47.8%) met criteria for SLD based on FLI ≥ 60. The prevalence varied considerably depending on the diagnostic framework, with MAFLD identifying the largest group (47.6%), followed by MASLD and NAFLD, both at 33.7%. Even among lean individuals, prevalence remained low but non-negligible. These findings collectively underscore the utility of MAFLD as a more inclusive and metabolically attuned classification. Specifically, MAFLD’s robust association with hepatic steatosis and metabolic risk factors highlights its ability to identify patients at greater risk for liver-related complications.
In our cohort, the prevalence of MASLD was identical to that of NAFLD, with both conditions diagnosed in 70.5% of individuals exhibiting SLD. In contrast, MAFLD encompassed 99.7% of all SLD cases. This significant difference highlights the widespread presence of metabolic dysfunction among individuals with hepatic steatosis in our population. Importantly, metabolic disturbances remained a key contributor to fat accumulation, even in those with coexisting viral hepatitis or alcohol-related liver conditions. A retrospective cohort in China reported a prevalence of 36.3% for NAFLD, 53.0% for MAFLD, and 36.9% for MASLD5. Similarly, a liver MRI–screened population at Severance Hospital exhibited a slightly higher MAFLD rate9. A meta-analysis confirmed that MAFLD is more prevalent and associated with more severe metabolic dysfunction than NAFLD10. In line, NHANES 2017–2020 data reflect a higher MAFLD prevalence of 41.1% compared to 38.0% for NAFLD and 37.9% for MASLD4. The Global Burden of MAFLD study (1990–2021) ranked Iran among the top countries in the MENA region for MAFLD prevalence, reinforcing our results11. By focusing on an inclusion-based framework for metabolic criteria, MAFLD facilitates a more comprehensive and clinically meaningful identification of at-risk individuals.
Our findings indicate that the metabolism-focused definition fully encompasses the traditional NAFLD group while sharpening emphasis on cardiometabolic risk. This pattern is echoed in large international studies. In a Japanese cohort, 96.7% of patients originally diagnosed with NAFLD could be reclassified as MASLD, and the concordance remained at 96.2% when local waist-circumference thresholds were applied12. The U.S. TARGET-NASH registry found roughly 99% overlap among patients with NAFLD, NASH, and NASH-related cirrhosis under MASLD criteria13. Similarly, a Mexican population survey reported that 97.6% of individuals with NAFLD also met MASLD criteria14. Taken together, these observations indicate that MASLD does not identify a novel disease entity but rather reclassifies existing cases by explicitly incorporating metabolic dysfunction, thereby enhancing clinical relevance and risk stratification. A separate retrospective study conducted in Turkey with 678 patients, all of whom had biopsy-confirmed NAFLD, found that both MAFLD and MASLD criteria identify a population similar to NAFLD. However, MASLD seems to include a broader group of patients with biopsy-proven NAFLD15.
SLD was uncommon among lean adults in our cohort, with MAFLD identifying a marginally higher proportion by emphasizing metabolic dysfunction. Although modest, these rates confirm that hepatic steatosis can occur independently of elevated BMI. In contrast, a large prospective study of lean cohorts found NAFLD in nearly 10% of participants but MAFLD in only about 4%, illustrating how differences in study design, population makeup, and metabolic profiles can affect prevalence estimates16. The results highlight the importance of developing diagnostic thresholds tailored to lean individuals.
Although MetALD individuals constituted a smaller subgroup (n = 471), they displayed a predominance of older males with liver-enzyme elevations. Analysis of NHANES III data shows MetALD prevalence at 3.2% in men versus 1.2% in women17. Similarly, UK Biobank data reveal a higher male proportion in MetALD (66%) compared to MASLD (60%)18. Moreover, men over 50 are at higher risk for progressive fibrosis, cirrhosis, and related complications, which may partly explain the increased MetALD prevalence in this demographic19.
