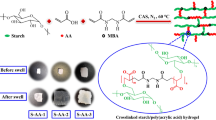
Abstract
This study focuses on synthesizing and characterizing a superabsorbent hydrogel based on xanthan cross-linked with glycerin. The synthesis tests revealed that the swelling percentage of the hydrogel is greatly influenced by the mass ratios of glycerin-to-xanthan and water-to-xanthan, as well as the curing temperature. The resulting hydrogels exhibited swelling ratios ranging from 52 to 229% after 120 min of immersion in water. Nitrogen adsorption-desorption analysis indicated a specific surface area of 63.78 m2/g, a total pore volume of 0.42 cm3/g, and an average mesopore diameter of 26.2 nm for the dried hydrogel. Scanning electron microscopy revealed macropores ranging from 50 to 400 μm on the as-synthesized hydrogel’s outer surface. Thermal durability tests showed a mass loss of 0.5% to 1% when the optimal hydrogel was exposed to temperatures between 40 and 160 °C for 1 h, highlighting its high thermal stability. In the nitrate removal tests, the optimal absorbent demonstrated a nitrate removal percentage exceeding 92.4% after 70 min for initial nitrate concentrations of 100, 150, and 200 mg/L, using an absorbent dose of 1 g/L at pH 5. Reusability assessments indicated that the absorbent could maintain approximately 85% of its initial performance after 10 regeneration cycles. The Langmuir isotherm model provided a better fit for the equilibrium nitrate absorption data, with a maximum absorption capacity of 370.37 mg/g. Overall, this study highlights the potential of xanthan-based superabsorbent hydrogel as an eco-friendly and reusable absorbent for nitrate removal from drinking water.
Similar content being viewed by others
Introduction
Nitrate has emerged as a critical global water pollutant due to its high water solubility and mobility in aquatic systems1,2,3,4. Major sources include agricultural runoff, industrial discharges, and urban wastewater, leading to widespread groundwater contamination. The ion’s environmental persistence and health impacts - including methemoglobinemia in infants, potential carcinogenic nitrosamine formation, and associations with diabetes and hypertension5,6 - have prompted strict regulatory limits (the World Health Organization (WHO): 50 mg/L; the US Environmental Protection Agency (USEPA): 45 mg/L)7. Additionally, nitrate promotes algal blooms, further compromising water quality. These factors collectively establish nitrate removal as a priority in water treatment initiatives worldwide.
Sorption is a well-established method for nitrate removal from contaminated water, employing materials that selectively retain nitrate ions. Process efficiency depends on key parameters including nitrate concentration, pH, and sorbent dosage8. Carbon-based sorbents demonstrate a capacity of 13.1 mg/g with optimal performance at pH 6.09. Alternative granular adsorbents from natural materials show 78.6% removal efficiency, achieving maximum adsorption at 50 mg/L initial nitrate concentration10.
Nitrate adsorption studies using sewage sludge-derived activated carbon showed a maximum capacity of 17–26 mg/g, following the Langmuir isotherm and pseudo-second-order kinetics (rate constant: 0.0003 g/mg min)11. Magnetic carbon nanocomposites (MCFs) demonstrated 25.4 mg/g capacity, with maximum adsorption at 500 mg/L initial concentration, also fitting the Langmuir model12. A novel amine-based resin achieved exceptional nitrate selectivity with 138.9 mg/g capacity, showing pH-dependent adsorption optimal at pH 2.0 while maintaining Langmuir behavior13.
Hydrogel-based adsorbents, particularly hydrogel-rice husk biochar composites, have emerged as promising nitrate removal materials due to their high water absorption capacity14,15,16. A magnetic cationic hydrogel (MCH) developed by grafting chitosan onto polyacrylamide with Fe₃O₄ nanoparticles demonstrated 17.6 mg/g nitrate sorption capacity through electrostatic and ion exchange mechanisms15. The MCH maintained excellent reusability, retaining 93.1% (batch) and 85.7% (column) efficiency after five cycles15.
Researchers developed poly(ethylene glycol) diacrylate (PEGDA)-based anion exchange hydrogels modified with methacryloxyethyltrimethyl ammonium chloride (MTAC) or 2-aminoethyl methacrylate hydrochloride (AMHC) for nitrate/nitrite removal17. Langmuir isotherm analysis revealed maximum capacities of 13.51 for PEGDA-MTAC and 13.16 mg NO₃⁻-N/g for PEGDA-AMHC, with corresponding nitrite capacities of 12.36 and 10.99 mg NO₂⁻-N/g. Remarkably, after 15 cycles, nitrate removal efficiency remained at 94.71% for PEGDA-MTAC and 83.02% for PEGDA-AMHC17.
Hydrogel-biochar composites demonstrate effective nitrate removal from aqueous solutions18,19. Optimized composites containing 5% biochar achieved 34.3% nitrate removal at pH 3 using 0.8 g/L of sorbent, following Temkin isotherm (R²>0.97) and first-order kinetics, with endothermic, spontaneous adsorption19. Alternatively, chitosan-Fe₃O₄ hydrogel composites exhibited superior 47 mg/g nitrate capacity via electrostatic interactions, maintaining 82% efficiency after 4 regeneration cycles18.
Despite available nitrate removal methods (sorption, reduction, ion exchange), critical gaps persist in efficiency and sustainability. Key challenges include developing cost-effective, eco-friendly sorbents to replace expensive conventional materials (activated carbon, ion-exchange resins) that generate waste during regeneration17,20. Novel solutions like hydrogels and biochar composites show promise for sustainable nitrate removal. Additionally, improving sorbent reusability remains crucial, as many current adsorbents degrade after single use or require uneconomical regeneration21,22.
The development of cost-effective, easily regenerable adsorbents is crucial for sustainable nitrate removal solutions. Additionally, there is a lack of understanding regarding the long-term performance and stability of sorbents used for nitrate removal. Many studies solely focus on the initial sorption capacity and efficiency of sorbents, but there is limited information on how these materials perform over extended periods of use. Conducting long-term studies is crucial in the durability, regeneration potential, and overall sustainability of sorbents for nitrate removal applications. Furthermore, the fate of nitrate after sorption onto various materials is not well understood. Understanding the mechanisms of nitrate sorption, desorption, and transformation is essential in predicting the overall effectiveness of nitrate removal processes. Studies investigating the fate of nitrate after sorption can provide insights into the potential release of nitrate back into the environment and inform strategies for safe disposal or reuse of spent sorbents23.
