Abstract
Capparis Spinoza extract (CPS) was evaluated as a green corrosion inhibitor for carbon steel (CS) in 1 M hydrochloric acid (HCl) solution. Weight loss (WL) measurement, potentiodynamic polarization (PDP), and electrochemical impedance spectroscopy (EIS) techniques were employed to assess its efficacy. CPS demonstrated excellent inhibition efficiency (% IE) By increasing both concentration and temperature. % IE reached 90.6% at 25 °C, 300 ppm, but it reached 95.2% at 45 °C and at the same concentration. Adsorption isotherm analysis indicated Temkin-type behavior, suggesting both physical and chemical adsorption mechanisms, with the latter being predominant. Thermodynamic calculations (-ΔG°ads = -38.1 kJ mol− 1 at 25 °C) confirmed the spontaneous adsorption process, the stability of the formed adsorbed layer and shows that the CPS is mixed (physical and chemical) adsorbed on CS surface. PDP data classified CPS as a mixed-type inhibitor. EIS spectra exhibited characteristic inductive and capacitive loops at low and high frequencies, respectively. The formation of a protective CPS film on the CS surface was confirmed by atomic force microscopy (AFM) and Fourier transform infrared spectroscopy (FT-IR). The corrosion inhibition mechanism was elucidated through a combination of experimental and surface characterization techniques.
Similar content being viewed by others
Introduction
Corrosion is a slow, continuous, spontaneous process that occurs naturally”. Lately, “there have been significant financial losses, environmental harm”, and even potential concealed concerns to human safety as a result of metal corrosion1. “Thus, effectively preventing metal corrosion has long been the primary goal of concerned researchers. High carbon steel is a type of carbon steel that has excellent mechanical and flexible capabilities. It can be used to make wire products, plates, and body parts for automobiles. Sadly, mild steel rusted easily over extended periods of service due to exposure to harsh and complex environments2.
The authors selected hydrochloric acid (HCl) solution as the corrosive medium due to its widespread industrial relevance and aggressive corrosive nature. HCl is commonly used in acid cleaning, pickling of metals, descaling, and oil well acidizing operations, where metallic materials are frequently exposed to highly acidic environments3. Also, HCl, H2SO4 are particularly significant because they promote uniform and localized corrosion, allowing researchers to assess inhibitor efficiency, material resistance, and surface film stability under severe chemical stress.
The purpose of inhibitor is to stop CS corrosion. The inhibitors’ ability to establish an adsorbing layer on CS by physisorption or chemisorption is connected to their efficacy in inhibiting corrosion4. The kind of corrosive media, the chemical makeup of the inhibitors, and the kind and state of the metal surface all have an impact on the adsorption5,6. Good corrosion inhibitors are heterocyclic organic compounds with plenty of bonds and heteroatoms like O, N, and S that can be adsorbed on the metal surface7. The primary outcome of corrosion inhibition is the mitigation of potential hazards resulting from the thinnest metal in tanks and pipes which could cause material leak and catastrophic events like explosions and fires. Stated differently, the suppression of corrosion raises concerns regarding environmental protection and safety8. Plant extracts encompass a variety of organic compounds such as polyphenols, flavonoids, tannins, and alkaloids, which have established corrosion inhibition properties9,10,11,12,13. Numerous studies have explored the corrosion inhibition potential of plant extracts on metal and its alloys across different media. These extracts are typically derived from leaves, stems, seeds, or fruits of various plant species. Some commonly studied plant extracts for metal alloy corrosion inhibition include those from neem, aloe vera, green tea, mango leaves, and garlic14,15,16,17,18. By creating a barrier on the metal surface, plant extracts prevent corrosion by slowing down the corrosion process. The adsorption of phytochemicals onto the metal surface, which prevents corrosive substances from entering and stabilizes the passive oxide layer, may be one of the specific mechanisms behind inhibition19,20,21,22. Therefore, CPS extract is eco-friendly substance to inhibit the corrosion of CS in acidic media”.
Capparis spinosa, the caper bush, also called Flinders rose is a perennial plant that bears rounded, fleshy leaves and large white to pinkish-white flowers. Capparis spinosa (Caper) leaf extracts have been studied in Egypt for various medicinal properties, including antioxidant, anti-inflammatory, anti-diabetic, anti-cancer, and antimicrobial activities. The extracts are rich in bioactive compounds like flavonoids and phenolic acids, which are responsible for these beneficial effects (Figs. S1-S4 see supplementary file)). Studies have focused on the anti-carcinogenic properties of Caper leaf extracts against specific cell lines and their potential use for gastrointestinal issues. The below Table 1 shows list of extract sources used for corrosion inhibition of metals in different media.