We demonstrate that NAFLD, MAFLD, and MASLD are all associated with increased risk scores of HSI and higher FIB-4 values, which are widely used as first-line tools for exclusion and stratification of advanced fibrosis rather than definitive detection20,21, with MAFLD showing the strongest association with HSI. The consistent association with FIB-4 across all definitions therefore indicates a higher estimated fibrosis risk burden in these populations, though confirmation of advanced fibrosis requires sequential noninvasive testing or histology. This supports broad screening efforts in at-risk populations. However, the markedly stronger association of MAFLD with HSI underscores its utility as a comprehensive framework for identifying patients with significant steatosis, aligning with its focus on metabolic dysfunction. The identical ORs for NAFLD and MASLD in this study are potentially due to overlapping diagnostic criteria between the two definitions. A recent study assessed the diagnostic accuracy of HSI within the MAFLD cohort, demonstrating good ability to detect steatosis and superior performance compared to other markers like HOMA-IR and waist-to-hip ratio22. Another study by Deng et al. directly compared MASLD vs. non-MASLD individuals, finding significantly higher HSI and FIB-4 scores among MASLD patients, confirming associations with both steatosis and fibrosis risk23. Similarly, a large population-based study that analyzed the UK Biobank, NHANES III, and FHCS data showed that MAFLD, MASLD, and MetALD all carry increased risks of fibrosis, cirrhosis, liver cancer, and mortality, with adjusted hazard ratios progressively elevated from MetALD to MASLD to MAFLD24. These findings broadly mirror our results that all definitions correlate with fibrosis risk (via FIB-4), and MAFLD aligns best with steatosis markers (HSI).
The adoption of MAFLD, MASLD, and MetALD in fatty liver disease nomenclature carries significant implications for public health and policy making in our community, as evidenced by the PGCS. By emphasizing metabolic dysfunction, these terms enhance diagnostic precision and inclusiveness, enabling better identification and management of at-risk individuals in diverse populations. This shift reduces stigma, promoting greater patient engagement and earlier interventions, which are critical for improving health outcomes.
The choice of diagnostic criteria has direct implications for clinical practice and public health strategy. Our findings suggest that MAFLD and MASLD/NAFLD serve complementary but distinct purposes. MAFLD, by capturing a broader population with metabolic dysfunction, appears superior for early identification and primary prevention at the population level. Its strong association with both steatosis (HSI) and fibrosis (FIB-4) risk scores highlights its utility in screening programs that target individuals for lifestyle modification and cardiometabolic risk control before progressive liver injury develops. In contrast, the high concordance between NAFLD and MASLD shows that these definitions identify a more homogeneous subgroup. This specificity is valuable for enriching patient populations in hepatology clinics and clinical trials of novel pharmacotherapies, ensuring continuity with historical NAFLD cohorts. Moreover, the recognition of MetALD as a distinct subgroup within the new MASLD framework is an important advance, emphasizing a population that requires integrated management of both metabolic dysfunction and alcohol use. Overall, our results indicate that the choice of definition should depend on the purpose: MAFLD for sensitivity-driven screening and prevention, and MASLD/NAFLD for specificity-driven management and therapeutic development.
The ample sample size and real-world context enhanced the external validity of our findings. However, several limitations should be acknowledged. First, the cross-sectional design restricts our ability to establish causal relationships or assess disease progression over time, highlighting the need for longitudinal follow-up studies. Second, although we used noninvasive indices (FLI, HSI, and FIB-4) that are practical for large-scale epidemiological research, they may fail to capture moderate steatosis or intermediate fibrosis, which could be detected more effectively with imaging or biopsy. While FIB-4 and HSI are convenient tools for large-scale epidemiological studies, their diagnostic performance is limited. FIB-4 primarily serves as a rule-out tool for advanced fibrosis, whereas HSI functions mainly as a rule-in tool for hepatic steatosis. Both indices may be affected by age, BMI, and comorbidities, and they cannot substitute for imaging or histological assessment. In addition, we did not apply the age-specific cut off values for HSI and FIB-4 in present study. Consequently, potential misclassification should be considered when interpreting results in population-based studies. Another important limitation is the potential under-reporting of alcohol consumption in our population, which may have led to misclassification between MASLD and MetALD groups. Given the cultural and legal restrictions on alcohol use in Iran, some individuals with moderate alcohol intake might have been misclassified as having MASLD or NAFLD instead of MetALD. This potential bias could slightly underestimate the prevalence of MetALD and overestimate that of MASLD; therefore, the reported overlaps should be interpreted with this context in mind.