The aim of this study is to synthesize and characterize a reusable superabsorbent hydrogel based on xanthan gum chemically cross-linked with glycerol, which is used to absorb nitrate from potable water. For this purpose, various parameters such as the mass ratio of glycerol to xanthan, the mass ratio of water to xanthan and the curing temperature are examined with regard to the swelling percentage of the synthesized hydrogels. First, the swelling percentages are evaluated based on the immersion time in water, and the hydrogel with the highest swelling percentage is selected to examine the mechanical strength, thermal stability, chemical bonding status, crystal structure, and microstructure. Nitrate absorption tests examine the effects of parameters such as immersion time, nitrate concentration, amount of absorbent and initial pH of the nitrate solution on the amount of absorption and the percentage removal of nitrate from aqueous solutions. Furthermore, the isotherm and kinetics of nitrate absorption by superabsorbent hydrogel are investigated. In addition, the reusability and fate of the spent superabsorbent as a nitrate fertilizer for plants were also investigated. This represents an environmentally friendly solution for both nitrate removal and disposal.
Materials and methods
Materials
Food-grade xanthan powder was supplied by the Guangzhou Factory in China. Glycerin (99%) was purchased from Neutron Company in Iran. Monoethanolamine (≥ 98%) and sodium nitrate (≥ 99%) were purchased from Merck.
Methods
Synthesis of superabsorbent hydrogel (SAH)
Various SAHs were synthesized according to the conditions outlined in Table 1. It is worth noting that in all the experiments presented in Table 1, the quantity of xanthan used was consistently 2.00 g.
To synthesize SAHs, glycerin which is a cross-linking agent was poured into a beaker according to the specified amounts in Table 1. Next, 2.00 g of xanthan powder was added and mixed with it. Then, the specified amount of water, also indicated in Table 1, was added and mixed. After the water was added to the mixture of xanthan and glycerin, but before it transformed into a sticky gel, 2.00 g of monoethanolamine (MEA) was introduced and vigorously stirred. Eventually, the mixture transformed into a sticky gel. MEA plays multiple roles in the synthesis of the hydrogels. Primarily, MEA acts as a reactive modifier and secondary crosslinking/coordinating agent. Its hydroxyl (–OH) and amino (–NH2) functional groups can form hydrogen bonds and weak ionic interactions with the hydroxyl and carboxyl groups of xanthan and the hydroxyl groups of glycerin. These interactions enhance the intermolecular association within the polymer network, resulting in a more cohesive and stable gel structure. In addition, the basic nature of MEA slightly increases the pH of the reaction medium, which promotes deprotonation of xanthan’s carboxylic acid groups and facilitates intermolecular electrostatic interactions. This contributes to better dispersion of xanthan chains before gelation and helps achieve a more uniform network structure.
A soluble fraction test (based on ASTM F2450-18) where the hydrogel was immersed in deionized water for 24 h, and any uncrosslinked (soluble) xanthan/glycerol residues were quantified gravimetrically after drying. The low soluble fraction (< 3% w/w) confirms successful crosslinking, as most polymer chains were incorporated into the insoluble network. The obtained gel was then cured at the desired temperature for cross-linking reactions. The resulting hydrogels’ swelling ratio was measured using Eq. (1).
Where, W1 and W2 are the masses of swollen and dry hydrogels in g, respectively.
Characterization techniques
The chemical bonds of materials were studied using Fourier Transform Infrared (FTIR) spectra with a PerkinElmer IR Spectrophotometer Spectrum100 (USA) within the wavenumber range of 400–4000 cm− 1. For the FTIR analysis, the as-synthesized hydrogel was dried at 40 °C. The 3D topography and physical properties were assessed using an Atomic Force Microscope (AFM) Brisk (Islamic Republic of Iran). Nitrogen gas sorption data were obtained employing the single-point method with a Belsorp Mini 2 apparatus (Japan) after sample degassing at 110 °C for 3 h. The microstructure of the synthesized superabsorbent hydrogels was examined using a VEGA II TESCAN scanning electron microscope (SEM) at 30 kV. For the SEM analysis, the as-synthesized and dried hydrogels were dried at 40 and 100 °C, respectively. X-ray diffractograms were generated at 2θ° of 5–80° using a Philips PW1730 X-ray diffractometer (Netherlands) at 40 kV and 30 mA. Molecular structure analysis was carried out via Fourier transform infrared (FTIR) spectroscopy (PerkinElmer Spectrum100, USA). Spectra were acquired in transmittance mode with 4 cm⁻¹ resolution, scanning the full mid-IR range (400–4000 cm⁻¹) to identify functional groups and chemical bonds.
The compressive strength of the hydrogel was measured using a simple load-bearing method. A hydrogel sample with dimensions of 1 × 1 × 1 cm3 was carefully cut and placed on a flat surface. Incremental weights were then applied vertically onto the sample’s surface until visible deformation or structural failure occurred. The compressive strength (σ) was calculated using the formula σ = F/A, where F is the applied force (equivalent to the weight of the loads) and A is the initial cross-sectional area of the hydrogel (1 cm² in this case). This method provided a practical assessment of the hydrogel’s mechanical stability under compressive stress.
Nitrate removal tests
The effectiveness of SAH in removing nitrates was studied. The variables studied included contact time, initial nitrate concentration, pH of the nitrate solution, and the dosage of SAH. All sorption experiments took place at room temperature (25 ± 3 °C). To prepare nitrate solutions, a 1000 mg/L stock solution was made using NaNO3 salt. This stock solution was then diluted with deionized water to create solutions of varying concentrations. A refrigerator was used to keep the prepared solutions at 4 °C until the sorption tests were conducted. For the nitrate removal tests, 100 mL of the desired nitrate solution was stirred with 1 g/L of SAH at 150 rpm under specified conditions of initial concentration and pH. To adjust the pH, 0.2 M NaOH and 0.2 M HCl were used. Each removal test was repeated three times. Table 2 presents equations and models used to describe the nitrate sorption by the synthesized SAH.
In the Freundlich model, it is assumed that the sorbent surface contains sites with varying energy levels24. The Langmuir model, on the other hand, shows that a monolayer sorption takes place on a uniform surface with no interaction between the adsorbate species25. In the Langmuir model, a value of 0 < RL < 1 indicates desirable sorption, while RL > 1, RL = 1, and RL = 0 indicate undesirable, linear, and irreversible sorption, respectively.