The novelty statement for a green inhibitor (such as a CPS extract) is the claim that the study closes a knowledge gap by offering a new, environmentally friendly, and efficient solution and/or by offering previously unheard-of, comprehensive information on its protective mechanism, thus contributing both theoretically and practically to sustainable corrosion control.
The present study offers an advantage by providing a detailed investigation of corrosion behavior in HCl medium, emphasizing the effectiveness of the proposed protection method under realistic acidic conditions using different methods [weight loss (WL) method, (PDP), and (EIS)]. This not only contributes to understanding the underlying corrosion mechanisms but also supports the development of more durable and sustainable corrosion control strategies for industrial applications.

Experimental techniques
Materials and solutions
“The CS (1018) sheets which were used in this study had the following chemical composition, expressed as a weight%”: “0.0003 Silicon, 0.024 Phosphorus, 0.35 Manganese, 0.20 Carbon, and Fe the remaining percentage. By diluting the 37% hydrochloric acid utilizing bidistilled water, a 1 molar of the corrosive solution was created. A 1 cm by 1 cm work surface was the sole part of the CS electrodes that was not sealed with epoxy and submerged in the corrosive test solution (HCl 1.0 mol./L). The CS electrode was sequentially polished using the emery sheets (grades 250–1200) before to electrochemical tests”. After rinsing with bidistilled water, the polished CS was allowed to cool before being dried with cold air.
Preparation of CPS extract
After being cleaned with deionized water, the (CPS) were dried. CPS was subsequently ground into tiny fragments. “Ten grams of dried leaves were weighed and placed to a 150 mL conical flask. The mixture was then mixed with one hundred milliliters of 70% ethanol. After being soaked for 48 hrs at 25 °C, the mixture was shaken for four hours at 25°C. After filtering the mixture through filter paper, it was kept at 0–5°C for later testing. Following the filtering process, the CPS extract was vacuum-concentrated to a precise volume of around 100 mL, and the extract dose was determined. A 1000 ppm stock solution was made by mixing one gram of the extract with one thousand milliliters of bi-distilled water. The extract used in the study was prepared by diluting CPS extract with bidistilled water, and its dose varied from 50 to 300 ppm”. The literature on the chemical components of CPS extract indicates that ferulic acid (c), p-coumaric acid (b), Syring aldehyde31. The toxicity of CPS extract is generally low under normal conditions. Capparis spinosa extract contains a diverse array of bioactive compounds, including flavonoids, alkaloids and other components like tocopherols, carotenoids, and various phenolic acids. These compounds are found in different parts of the plant, including the flowers, fruits, leaves, stems, and roots, and contribute to its various medicinal properties, such as antioxidant, anti-inflammatory, and antimicrobial effects. Quercetin, part of a subclass of flavonoids called flavonols, has received considerable attention because of its.
beneficial impact on health. Its biochemical activity is well documented. It is one of the most potent antioxidants among polyphenols32. Quercetin has also been to demonstrate to display the antiviral, antibacterial, anticarcinogenic and anti-inflammatory effects33. Also, is a strong antioxidant due to its ability to scavenge free radicals and bind transition metal ions. These properties of quercetin allow it to inhibit lipid peroxidation34. Its structural formula is presented in Scheme 1.

Scheme 1: List of some phytochemical constituents isolated from CPS extract
WL experiment
The CS coupons used in this WL method were established in accordance with the ASTM G 31–7235 standard. “The CS samples sized at 2 cm x 2 cm x 0.01 cm, the CS specimens underwent mechanical polishing and abrading emery sheets with a grade range of 250–1200. After being cleaned utilizing bi-distilled water and acetone, they were dried using filter sheets in accordance with standard procedure. The specimens were weighted and then immersed in 100 mL solution in 150 mL beakers with varying concentrations of CPS extract and 1 M HCl at temperatures between 25 and 45°C. The CS specimens were removed from the solution, cleaned, dried, and weighed again after different durations” (30 to 180 min)36. All experiments were carried out in stagnant aerated solutions. The following equation was used to determine the metal sample’s corrosion rate (CR)37:
where A (cm2) is the area of the specimen, t (min) is the time of immersion, and ∆W (mg) is the weight reduction. The surface coverage (Ɵ) and inhibition effectiveness (% IE) can be derived as described below38:
where ∆W1 represents WL while the extract is present and ∆W2 represents WL when it is not.