Another important limitation is the potential under-reporting of alcohol consumption in our population, which may have led to misclassification between MASLD and MetALD groups. Given the cultural and legal restrictions on alcohol use in Iran, some individuals with moderate alcohol intake might have been misclassified as having MASLD or NAFLD instead of MetALD. This potential bias could slightly underestimate the prevalence of MetALD and overestimate that of MASLD; therefore, the reported overlaps should be interpreted with this context in mind.
Conclusion
This large, population-based study shows that the ongoing shift in fatty liver disease terminology represents more than a change in wording. Nearly half of Iranian adults in our cohort met the criteria for steatotic liver disease, with MAFLD identifying the largest proportion compared with MASLD and NAFLD. The strong overlap between NAFLD and MASLD indicates that these definitions capture similar populations with comparable metabolic risk profiles. In contrast, participants classified as MAFLD had higher FIB-4 and HSI values, reflecting a greater burden of metabolic dysfunction and possible liver injury.
It should be noted, however, that the stronger associations observed for MAFLD with metabolic and fibrosis indices may, in part, arise from shared components between the MAFLD definition and these indices rather than from an independent causal effect. Therefore, our findings should be interpreted as reflecting associations, not as evidence of superiority of one definition over another. Taken together, the results suggest that MAFLD may serve as a practical framework for identifying individuals at increased metabolic risk in population-level screening, while NAFLD and MASLD remain useful for more specific clinical and research settings. Longitudinal studies are needed to clarify the prognostic implications of these classifications and to guide prevention and management strategies across different phenotypes, including lean MAFLD and MetALD.
Methods
Data source
This study utilized data from the first phase of the Prospective Epidemiological Research Studies in Iran (PERSIAN)Guilan Cohort Study (PGCS) (2014–2017), encompassing 10,520 participants of Guilan province in the North of Iran8. PGCS is one of the 14 branches of the PERSIAN cohort study, aiming to study the risk factors of non-communicable diseases among 180,000 Iranian adults25. Both the Persian Guilan Cohort Study (PGCS) and the present study were conducted according to the Helsinki Declaration. The PGCS design was approved by the ethics committees at the Ministry of Health and Medical Education, the Digestive Diseases Research Institute (Tehran University of Medical Sciences), and also
Guilan University of Medical Sciences (P/3/132/215). All participants completed written informed consent. The present study received ethical approval from the Digestive Disease Research Institute at Tehran University of Medical Sciences (IR.TUMS.DDRI.REC.1403.008).
Data collection
In the PGCS, the structured questionnaires were completed by face-to-face interviews for each participant, including socio-demographic, dietary intake, personal and family medical histories, reproductive health, smoking and alcohol consumption, and medication use information. Physical activity status was evaluated using the validated Persian version of the International Physical Activity Questionnaire (IPAQ-7). Body weight and height were measured using SECA 755 scale and SECA 204 stadiometer, respectively. Waist circumference (WC) was measured at the midpoint between the lowest rib and the iliac crest. Body mass index (BMI) was calculated as weight (kg) divided by height (m2).
After a 12–14-hour fast, plasma glucose (FPG); high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), total cholesterol (TC), γ-glutamyl transferase (GGT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), were measured using Pars Azmoon kits (Tehran, Iran) by an automated analyzer (BT 1500, Biotecnica Instruments Spa, Rome, Italy); and low-density lipoprotein cholesterol (LDL-c) was calculated by the Friedewald formula. Quality control was maintained with intra- and inter-assay coefficients of variability ≤ 4.3% for all biochemical tests.
Definitions & Thresholds
- Definition of SLD
This study defined SLD as the presence of hepatic steatosis based on the fatty liver index (FLI) ≥ 60 using the following formula:
- Definition of HSI and FIB-4 and K-NAFLD score
Hepatic steatosis index (HSI)28 and Fibrosis-4 (FIB-4)29 scores were used as the estimated indices to rule in liver steatosis and rule out advanced liver fibrosis, respectively, and calculated as follows:
HSI = 8 x ALT/AST + BMI (+ 2, if DM; +2, if female).
HSI ≥ 36 indicates the presence of hepatic steatosis.
HSI < 30 suggests the absence of hepatic steatosis.
Values 30–36 are considered an indeterminate zone.