Measurement of nitrate concentration
Nitrate concentrations were measured according to the procedure outlined in the book “Standard Methods for Examination of Water and Wastewater”26. UV-Vis spectroscopy was employed to determine the concentration of nitrate anions in an aqueous solution. This was done using a GBC Cintra 40 spectrometer equipped with a 1 cm diameter quartz cell. In UV-Vis spectroscopy, the measurement of nitrate sorption was performed at a wavelength of 220 nm. Subsequently, the absorbance at 275 nm, which is influenced by the interactions of dissolved organic matter was also recorded. It is important to note that the absorbance at 275 nm should not exceed 10% of the absorbance at 220 nm. Therefore, the absorbance at a wavelength of 275 nm is multiplied by two. If the resulting value is less than or equal to 10% of the absorbance at 220 nm, it is subtracted from the absorbance at 220 nm to obtain the final absorbance. However, this method should not be used if the correction value exceeds 10% of the absorbance at 220 nm.
Results and discussion
Characterization of SAHs
Figure 1 shows the swelling ratio of various SAH samples based on the duration of immersion in water.
As shown in Fig. 1, all SAHs reached their maximum swelling ratio between 52% and 229% after 120 min. Another important point to consider is that the SAH1, SAH2, and SAH3 samples cured at ambient temperature had a significantly lower swelling ratio compared to the samples cured at 100 °C. This is because the treatment at a higher temperature increases the rate of cross-linking reactions between the xanthan and glycerin branches27. In the case of samples SAH4, SAH5, and SAH6, the amount of cross-linking agent (glycerin) and curing temperature are the same. The reaction medium’s water content is the only variation. The varying behavior of these samples in terms of swelling indicates that as the water content increases, the swelling ratio of the hydrogel also increases. This could be due to an increased influx of water molecules into the hydrogel structure, occupying a larger volume. This increased porosity after the hydrogel is cured at high temperatures, allows water molecules to escape, providing more room for water molecules during the absorption process28. In samples SAH7, SAH8, and SAH9, the water content and curing temperature remain constant, with the only variation being the amount of cross-linking agent (glycerin). As shown in Fig. 1, the swelling ratio decreases with an increase in the amount of glycerin. Another study on xanthan gum derivatives confirmed similar results, where increasing amounts of glycerol resulted in reduced swelling ratios due to changes in hydrogel structure and interactions with water27.
Due to the desirable high swelling ratio observed in the SAHs, the SAH6 sample was chosen as the optimal sample for further tests The macroscopic morphology of the synthesized SAH6 hydrogel is shown in Figure S1. The images demonstrate the hydrogel’s uniform structure and physical integrity, which are critical for its intended applications. Figure S1 also shows the SAH6 hydrogel sample after being divided into smaller fragments for testing purposes. The images clearly illustrate the hydrogel’s ability to maintain structural integrity even when sectioned.
Figure 2 visually compares the SAH6 hydrogel in its as-synthesized, dried, and swollen states. As can be seen, the SAH6 sample swelled after being immersed in water for 120 min, and the penetration of water into the hydrogel network resulted in the transparency of the sample. The as-synthesized sample (Fig. 2b) exhibits a homogeneous, translucent appearance, confirming successful crosslinking. Upon drying (Fig. 2a), the hydrogel shrinks but retains its structural framework, while the swollen state (Fig. 2c) reveals a significant volumetric expansion. This macroscopic behavior correlates with the porous network (which is further confirmed by SEM images), where swelling facilitates fluid penetration without material fragmentation.
The following tests designed by the authors were conducted to evaluate the mechanical strength of the SAH6 sample. The objective was to determine if the absorbent has sufficient mechanical strength to withstand the stresses encountered in the water treatment process. The compressive strength was measured as about 0.5 MPa. As shown in Fig. 3, the SAH6 sample demonstrated excellent resistance to tensile, twisting, knotting, and compressive stresses. Notably, a cubic SAH6 sample measuring 3 mm in thickness was able to bear a weight 4000 times its own mass and recover to its original state once the weight was removed. This indicates that the hydrogel also possesses elastic properties.
Figure 4 shows the FTIR spectra of xanthan, glycerin, and SAH6 hydrogel. In the FTIR spectrum of xanthan, the absorption band at 3403 cm− 1 corresponds to the stretching vibration of the alcoholic –OH group. The absorption band at 2918 cm− 1 is associated with aliphatic –CH2 stretching vibration. The absorption band at 1734 cm− 1 is assigned to the C = O bond vibration. The absorption bands appearing at the wave numbers 1621 cm− 1 and 1419 cm− 1 are related to the symmetric and asymmetric vibrations of COO−, respectively. Moreover, the absorption band at 608 cm− 1 appeared due to COO− stretching vibration29,30,31,32,33.
In the FTIR spectrum of glycerin, the absorption band at 678 cm− 1 can indicate the presence of structural characteristics related to the polysaccharide backbone or modifications due to the incorporation of glycerin. The absorption peak observed at 1652 cm⁻¹ in the FTIR spectrum of glycerin most likely corresponds to the bending vibration (δ H-O-H) of water molecules absorbed by the sample. This observation is attributed to glycerin’s extremely hygroscopic nature - its three hydroxyl groups readily absorb moisture from the environment. The absorption band appearing at 3380 cm− 1 is due to the stretching vibration of the OH group resulting from intramolecular or intermolecular hydrogen bonding. Additionally, the absorption band at 2943 cm− 1 is caused by the bending vibration of the C-C-H bond. The C-O stretching vibration is observed at wavenumbers of 1111 cm− 1 and 1218 cm− 1. The absorption band at 1047 cm− 1 is related to the CH2-OH bond34.
Generally, the relative amount of the corresponding functional groups can be determined by examining the area under the absorption bands35. In the case of glycerin, the area under the C-H absorption bands for glycerin is larger than the area of hydrogel and xanthan. The area under the C = O absorption bands of the hydrogel is smaller than the area under the C = O absorption band of xanthan, suggesting the formation of a crosslink between xanthan and glycerin. The C = O functional groups are reactive sites that can participate in crosslinking reactions. When these groups form covalent bonds during crosslinking, their vibrational modes may shift or weaken, leading to a decrease in the corresponding FTIR peak area36. Furthermore, the area under the C-O-C absorption bands of the hydrogel is smaller than the area under the C-O-C absorption bands of xanthan, indicating the crosslinking between xanthan and glycerin monomers and oligomers29.