Electrochemical measurements
“The Gamry device cell (PCI4/750). Potentiostat./Galvanostat./ZRA comprises three electrodes”: “a SCE reference electrode, the counter electrode, which is a platinum wire, and a CS serving as the working electrode. A 1 × 1 centimeter square-shaped sample was connected electrically using a copper wire weld. It was then placed inside a Teflon tube and secured with an adhesive to function as a working electrode. Similar to the WL method, the electrode’s surface underwent treatment. Every electrochemical measurement was run at 25°C. The testing was started after allowing the CS to stabilize for 30 min prior to measurement. Tafel curves were developed using PDP analysis, applying potentials between − 1.5 and 0.5V versus OCP with sweep rate of 0.2 mVs[─ 1. Using the Tafel extrapolation method, the values of the corrosion potential (Ecorr) and corrosion current density (icorr) were calculated”. The PDP test yielded the following values for ʔ and % IE, as indicated below39:
“where icorr(inh) represents the density of the corrosion current while the extract is present and icorr(free) represents the density of the corrosion current when it is absent. The equation Ɵ = [1 – (icorr(inh)/icorr(free))] is derived from the postulate that the inhibitor operates via a blocking mechanism. The EIS tests were run at the OCP over the frequency range 105 – 10− 2 Hz with a signal amplitude perturbation of 10 mV”. Below is the equation used to determine the values of θ and % IE from EIS tests40:
where the charge transfer resistance in the blank solution is denoted by Rct(free), and the charge transfer resistance in the inhibited solution by Rct(inh). To determine the impedance parameters Rct and Cdl, the impedance data was fitted to a suitable equivalent circuit. All tests were conducted in air-exposed stationary solutions. We conducted experiments in triplicate to ensure the reliability of our results.
Surface morphology
AFM examination
An effective method for examining the form of metal surfaces at the nano and micro scales is the AFM technique. This analysis’s ability to measure surface roughness is its main benefit. The 2 × 2 cm CS specimen was prepared as previously described in the WL method, and it was then submerged in 1.0 M HCl for three hours with and without a 300 ppm extract from CPS. The Japanese-made Shimadzu Wet-SPM (Scanning Probe Microscope) type was used for AFM testing.
FT-IR spectroscopy analysis
FT-IR investigations were carried out to determine the spectrum inhibitors structure that produces peaks with values in order to “learn more about the functional groups” of the extract both before and after its adsorption on CS. Following the WL technique, the 2 × 2 cm CS specimen was prepared, immersed for 3 h in 1 M HCl containing 300 ppm CPS extract and then directly investigated utilizing FT-IR spectra by (ATR-IR Affinity-1, Shimadzu, Japan).
Results and discussion
WL measurements
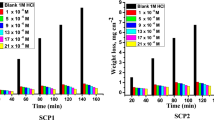
“The effect of adding varying amounts (50–300 ppm) of CPS extract on CS corrosion in a 1.0 M HCl solution was evaluated utilizing the WL method”. “The association between the impact of various doses of CPS extract on CS surface WL- time curves at 25 °C was depicted in Fig. 1. It is evident that the WL of CS with the extract present was significantly lower than the WL in the free extract solution. As the extract dose grow, the layer created by the extract molecules adsorbed on the metal surface causes the WL to drop. This layer protects the metal’s corrosive sites and keeps the metal from corroding in the corrosive environment. The WL-time curves are roughly linear, signifying that the CS surface is free of oxide films41. Exceeding the optimal extract dose of 300 ppm results in solution saturation. Additionally, the possibility exists of powerful interactions between extract molecules anchored to the metal and those within the solution, as reported in the literature42 This interaction may induce desorption of the adsorbed extract layer into the solution”.
Temperature effect
Using WL tests, the impact of different temperatures (25–45 °C) on “CS corrosion in 1.0 M HCl with and without varying extract doses was investigated”. “Table 2 displays the evaluations of CR and % IE derived from the WL tests for varying extract doses in 1 M HCl solution at altered temperatures. The findings showed that a rise in the dose of the extract leads to a reduction in CR value and a growth in % IE. This is an outcome of the extract’s growing degree of adsorption and subsequent covering of the CS surface as extract dose increases”. “The percentage of IE that increases as temperature rises shows that the extract may be chemisorbed on the CS surface”43.