FIB-4 = \(\:\frac{\text{A}\text{g}\text{e}\:\left[\text{y}\text{r}\right]\:\text{x}\:\text{A}\text{S}\text{T}\:[\text{U}/\text{L}]}{\text{P}\text{L}\text{T}\:\left[\frac{10\left(9\right)}{\text{L}}\right]\:\text{x}\:\sqrt{\text{A}\text{L}\text{T}\:[\text{U}/\text{L}]}}\)
FIB-4 < 1.3: Low probability of advanced liver fibrosis.
FIB-4 ≥ 2.67: High probability of advanced fibrosis.
In present study we consider the FIB-4 > 1.3 as a moderate or high probability of having advanced fibrosis.
K-NAFLD score = 0.913 x sex (female = 2, male = 1) + 0.089 x (WC) + 0.032 (SBP + FPG) + 0.007 x TG + 0.105 x ALT – 20.92930.
K-NAFLD ≥ 0.884 Indicates the presence of hepatic steatosis (NAFLD-positive).
K-NAFLD < 0.884 Suggests the absence of hepatic steatosis.
- Definition of NAFLD
NAFLD is defined by the excessive fat accumulation within hepatocytes (> 5% of hepatocytes weight) characterized by liver biopsy, imaging (ultrasound, CT, MRI, CAP) or validated non-invasive surrogate such as FLI > 60, in individuals with no or minimal alcohol consumption and no other causes of steatosis, including chronic hepatitis B or C, cancer, inflammatory bowel disease (IBD), celiac disease, Wilson’s disease, alcoholic fatty liver, other liver diseases, organ transplantation or current use of any of the following medications that have been associated with NAFLD: valproate sodium, amiodarone, methyldopa, methotrexate, tamoxifen, naproxen, fluoxetine, nifedipine, gentamicin, tetracycline, and interferons. Consumption more than 30 g/day ethanol for men and > 20 g/day ethanol for women was considered as exclusion criteria.
- Definition of MAFLD
MAFLD is characterized by liver steatosis accompanied by one of these conditions, including being overweight or obese (BMI ≥ 25 kg/m2), a diagnosis of T2DM (HbA1c ≥ 6.5% or being on any diabetes medication), or having any evidence of metabolic dysregulation. Metabolic dysregulation among individuals with hepatic steatosis and lean/normal weight who were not diagnosed with T2DM was defined as having at least two of the following metabolic risk abnormalities: (1) WC ≥ 102 cm among men, and ≥ 88 cm among women; (2) BP ≥ 130/85 mmHg or an antihypertensive medication; (3) plasma TG ≥ 150 mg/dL or on a lipid-lowering medication; (4) HDL-C < 40 mg/dL among men and < 50 mg/dL among women; and (5) pre-diabetes2.
- Definition of MASLD
MASLD is classified into four subgroups, including pure-MASLD, MetALD, Cryptogenic SLD, and other specific SLDs. In this definition, cardiometabolic criteria is characterized by having at least one metabolic abnormality, including overweight/obesity (BMI ≥ 25 kg/m2 or WC ≥ 102 cm for men and ≥ 88 cm for women); glucose dysregulation (FPG 5.6–6.9 mmol/L, HbA1c 5.7–6.4%, established T2DM, or current diabetes treatment); hypertension (PB ≥ 130/85 mm Hg or antihypertensive medication use); hypertriglyceridemia (TG ≥ 1.70 mmol/L requiring pharmacological intervention); or reduced HDL-c (< 1.0 mmol/L for men and < 1.3 mmol/L for women, or current pharmacological treatment). Patients meeting cardiometabolic criteria without any other cause of steatosis, with or without the excessive consumption of alcohol, were grouped as MetALD and Pure-MASLD, respectively31,32.
Statistical analysis
We used SPSS software version 22.0 (SPSS Inc. Chicago, IL) for statistical analysis. A significance level of 0.05 was employed for all statistical tests. Continuous variables were presented as the mean and standard deviation, and categorical variables as numbers and percentages. A logistic regression model was used to assess the association between SLD—defined according to NAFLD, MAFLD, and MASLD criteria with FIB-4 and an HSI. In all logistic regression analysis, participants without each condition (non-NAFLD, non-MAFLD, non-MASLD) were used as a reference group. We used three models in present analysis; model 1 (unadjusted or crude), model 2 with adjusted for age (years) and gender (men/women), and model 3 with additionally adjusted smoking status (yes/no), physical activity status (METs/day), and BMI (kg/m2). The same covariates structure was applied consistency across all definitions to ensure comparability of the estimated odds ratios (ORs).