Figure S2 shows the XRD patterns of xanthan and SAH6 hydrogel. In Figure S2a, a significant peak can be observed at approximately 20.19° 2θ angle, indicating the amorphous nature of xanthan. As shown in Figure S2b, the hydrogel also retains its amorphous nature during cross-linking process between xanthan and glycerin. In other words, the crystal structure remains unaffected by the formation of crosslinks and subsequent hydrogel formation15.
To determine the porosity and specific surface area of the obtained SAH6 hydrogel, nitrogen sorption analysis was performed. The N2 sorption isotherms and pore size distribution are illustrated in Fig. 5. According to the IUPAC classification of physical adsorption isotherms, the SAH6 hydrogel exhibits a type IV isotherm with an H2-type hysteresis loop. This type is characteristic of mesoporous materials, indicating capillary condensation within mesopores37. The presence of H2-type hysteresis loop suggests the existence of bottle-shaped pores in the hydrogel38. According to IUPAC, mesoporous materials have pores that range in diameter from 2 nm to 50 nm. The results of this research demonstrate that the SAH6 hydrogel is mesoporous, with a multi-modal pore size distribution.
Based on the N2 sorption isotherms, the specific surface area of the hydrogel was determined to be 63.78 m²/g. Additionally, the total mesopore volume was found to be 0.42 cm³/g, and the mean pore size was 26.2 nm.
SEM images of the SAH6 hydrogel are shown in Fig. 6. In Figs. 6a,b, pores ranging from 50 μm to 400 μm are visible on the outer surface of the hydrogel. These pores facilitated the initial penetration of the aqueous solution into the hydrogel network. As the solution infiltrates the network through these pores, the hydrogel swells. Additionally, these apertures act as conduits for the discharge of the aqueous solution during regeneration phase of the absorbent, allowing the hydrogel contract39. Figures 6c,d, reveal a rough surface, likely resulting from the cross-linking of xanthan with glycerin. Both Figs. 6c,d are images of the dried SAH6 surface where the porous network collapsed during drying. Figure 6c is a lower-magnification overview of the dried surface showing a generally compact, cracked morphology, while Fig. 6d is a higher-magnification view that reveals the dense, smooth polymer matrix and fine surface texture produced by collapse and consolidation.
SEM images of SAH6 hydrogel. (a,b) As-synthesized gel morphology. (c) Dried sample — low-magnification view showing an overall compacted surface with drying-induced cracking and loss of the open porous network. (d) High-magnification view of the dried surface showing the consolidated polymer matrix (smooth regions and micro-texture) produced by capillary collapse during drying.
In general, hydrogel superabsorbents can be used in water purification processes in two ways. One scenario occurs when clean water enters the hydrogel network from the polluted water environment in an increased concentration of pollutants in the remaining water. In this situation, clean water is effectively extracted from the contaminated environment. The second scenario occurs when pollutants are absorbed into the hydrogel network, reducing their concentration in the initial solution. In the first scenario, it is essential to extract and utilize the clean water that has infiltrated the hydrogel network. In the second scenario, the hydrogel must be regenerated and reused in the purification process. In both cases, applying heat to the hydrogel is a common method for extraction and regeneration, necessitating good thermal durability40. Figure 7 shows the mass loss of synthesized SAH6 after exposure to different temperatures ranging from 40 to 160 °C for 1 h. As illustrated, SAH6 experienced approximately a 1% mass loss after being exposed to 160 °C for 1 h, indicating the high thermal durability of the hydrogel.
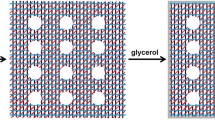
Mechanism of hydrogel formation
Glycerin has the ability to cross-link with all carboxyl groups in biopolymers like xanthan41. Additionally, glycerin monomers can partially cross-link with other functional groups in the xanthan structure. The xanthan side chains consist of carboxyl groups, which are likely to react with glycerin and form xanthan-glycerin crosslinks. The presence of -CH2 and -CH3 groups in the xanthan structure reduces the reactivity of the acetyl group. When xanthan-glycerin mixture is heated, the double helix structure of xanthan is lost, allowing the functional groups to interact with glycerin and form crosslinks. Glycerin can create crosslinks between carboxyl groups within in the same branch or between two carboxyl groups in neighboring branches (see Fig. 8). Moreover, glycerin can also react with itself to form double, triple, or multiple oligomers that contribute to the cross-linking xanthan. The acetyl content of xanthan, the degree of cross-linking, and other characteristics of xanthan all have an impact on cross-linking process41.
Nitrate removal tests
Impact of initial nitrate concentration and immersion time
Nitrate sorption by the SAH6 superabsorbent was studied by varying the initial nitrate concentration and contact time. Figure 9 shows the results. The standard deviation of the measured absorption data ranges between 3% and 5%. These experiments were conducted with a sorbent amount of 1 g/L (0.1 g of SAH6 in 100 mL of nitrate solution) and a pH value was 7.0. The concentrations of 100, 150, and 200 mg/L were chosen because they fall within the range of nitrate concentrations found in many contaminated underground waters. Figure 9 illustrates that as contact time and initial nitrate concentration increase, so does the amount of nitrate absorption. Initially, the rate of nitrate absorption is high, but it slows down progressively until it stabilizes. For an initial nitrate concentration of 100 mg/L, the nitrate absorption increases from 32 mg/g to 60 mg/g as the contact time increases from 5 min to 40 min. Nevertheless, the rate of nitrate absorption decreases with additional contact time increases, finally reaching a constant value of 72.5 mg/g after 60 min. This constant value is equivalent to a nitrate removal rate of 72.5%. For the initial nitrate concentration of 150 mg/L, the amount of nitrate absorption increases from 60 mg/g to 88 mg/g as the contact time increases from 5 min to 40 min. Subsequently, nitrate absorption increases rapidly with longer contact time, reaching a constant value of 120 mg/g after 70 min. This constant value corresponds to an 80% nitrate removal rate. For an initial nitrate concentration of 200 mg/L, the nitrate absorption increases from 140 mg/g to 160 mg/g as the contact time increases from 5 min to 40 min. After that, the rate of nitrate absorption decreases at a slower rate, reaching a constant value of 175 mg/g after 60 min. This constant value corresponds to an 87.5% nitrate removal rate. The obtained results indicate that the equilibrium contact time for all three initial nitrate concentrations is 70 min.