“Using the Arrhenius relationship, we can estimate the energy of activation (Ea*) as follows”44:
A is the pre-exponential multiplier. As displayed in Fig. 2, “the log C.R against 1/T plots attendance and without doses of CPS extract produced straight lines with slopes from which the Ea* values were obtained”. “The next equation was used to calculate ΔH* and ΔS* of activation for the corrosion of CS[“ 45:
“Plot (1/T) vs. log (CR/T) produced linear graphs (Fig. 3) and from their slopes and intercepts”, “the values of ΔH* and ΔS* were obtained, respectively. Table 3 contains the computed values for Ea*, ΔH*, and ΔS*, both with and without the extract present. The results in Table 3 indicates that the values of Ea*of the molecules adhered to the metal surface through chemical adsorption. It is suggested that disorder arises when the reactant changes into the activated complex due to the negative ΔS* values, which showed that an association phase rather than a dissociation phase is included in the activated complex within the rate-limiting step46. The positive ΔH values indicate that the adsorption of CPS molecules onto the CS surface is endothermic. A decrease in ΔH* in the presence of the extract compared to the blank solution suggests a lower energy barrier for the corrosion reaction”. The close agreement between Ea* and ΔH* values (differing by approximately RT) supports a single-step, single-molecule dissolution mechanism for CS in the acidic medium47.
Isotherms of adsorption
The information required to comprehend the corrosion mechanism was obtained by the analysis of adsorption isotherms. “The Temkin adsorption isotherm equation (Eq. 7)48 is the most appropriate isotherm to fit the data after applying the different adsorption isotherms.” This isotherm demonstrates that the part of the CS surface that is protected by the inhibitor, θ, is determined by the inhibitor dose, Cinh.
Kads represents the equilibrium constant for adsorption in the adsorption–desorption process. Three presumptions underpinned the Temkin adsorption isotherm: (i) a single layer of the adsorbate was adsorbed at a predetermined number of adsorption locations; (ii) there was no lateral interaction or steric hindrance among the adsorbed molecules; and (iii) all adsorption points on the adsorbent had constant activation energy, adsorption, and enthalpy. Temkin isotherm diagrams at different temperatures result in straight lines with R2 almost equal to unity, as seen in Fig. 4. The adsorption’s free energy (ΔGoads)49 values were obtained using the following equation:
where 55.5 is the dose of water, in mol/L, at the metal/solution interface. Adsorption free energy (ΔG°ads) values were calculated, considering water concentration (55.5 mol/L) at the metal/solution interface”. The negative ΔG°ads values in Table 4 indicate spontaneous and stable adsorption of the extract onto the CS surface. Based on general guidelines, adsorption is considered physical for values around − 20 kJ/mol and chemical for values below − 40 kJ/mol. The calculated ΔG°ads values between − 38.1 and − 49.3 kJ/mol suggest a predominantly chemisorptive mechanism50. Enthalpy of adsorption (ΔH°ads) was determined using51:
“Plots log Kads vs. 1/T for dissolving of CS in a 1 M HCl solution contains CPS is displayed in” Fig. 5. show straight line with regression constant R2 = 0.9139 “offerings positive ΔHoads data, suggesting an endothermic adsorption process”. “This elucidates the reason for the experimental results where the IE rises with temperature”. Finally, the entropy of adsorption (ΔSoads) can be found using the following formula25:
The negative signs of ΔS° indicate reduced disorder upon transition from the reactant to the adsorbed metal. This supports the potency and adsorption power of extract.