Data availability
These data will be available on request from the corresponding author.
References
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77(4), 1335–1347 (2023).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73(1), 202–209 (2020).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 78 (6), 1966–1986 (2023). PubMed PMID: 37363821. Pubmed Central PMCID: PMC10653297.
Zou, H., Ma, X., Pan, W. & Xie, Y. Comparing similarities and differences between NAFLD, MAFLD, and MASLD in the general U.S. Population. Front. Nutr. 11, 1-11, 1411802 (2024)
Huang, X. et al. Comparison of NAFLD, MAFLD, and MASLD Prevalence and Clinical Characteristics in Asia Adults. J. Clin. Experimental Hepatol. 15(1), 102420 (2025).
Tabaeian, S. P. et al. Prevalence of Non-alcoholic Fatty Liver Disease in Iran: A Systematic Review and Meta-analysis. J. Clin. Exp. Hepatol. 14(1), 101209 (PubMed PMID: 38076354. Pubmed Central PMCID: PMC10709132. Epub 2023/12/11. eng.).
Jafari, E. et al. Prevalence and characteristics of metabolic Dysfunction-Associated fatty liver disease among an Iranian adult population with ethnic and genetic diversity: results of the PolyIran-Liver study. Middle East. J. Dig. Dis. 16 (2), 86–95 (2024). PubMed PMID: 39131113. Pubmed Central PMCID: PMC11316194. Epub 2024/08/12. eng.
Mansour-Ghanaei, F. et al. The PERSIAN Guilan cohort study (PGCS). Arch. Iran. Med. 22 (1), 39–45 (2019). PubMed PMID: 30821159. Epub 2019/03/02. eng.
Park, H. J., Lee, S. & Lee, J. S. Differences in the prevalence of NAFLD, MAFLD, and MASLD according to changes in the nomenclature in a health check-up using MRI-derived proton density fat fraction. Abdom. Radiol. (NY). 49 (9), 3036–3044 (2024). PubMed PMID: 38587630. Epub 2024/04/08. eng.
Lim, G. E. H. et al. An observational data Meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin. Gastroenterol. Hepatol. 21 (3), 619–629 (2023). e7. PubMed PMID: 34871813. Epub 2021/12/07. eng.
Guo, Z. et al. Global burden of MAFLD, MAFLD related cirrhosis and MASH related liver cancer from 1990 to 2021. Sci. Rep. 15(1), 7083 (2025).
Suzuki, K. et al. Concordance between metabolic dysfunction-associated steatotic liver disease and nonalcoholic fatty liver disease. Hepatol. Res. 54 (6), 600–605 (2024). PubMed PMID: 38234088. Epub 2024/01/18. eng.
Barritt ASt, Yu, F. et al. High concordance between nonalcoholic fatty liver disease and metabolic Dysfunction-Associated steatotic liver disease in the TARGET-NASH Real-World cohort. Am. J. Gastroenterol. 119 (8), 1624–1627 (2024). PubMed PMID: 38587293. Epub 2024/04/08. eng.
Priego-Parra, B. A., Bernal-Reyes, R., Icaza-Chávez, M. E., Martínez-Vázquez, S. E. & Remes-Troche, J. M. Transitioning from NAFLD to MAFLD to MASLD in the Mexican population. Revista de Gastroenterología de México (English Edition). 2025/01/01/;90(1):153–156. (2025).
Kaya, E. & Yilmaz, Y. Deciphering the implications of MAFLD and MASLD definitions in the NAFLD population: results from a single-center biopsy study. Chin. Med. J. 137 (05), 616–618 (2024).
Ng, C-H. et al. IDDF2022-ABS-0132 Mafld and Nafld in lean individuals. A prospective analysis of 12,505 lean individuals. Gut.17(2), A50.1-A50 (2022).
Ji, H. & Cheng, S. Sex differences in prevalence and prognosis of steatotic liver disease phenotypes: Biological sex matters. J Hepatol. 80(2), e68-e9. PubMed PMID: 37619929. Pubmed Central PMCID: PMC10873108. Epub 2023/08/25. eng. (2024).