Effect of absorbent amount
The effect of hydrogel superabsorbent dosage on absorption amount and nitrate removal efficiency is shown in Fig. 10. These results were obtained at the pH of 7.0, the initial nitrate solution concentrations of 100, 150, and 200 mg/L, and the contact time of 70 min. From the results, it is evident that increasing the amount of SAH6 leads to higher efficiency in nitrate removal. Specifically, when the amount of SAH6 is increased from 0.5 g/L to 2 g/L, the percentage of nitrate removal increases from 65 to 78% to approximately 95%. This is because a larger amount of SAH6 provides more space in the three-dimensional network of the hydrogel for nitrate anions. As a result, the contact surface between the sorbent and the absorbed nitrate anions increases, and more active binding sites are available to accept absorbed ions. However, increasing the amount of absorbent beyond 2 g/L does not significantly affect nitrate removal efficiency. This means that using more absorbent does not necessarily increase the nitrate removal efficiency. On the other hand, increasing the amount of absorbent under constant volume and initial concentration of nitrate anion leads to an increase in the number of remaining unsaturated sites during the absorption process. Consequently, this reduces the amount of nitrate absorption from 130.6 mg/g to 47.15 mg/g for an initial nitrate concentration of 100 mg/L and from 215.7 mg/g to 71.77 mg/g for a nitrate concentration of 150 mg/L and from 312.4 mg/g to 96.4 mg/g for an initial nitrate concentration of 200 mg/L. To determine the optimal absorbent dosage, it is crucial to consider both the removal percentage and the quantity of nitrate absorption. According to Fig. 10, the intersection of the removal percentage diagram and the absorption value diagram indicates the appropriate absorbent dose, which is about 1 g/L.
Effect of initial pH value of nitrate solution
One of the key factors that affect the absorption process is the initial pH of the solution. Figure 11 demonstrates the impact of pH on nitrate absorption for three different initial concentrations of this anion. The standard deviation of the measured absorption of nitrate, considering three different initial concentrations of 100, 150, and 200 mg/L is less than 3.8%, 4.8%, and 5.2% respectively. These results were obtained assuming that the other parameters remained constant, with the absorbent value set at 1 g/L and the contact time at 70 min. Specifically, the results indicate that for an initial nitrate concentration of 100 mg/L, increasing the pH of the solution from 3 to 5 leads to an increase in the amount of absorbed nitrate from 68.3 mg/g to 93.4 mg/g, followed by a decline as the pH rises further. Similarly, for the initial nitrate concentration of 150 mg/L, increasing the pH of the solution from 3 to 5 results in an increase in the amount of nitrate absorbed by the hydrogel superabsorbent from 110.4 mg/g to 130.6 mg/g, and then it decreases at pH values higher than 5. For the initial nitrate concentration of 200 mg/L, increasing the pH from 3 to 5 leads to an increase in nitrate absorption from 165.6 mg/g to 188.3 mg/g. However, with further increase in the pH, the nitrate absorption decreases. The combined results demonstrate that the highest amount of nitrate absorption occurs at a pH of 5. This is because the xanthan chains are always in a double helix state in an acidic environment, allowing them to provide more space in the hydrogel network. Conversely, xanthan chains in an alkaline environment undergo a slight structural change to a more compact coiled state, resulting in less space within the network42. In addition, the pH-dependent uptake behavior reveals a critical electrostatic exclusion mechanism under alkaline conditions. Deprotonation of hydroxyl/carboxyl functional groups generates fixed negative charges within the hydrogel matrix, establishing a Donnan potential barrier43 that effectively repels nitrate anions (NO₃⁻). This charge-assisted molecular exclusion becomes the dominant mechanism, significantly reducing nitrate absorption capacity at elevated pH while simultaneously suppressing potential surface interactions.
Nitrate absorption isotherm
Sorption isotherms play a crucial role in studying the performance of sorbents in sorption processes. They provide information about the distribution of the absorbed substance between the solution and the equilibrium state, as well as the characteristics of the absorbent44. Two widely recognized and commonly used absorption isotherms are the Langmuir and Freundlich isotherms. In this study, absorption experiments were conducted to determine the absorption isotherm of the nitrate absorption process using SAH6 superabsorbent. The initial nitrate concentrations ranged from 50 mg/L to 250 mg/L. The tests were performed under specific conditions, including a pH value of 5, a contact time of 70 min, and an absorbent amount of 1 g/L. Figure 12 illustrates the Langmuir and Freundlich absorption isotherms for the equilibrium data of nitrate absorption by the hydrogel superabsorbent.
Each isotherm is based on a set of assumptions regarding the homogeneity or heterogeneity of the absorbent surface and the potential interaction between the absorbent and the absorbed species45. The Langmuir isotherm assumes that the sorbent surface is homogeneous, and that absorption takes place on the homogeneous sites of the absorbent. Furthermore, it assumes that the absorbed species do not interact with each other. The Langmuir isotherm is utilized when absorption occurs as a monolayer. By plotting 1/qe values against 1/Ce values, a line can be obtained that extends 1/qmax units from the origin. This line allows for the estimation of the maximum theoretical absorption capacity of the sorbent. The slope of the above line represents 1/(qmaxb), where b represents the Langmuir constant and is associated with absorption energy. According to Fig. 12, the values of qmax and b are equal to 370.37 mg/g and 0.06 L/mg, respectively, based on the width from the origin and the slope of line in (Fig. 12a). The validity of the Langmuir isotherm is determined by the separation parameter RL, which is calculated using formula RL = 1 / (1 + bCi). When RL is less than one, absorption is considered favorable. In this research, RL value for all initial concentrations of nitrate (ranging from 50 mg/L to 250 mg/L) are less than one, indicating the optimal absorption of nitrate anions by the hydrogel superabsorbent. The Freundlich isotherm is used for sorption on heterogeneous surfaces. By plotting log(qe) against log(Ce), a line is obtained with a distance of log(Kf) from the origin and a slope of 1/n. The value of Kf represents absorption capacity, while 1/n indicates the intensity of absorption. Based on the slope and y-intercept of line in (Fig. 12b), the values of Kf and n were obtained as 23.68 and 1.28, respectively. A value of n between 1 and 10 indicates the optimal absorption of nitrate by the hydrogel superabsorbent. To determine the optimal absorption isotherm model, it is important to consider the proximity of the R2 correlation coefficients. In this case, we utilize the non-linear statistic χ2 (Eq. 2).