Electrochemical measurements
PDP curves
PDP curves were obtained for carbon steel (CS) in 1 M HCl solution with and without various concentrations of CPS extract at 25 °C (Fig. 6). “A shift of both anodic and cathodic curves to more positive and negative potentials, respectively, indicates the mixed-type inhibitory behavior of the extract. Corrosion current density (icorr) and corrosion potential (Ecorr) values were determined from the extrapolation of the linear portions of the polarization curves. These data were subsequently used to investigate the adsorption mechanism. The corrosion rate will be computed using these parameters. The results of electrochemical parameters at different CPS extract doses were recorded in Table 5. The addition of 300 ppm produced the best inhibitory efficiency value of 79.7% (Table 5). “This finding suggests that raising the dose to 300 ppm will effectively slow down the step of CS corrosion”. “The connection between the rise in inhibition efficiency and the fall in current density is linear”. “The adsorption mechanism was examined using the PDP measurement findings”. The anodic (βa) and cathodic (βc) Tafel slope values are essentially unchanged, as demonstrated in Table 5, resulting in an almost parallel collection of cathodic and anodic lines. Consequently, in these solutions, the adsorbed extract molecules decrease the surface area susceptible to corrosion without affecting the underlying corrosion mechanism of CS52,53. Conversely, the Ecorr data does not show any regular displacement pattern, indicating that the CPS extract functions as a mixed-type inhibitor. According to multiple studies54, the behavior of CPS extract as anodic, cathodic, or mixed-type inhibitor depends on the magnitude of Ecorr displacement relative to the blank”. The extract has an anodic or cathodic function if this displacement beats 85 mV. However, when the displacement is less than 85 mV, as in our case (27 mV), the extract exhibits mixed-type inhibition characteristics. As shown in Table 5, icorr dropped from 205 to 47.4 mA/cm2 and the CR altered from 93.8 to 21.8 mpy in the existence of 300 ppm of the extract. It’s owed to the molecules of the extract covering a great portion of the metal surface, impeding its corrosion”. A comparison of PDP curves with and without varying doses of CPS extract reveals that increasing the extract’s dose shifts the Ecorr towards less negative values and diminishes the anodic process55.
EIS measurements
EIS was employed to investigate the kinetics and surface properties of the carbon steel (CS) electrode. Nyquist and Bode plots obtained at 25 °C (Figs. 7 and 8) illustrate the behavior of CS in 1.0 M HCl solution in the absence and presence of various CPS extract concentrations. “EIS parameters, including double-layer capacitance (Cdl) and charge transfer resistance (Rct), were extracted from the data and are summarized in Table 6. The increasing diameter of the semicircle in the Nyquist plots (Fig. 7) with increasing extract concentration indicates that the corrosion process is predominantly controlled by charge transfer. The semicircular Nyquist plot form (Fig. 7) indicates the roughness and inhomogeneity properties of the electrode surface. “Moreover, the semicircle at the top of the frequency area is related to the time constant, which is also connected to CPE (Y0 is its magnitude) which is considered a surface irregularity of the electrode and ct. The values of Y0 decrease as dl by improving doses of extract. The analogous circuit model (Fig. 9) shows a general instance of the frequency response of an interface defined by charge transfer and diffusion processes. The development of a protective layer on the surface of CS is responsible for the increase in log (Z) values for varying inhibitor concentrations in the Bode diagram (Fig. 8), which also shows a drop in frequency values. However, when compared to the blank (40o), the phase angle values rise to a more negative value of roughly 60o for an ideal concentration of 300 ppm, suggesting that the tested inhibitor is effective. Table 6 illustrates that when extract dose rises, Rct values rise and Cdl values drop. This is explained by water molecules exchanging with extract-adsorbed molecules and/or thicken the double layer”. Higher Rct values with increasing CPS extract doses are consistent with superior resistance to electrochemical corrosion at elevated extract doses. But when the extract is added to 1.0 M HCl, the “n” values decrease (from 0.985 to 0.977) in comparison to the results obtained in the reference electrolyte (0.991). This means that the “n” value lessens with the increase in extract dose, suggesting that the extract does not adsorb onto the CS surface uniformly, hence the surface is comparatively homogeneous56,57. The precision of the fitting results was assessed utilizing the chi-squared method”. “The achieved results for all outcomes reveal that the fitted results strongly concur with the experimental data, as indicated by the modest chi-squared values” (Table 6). “This method’s 83.7% inhibitory efficiency (Table 6) is in good accord with the PDP method”.
Examining the surface
AFM tests
When discussing the inhibitory effect on the metal/solution interface, “AFM has proven to be a useful instrument for estimating surface morphology investigations58. An essential test for determining a metal’s surface roughness at the maximum resolution in nanometer fraction is AFM. This method can provide information about the metal surface’s shape, which is helpful for studies on corrosion science. Figure 10 displays three-dimensional AFM pictures. In contrast, the CS sample treated with 300 ppm CPS has low roughness (147 nm) and a smoother surface when compared to the blank sample”. This is significant because the CPS adsorbed on the surface of the CS metal creating a protective film59. The average roughness (Ra) shows that the metal surface after being submerged in 1.0 M HCl is destroyed and the roughness rises (581 nm) as in Table 7.