Schneider, K. M. & Schneider, C. V. A new era for steatotic liver disease: evaluating the novel nomenclature in the UK biobank. J. Hepatol. 80 (2), e58–e60 (2024).
Polyzos, S. A. & Goulis, D. G. Menopause and metabolic dysfunction-associated steatotic liver disease. Maturitas 186, 108024 (2024). PubMed PMID: 38760254. Epub 2024/05/18. eng.
Chen, J., Mao, X., Deng, M. & Luo, G. Validation of nonalcoholic fatty liver disease (NAFLD) related steatosis indices in metabolic associated fatty liver disease (MAFLD) and comparison of the diagnostic accuracy between NAFLD and MAFLD. Eur. J. Gastroenterol. Hepatol. 35(4), 394–401 (2023).
Xu, X. et al. The role of fibrosis index FIB-4 in predicting liver fibrosis stage and clinical prognosis: A diagnostic or screening tool? J. Formos. Med. Assoc.121(2):454–466. (2022).
Priego-Parra, B. A. et al. Hepatic steatosis index (HSI): A valuable biomarker in subjects with metabolic Dysfunction-associated fatty liver disease (MAFLD). Ann. Hepatol. 2024/02/01/;29:101391. (2024).
Deng, Y., Chen, W. & Xie, W. MASLD identifies patients with significant hepatic fibrosis and steatosis in fatty liver population. Ann. Hepatol. 29(3):101488. (2024).
Zeng, H. et al. Prognostic and predictive effects of new steatotic liver disease nomenclatures: a large population-based study. MedComm. 13: 6(2):e70087 (2025). PubMed PMID: 39949980. Pubmed Central PMCID: PMC11822458. Epub 2025/02/14. eng. (2020).
Poustchi, H. et al. Prospective epidemiological research studies in Iran (the PERSIAN cohort Study): rationale, objectives, and design. Am. J. Epidemiol. 187 (4), 647–655 (2018).
Han, A. L. Validation of fatty liver index as a marker for metabolic dysfunction-associated fatty liver disease. Diabetol. Metab. Syndr. 14(1), 44 (2022).
Nomura, T. et al. Validation of fatty liver index as a predictor of hepatic steatosis in Asian populations: impact of alcohol consumption and sex. Hepatol. Res. 53 (10), 968–977 (2023).
Lee, J-H. et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Disease. 42 (7), 503–508 (2010).
Sugiyama, A., Kurisu, A., Ouoba, E. B., Ko, S. & Rakhimov, K. Distribution of FIB-4 index in the general population: analysis of 75,666 residents who underwent health checkups. BMC Gastroenterol. 22(1), 241 (2022).
Jeong, S. et al. Development of a simple nonalcoholic fatty liver disease scoring system indicative of metabolic risks and insulin resistance. Ann. Transl Med. 8(21), 1–12 (2020).
Yilmaz, Y. (ed) Editor the Heated Debate Over NAFLD Renaming: an Ongoing Saga. Hepatology Forum, 7; 4(3): 89-91(2023).
Chen, L., Tao, X., Zeng, M., Mi, Y. & Xu, L. Clinical and histological features under different nomenclatures of fatty liver disease: NAFLD, MAFLD, MASLD and MetALD. J. Hepatol. 80 (2), e64–e6 (2024).
Funding
The authors received no funding for this work.
Author information
Authors and Affiliations
Contributions
Conceptualization, E.T.; methodology, E.T., F.J.; data curation and formal analysis, E.T., S.G.S.; writing—original draft preparation, E.T., M.M.I.; writing—reviews and editing, E.T., M.M.I., S.G.S; supervision, E.T.; project administration, E.T., F.M.G. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The present study was approved by the review board of the Digestive Disease Research Institute (DDRI) of Tehran University of Medical Sciences (TUMS) (IR.TUMS.DDRI.REC.1403.008).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Isfahani, M.M., Sepanlou, S.G., Joukar, F. et al. Comparison of prevalence of fatty liver disease according to the different nomenclatures of NAFLD, MAFLD, and MASLD in a large Iranian Population-based study. Sci Rep 16, 742 (2026). https://doi.org/10.1038/s41598-025-30226-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-30226-8