This non-linear statistic was obtained for Langmuir and Freundlich absorption isotherms as 0.00065 and 0.0112, respectively. These results suggest that the Langmuir absorption isotherm can better predicting the equilibrium absorption data. The excellent fit of nitrate absorption data to the Langmuir isotherm strongly supports a chemisorption-dominated process occurring through specific interactions between nitrate ions and the hydrogel’s functional groups. The model parameters propose mechanism of hydrogen bonding between nitrate anions and the hydroxyl/carboxyl groups of the xanthan-glycerol network. The findings align with the hydrogel’s structural properties, where glycerol crosslinking creates defined binding pockets while maintaining sufficient swelling for nitrate access.
Figure 12c demonstrates the correlation between initial nitrate concentration in water (mg/L) and two critical parameters: nitrate absorption amount (mg/g) and removal efficiency (%). The absorption amount shows a linear increase against nitrate concentrations (50–250 mg/L). Meanwhile, the removal efficiency maintains an impressively high level (92–96%) across the entire concentration range, with only a marginal decrease (1–3%) observed at concentrations of higher than 50 mg/L.
The consistent removal efficiency suggests the absorbent material performs reliably under varying contaminant loads, making it suitable for broad water treatment applications. The absorption trend follows classical adsorption behavior, where available binding sites become progressively occupied as nitrate concentration increases. These results highlight the material’s effectiveness for nitrate removal, particularly in scenarios with moderate contamination. For systems dealing with very high nitrate concentrations, operational adjustments such as increased absorbent dosage or extended contact time may further optimize performance. The minimal efficiency loss at elevated concentrations underscores the robustness of this absorption approach for diverse water treatment needs.
As shown in Fig. 13, The nitrate uptake occurs through bulk absorption within the three-dimensional hydrogel matrix rather than adsorption. Nitrate ions penetrate the swollen hydrogel network during immersion in water, becoming physically entrapped within the interstitial spaces of the crosslinked xanthan structure. The helical conformation of xanthan chains creates a molecular sieve effect, while hydroxyl groups from both xanthan and glycerol form weak hydrogen bonds with the nitrate anions, further stabilizing their retention. This combination of physical confinement and weak chemical interactions explains the observed nitrate uptake capacity.
Atomic force microscopy (AFM) test
An AFM test was conducted to analyze the properties and surface structures of the SAH6 absorbent at the nanometer scale. The micrographs obtained from this test, both two-dimensional and three-dimensional are depicted in Fig. 14. According to Fig. 14, the roughness height of the sorbent surface before sorption is higher, measuring 250.8 nm. The porous structure becomes filled after nitrate sorption, resulting in a smoother surface. After the sorption of nitrate, the surface roughness decreases to 219.8 nm. The three-dimensional images after sorption also reveal that the surface structure becomes more defined due to the presence of nitrate. Because sharp protrusions have higher surface energy thermodynamically than wider protrusions, and the surface has many of these sharp protrusions after sorption, the total sorbent surface energy also increases after absorption.
Investigation of superabsorbent reusability
The reusability of an absorbent is crucial for the economic efficiency of the absorption process. In this study, the hydrogel superabsorbent was regenerated 10 times. The absorbent was regenerated by immersing it in a 0.1 M NaOH solution for 2 h after each use in the nitrate absorption process. Following immersion, the absorbent was rinsed with a 0.1 M HCl solution and then with deionized water.
The amount of nitrate absorption was calculated after each regeneration of the absorbent. It is important to note that the performance of the regenerated absorbent was evaluated by using to absorb nitrate with an initial concentration of 100 mg/L under the following conditions: pH = 5, absorbent amount of 1 g/L, and an equilibrium contact time of 70 min. The results are shown in Fig. 15. The data has a standard deviation of less than 1.4%. After the first regeneration of the absorbent, the amount of nitrate absorption decreased by 2.2%, from the initial value of 93.4 mg/g to 91.3 mg/g. In subsequent regeneration of absorbent, the decrease in nitrate absorption ranged from 3% to 15%. After 10 regenerations of the absorbent, the amount of absorbed nitrate reached 79.4 mg/g. These results demonstrate that the regenerated hydrogel superabsorbent exhibits good nitrate absorption performance.
As shown in Fig. 16, comparative FTIR analysis of the superabsorbent hydrogel after the first and last nitrate absorption cycles reveals only minor variations in peak shapes, intensities, and widths, with no significant structural differences observed. This remarkable spectral consistency demonstrates the exceptional stability of the xanthan-glycerol hydrogel network throughout multiple nitrate uptake-regeneration cycles. The preserved characteristic absorption bands, particularly in the 1000–1200 cm− 1 region (C-O-C stretching) and 1600–1700 cm− 1 range (hydrogen-bonded C = O), confirm the maintenance of the hydrogel’s core polymeric architecture despite repeated use. These findings strongly support the hydrogel’s robust reusability, as neither the primary functional groups responsible for nitrate binding nor the fundamental crosslinked matrix undergo substantial degradation during cyclic operations. The minimal spectral changes observed suggest only superficial modifications to the hydrogel’s surface chemistry, without compromising its bulk structural integrity or absorption capacity. This stability is particularly noteworthy given the alternating acidic/alkaline conditions employed during regeneration, further highlighting the material’s potential for long-term environmental applications.
Exploring the potential of spent superabsorbent in alternative applications
A significant challenge of the absorption process is identifying methods to collect and repurpose spent absorbent for other uses. The superabsorbent examined in this research is biodegradable, ensuring that it does not pose environmental risk post-absorption. Preliminary findings suggest that this superabsorbent could serve as a nitrate fertilizer in agricultural after it has absorbed nitrates from drinking water. To investigate this, 100 g of farm soil with a pH level between 6 and 7 was prepared in suitable containers. The same number of bean seeds were placed in the containers at an appropriate depth in the soil. An equal number of bean seeds were sown at the correct depth in the soil surface. An identical mass of spent superabsorbent was applied to the surface of two containers, with one serving as a control (lacking the spent superabsorbent). The ideal temperature range for bean cultivation is between 20 and 28 °C during the day and 15–20 °C at night, thus the laboratory temperature was maintained 17 to 25 °C. The growth results of plants growth after 14 and 30 days are illustrated in Figure S3. It is clear that the plants treated with the spent superabsorbent exhibited enhanced growth after 14 days and remained vibrant even after 30 days, while the control group showed signs of wilting. It is important to note that this aspect is not the primary focus of the research. It was raised solely for the purpose of a preliminary investigation into the idea of utilizing superabsorbent but was introduces as an initial exploration into potential of superabsorbent materials as nitrate fertilizers. This idea warrants further investigation as a promising avenue for developing smart fertilizers and stabilizing fine desert dust particles.
Conclusions
In this study, a superabsorbent hydrogel intended for the efficient removal of nitrate from drinking water is developed and analyzed. The superabsorbent was synthesized through a chemical crosslinking process utilizing glycerin and were further modified with monoethanolamine. The synthesis tests indicated that the ratio of water to xanthan, glycerin (cross-linking agent) to xanthan, and the curing temperature significantly influenced the swelling ratio of the resulting hydrogels. The optimal swelling ratio of 229% (on mass basis) was achieved with a glycerin-to-xanthan ratio of 2.5, water-to-xanthan ratio of 5, and curing temperature of 100 °C. Characterization tests demonstrated that this optimal superabsorbent possesses adequate mechanical and thermal stability for the absorption process, as exposure to 160 °C for one hour resulted in only a 1% mass loss, indicating its high thermal resistance. The superabsorbent also displayed strong resistance to tensile, torsional, compressive, and knotting stresses. Additional characterization techniques including FTIR spectroscopy, XRD, SEM, and nitrogen sorptometry were conducted. FTIR spectroscopy confirmed the formation of new chemical bonds within the hydrogel, indicating successful chemical cross-linking. XRD analysis revealed that the amorphous characteristics of xanthan remained unchanged post- hydrogel formation. SEM analysis identified macropores ranging from 50 to 400 μm in size. Nitrogen sorptometry analysis and BET isotherm analysis indicated that the hydrogel microstructure comprises mesopores with a diameter of 26.2 nm and a total pore volume of 0.42 cm3/g. The dry hydrogel exhibited a specific surface area of 63.78 m2/g. Nitrate absorption experiments were conducted with nitrate concentrations of 100, 150, and 200 mg/L, examining the effects of contact time, absorbent dosage and pH. The findings indicated that for all three mentioned nitrate concentrations, the nitrate absorption reached a plateau after 70 min corresponding to removal efficiencies of 72.5%, 80.0%, and 87.5% for the initial concentrations of 100, 150, and 200 mg/L, respectively. An analysis of the impact of absorbent dosage and the pH of the initial nitrate solution on both the removal efficiency and the amount of nitrate absorbed identified 1 g/L as the optimal dosage and a pH of 5 as the ideal condition. When the equilibrium absorption data for initial nitrate concentrations ranging from 50 to 250 mg/L were fitted to the Langmuir and Freundlich isotherms, the Langmuir isotherm demonstrated a superior fit for data. Furthermore, the maximum nitrate absorption capacity of the superabsorbent was determined to be 370.37 mg/g. Tests on the reusability of the absorbent revealed that it could retain approximately 85% of its initial efficacy after 10 absorption cycles. Initial cultivation trials with bean plants provided encouraging evidence that used sorbent could serve as an agricultural fertilizer, although this concept warrants further exploration in subsequent research.
Data availability
All data generated or analyzed data for the experimental part of this study are included in this published article. The data that support the findings of this study are available from the corresponding author, [Elham Abdollahzadeh Sharghi], upon reasonable request. Moreover, all other data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Jain, S. et al. Enhancing adsorption of nitrate using metal impregnated alumina. J. Environ. Chem. Eng. 3, 2342–2349 (2015).
Ergas, S. J. & Rheinheimer, D. E. Drinking water denitrification using a membrane bioreactor. Water Res. 38, 3225–3232 (2004).
Kapoor, A. & Viraraghavan, T. Nitrate removal from drinking water. J. Environ. Eng. 123, 371–380 (1997).
Liu, A., Ming, J. & Ankumah, R. O. Nitrate contamination in private wells in rural Alabama, united States. Sci. Total Environ. 346, 112–120 (2005).
Dietrich, A. M. Aesthetic issues for drinking water. J. Water Health. 4, 11–16 (2006).
Rahman, A., Mondal, N. C. & Tiwari, K. K. Anthropogenic nitrate in groundwater and its health risks in the view of background concentration in a semi arid area of Rajasthan, India. Sci. Rep. 11, 9279 (2021).
Ward, M. H. et al. Drinking water nitrate and human health: an updated review. Int. J. Environ. Res. Public. Health. 15, 1557 (2018).
Bhatnagar, A. & Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 168, 493–504 (2011).
Gierak, A. & Łazarska, I. Adsorption of nitrate, nitrite, and ammonium ions on carbon adsorbents. Adsorpt. Sci. Technol. 35, 721–727 (2017).
Hosseini Nami, S. & Mousavi, S. B. Nitrate removal performance of different granular adsorbents using a novel fe-exchanged nanoporous clinoptilolite. Ind. Eng. Chem. Res. 62, 3659–3671 (2023).
Masengo, J. L. & Mulopo, J. Synthesis and performance evaluation of adsorbents derived from sewage sludge blended with waste coal for nitrate and Methyl red removal. Sci. Rep. 12, 1670 (2022).
Lee, H. K., Yoo, D. H., Jo, S. E. & Choi, S. J. Removal of nitrate from radioactive wastewater using magnetic multi-walled carbon nanotubes. Prog Nucl. Energy. 140, 103893 (2021).
Sun, Y., Zheng, W., Ding, X. & Singh, R. P. Selective removal of nitrate using a novel asymmetric amine based strongly basic anion exchange resin. Adsorpt. Sci. Technol. 38, 271–285 (2020).
Chen, C. et al. Biodegradable chitosan-ethylene glycol hydrogel effectively adsorbs nitrate in water. Environ. Sci. Pollut Res. 27, 32762–32769 (2020).
Li, J., Dong, S., Wang, Y., Dou, X. & Hao, H. Nitrate removal from aqueous solutions by magnetic cationic hydrogel: effect of electrostatic adsorption and mechanism. J. Environ. Sci. 91, 177–188 (2020).
Mahinroosta, M., Jomeh Farsangi, Z., Allahverdi, A. & Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 8, (2018).
Dharmapriya, T. N., Shih, H. Y. & Huang, P. J. Facile synthesis of hydrogel-based ion-exchange resins for nitrite/nitrate removal and studies of adsorption behavior. Polym. (Basel). 14, 1442 (2022).
Nasir, M. et al. Regenerable chitosan-embedded magnetic iron oxide beads for nitrate removal from industrial wastewater. Environ. Sci. Adv. 3, 572–584 (2024).
Sadeghi Afjeh, M., Bagheri Marandi, G. & Zohuriaan-Mehr, M. J. Nitrate removal from aqueous solutions by adsorption onto hydrogel‐rice husk Biochar composite. Water Environ. Res. 92, 934–947 (2020).
Wang, L., Liu, S., Xuan, W., Li, S. & Wei, A. Efficient nitrate adsorption from groundwater by Biochar-Supported Al-Substituted goethite. Sustainability 14, 7824 (2022).
Tyagi, S., Rawtani, D., Khatri, N. & Tharmavaram, M. Strategies for nitrate removal from aqueous environment using nanotechnology: a review. J. Water Process. Eng. 21, 84–95 (2018).
Khatamian, M., Derakhshan, S. K., Nami, S. H. & Fazli-Shokouhi Nitrate removal study of synthesized nano γ-alumina and magnetite-alumina nanocomposite adsorbents prepared by various methods and precursors. Sci. Rep. 14, 7673 (2024).
Dai, Y. G., Guo, X. H., Ma, G. W., Gai, W. Z. & Deng, Z. Y. Efficient removal of nitrate in neutral solution using zero-valent al activated by soaking. ACS Omega. 8, 24922–24930 (2023).
Vigdorowitsch, M., Pchelintsev, A., Tsygankova, L. & Tanygina, E. Freundlich isotherm: an adsorption model complete framework. Appl. Sci. 11, 8078 (2021).
Islam, M. A., Chowdhury, M. A., Mozumder, M. S. I. & Uddin, M. T. Langmuir adsorption kinetics in liquid media: interface reaction model.ACS Omega 6, 14481–14492. (2021).
Rice, E. W., Bridgewater, L. & Association, A. P. H. Standard Methods for the Examination of Water and Wastewater. vol. 10 (American Public Health Association, 2012).
Patel, J., Maji, B., Moorthy, N. S. H. N. & Maiti, S. Xanthan gum derivatives: review of synthesis, properties and diverse applications. RSC Adv. 10, 27103–27136 (2020).
Gupta, N. V. & Shivakumar, H. G. Investigation of swelling behavior and mechanical properties of a pH-sensitive superporous hydrogel composite. Iran. J. Pharm. Res. IJPR. 11, 481 (2012).
Bilanovic, D., Starosvetsky, J. & Armon, R. H. Preparation of biodegradable xanthan–glycerol hydrogel, foam, film, aerogel and xerogel at room temperature. Carbohydr. Polym. 148, 243–250 (2016).
Malik, N. S. et al. Chitosan/xanthan gum based hydrogels as potential carrier for an antiviral drug: Fabrication, characterization, and safety evaluation. Front. Chem. 8, 50 (2020).
Osiro, D., Franco, R. W. A. & Colnago, L. A. Spectroscopic characterization of the exopolysaccharide of Xanthomonas axonopodis pv. citri in Cu2 + resistance mechanism. J. Braz Chem. Soc. 22, 1339–1345 (2011).
Said, M. et al. Modification of Xanthan gum for a high-temperature and high-salinity reservoir. Polym. (Basel). 13, 4212 (2021).
Sethi, S. et al. Cross-linked Xanthan gum–starch hydrogels as promising materials for controlled drug delivery. Cellulose 27, 4565–4589 (2020).
Kachel-Jakubowska, M., Matwijczuk, A. & Gagoś, M. Analysis of the physicochemical properties of post-manufacturing waste derived from production of Methyl esters from rapeseed oil. Int. Agrophys. 31, (2017).
Wang, T., Tan, Y., Chen, Y. Z. & Tan, C. Infrared spectral analysis for prediction of functional groups based on Feature-Aggregated deep learning. J. Chem. Inf. Model. 63, 4615–4622 (2023).
Saadatlou, G. A. & Pircheraghi, G. Concentrated regimes of xanthan-based hydrogels crosslinked with multifunctional crosslinkers. Carbohydr. Polym. Technol. Appl. 2, 100047. https://doi.org/10.1016/j.carpta.2021.100047 (2021).
ALOthman, Z. A. A. & Review Fundamental aspects of silicate mesoporous materials. Mater. (Basel). 5, 2874–2902 (2012).
Cychosz, K. A. & Thommes, M. Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 4, 559–566 (2018).
Slaughter, B. V., Khurshid, S. S., Fisher, O. Z. & Khademhosseini, A. Peppas, N. A. Hydrogels in regenerative medicine. Adv. Mater. 21, 3307–3329 (2009).
Yang, Y., Zhu, H., Tsai, Y., Bai, L. & Deng, J. Study on the thermal stability of thermosensitive hydrogel. Procedia Eng. 135, 501–509 (2016).
Bilanovic, D., Starosvetsky, J. & Armon, R. H. Cross-linking Xanthan and other compounds with glycerol. Food Hydrocoll. 44, 129–135 (2015).
Zhang, Q. et al. Synthesis and performance characterization of poly(vinyl alcohol)-xanthan gum composite hydrogel. React. Funct. Polym. 136, 34–43 (2019).
Gao, K. W., Xiaopeng, Y., Darling, R. M., Newman, J. & Balsara, N. P. Increased Donnan exclusion in charged polymer networks at high salt concentrations. Soft Matter. 2, 282–292 (2022).
Fu, F. & Wang, Q. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage. 92, 407–418 (2011).
Salunkhe, B. & Schuman, T. P. Super-adsorbent hydrogels for removal of methylene blue from aqueous solution: dye adsorption isotherms, kinetics, and thermodynamic properties. Macromol 1, 256–275 (2021).
Acknowledgements
This work was financially supported by the Materials and Energy Research Center (MERC) under the Postdoctoral Project No. 511402003 [Grant numbers 14036054 and 396589].
Author information
Authors and Affiliations
Contributions
M.M: Conceptualization, Formal analysis, Investigation, Writing—original draft, Writing—review & editing, Project administration. E.A: Supervision, Conceptualization, Methodology, Formal analysis, Investigation, Writing—review & editing, Validation. M.P: Project administration, Resources, Writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahinroosta, M., Abdollahzadeh Sharghi, E. & Pazouki, M. Sustainable xanthan-based superabsorbent hydrogel with enhanced durability for repeated nitrate remediation in drinking water. Sci Rep 16, 963 (2026). https://doi.org/10.1038/s41598-025-30421-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-30421-7