FT-IR analysis
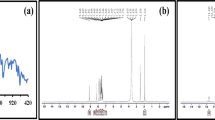
FT-IR spectroscopy was employed to identify functional groups involved in the formation of the protective layer on the metal surface. Comparative spectra of pure CPS extract and extract adsorbed on the CS surface (Fig. 11) revealed key functional groups. A broad band at 3272 cm⁻¹ indicated the presence of O-H, C-H, and N-H groups, while bands at 2905 cm⁻¹ and 2798 cm⁻¹ corresponded to C-H stretching vibrations. The peak at 1262 cm⁻¹ was attributed to C = O and C-O (acidic) groups, and the band at 1067 cm⁻¹ was assigned to C-O stretching. A shift in peak positions between the spectra of pure and adsorbed extract suggests the involvement of these functional groups in the interaction between CPS and the CS surface60.
Theoretical calculations
Quantum chemical parameters
It was found that the content of quercetin varied from 1.7 mg/g to 12.8 mg/g among different parts of caper. So, we try to calculate the DFT and MC for this main component in caper extract (see Fig. S2 and Fig S3) in the supplementary files. According to the DMol3 module built within the Materials Studio version 7.0 software, quantum chemistry was used for all calculations in the current study. “Fig. 12 displays the examination of the inhibitors’ optimal geometry, Molecular electrostatic potential (MEP) map, highest occupied molecular orbital EHOMO denotes the ability of the molecule to donate electron, whereas ELUMO describes the ability of the molecule to accept electron. Thus, the highest value of EHOMO refer to a major affinity for the donation of electrons to unoccupied molecular orbital that was d-orbital of iron atoms and lowest unoccupied molecular orbital (LUMO) density distributions. HOMO and LUMO can determine the donation-acceptance capacity and the molecular reactivity of the inhibitor. The inhibition efficiency increases with an increase in EHOMO values along with a decrease in ELUMO values. The increasing values of EHOMO imply a superior tendency to donate electrons to the molecule with empty orbitals. The dipolar moment (µ) is a measure of the polarity with the covalent bond. The energy band gap ΔEg was defined as: ΔE = EHOMO − ELUMO. A smaller ΔE value indicates greater molecular reactivity. Generally, a smaller energy gap means a molecule can more easily donate or accept electrons, which facilitates stronger adsorption onto the metal. Therefore, a lower energy gap value corresponds to a highly reactive molecule with good corrosion inhibition efficiency on the metal surface61. Table 8 displays the calculated dipole moments (µ), which indicate the polarity of the covalent bonds within the studied compounds. The higher µ value of inhibitor (15.94 Debye) suggests greater asymmetry in its charge distribution. This larger dipole moment increases inhibitor polarity, leading to stronger electrostatic interactions between the inhibitor and the charged CS surface in acidic HCl media.
Monte Carlo (MC) simulation
The side and top observations of the most suitable adsorption formations for the “inhibitor tested on CS surface obtained from the adsorption locator module are thus shown in Fig. 13. Adsorption energy is characterized as declining energy, when materials are mixed during the adsorption process in which an electron, ion, or molecule (adsorbent) is bound to the solid surface. Table 9shows that inhibitor has a greater adsorption energy, indicating that unused inhibitor will heavily adsorb on the toughened surface of CS to form adsorbed stable layers that will prevent corrosion62.”.
This configuration, leading to greater surface coverage than other orientations, improves the overall inhibition efficiency (η). These findings support previous research in this study, which indicates that improved surface coverage results in more effective corrosion protection. Table 9 quantitatively illustrates this interaction. The most favorable adsorption interactions are associated with the lowest adsorption energies, which correspond to the most stable configurations observed in the system. The adsorption energy of inhibitor is calculated as 1566.677 kJ mol− 1, led to interact strongly with the surface63. The greater magnitude of adsorption energies in the aqueous phase indicates stronger adsorption interactions compared to the gas phase, as solvation effects in water enhance the stabilization of the inhibitor-metal complex. The results demonstrated that inhibitor with its higher dipole moment, lower HOMO-LUMO gap, and greater electron-donating ability, exhibits superior adsorption and interaction with the Fe (1 1 0) surface. This is further supported by the adsorption energies obtained from Monte Carlo simulations,
Mechanism of corrosion Inhibition
Based on electrochemical and WL studies, “it was found that the examined extract works by adsorbing on the metal surface in accordance with the Temkin isotherm, providing strong protection against corrosion of CS in a 1.0 M HCl solution. The co-adsorption of cationic species and Cl−ions may account for the excellent inhibitive qualities of the CPS extract in HCl. Chemisorption and physisorption are the two types of coupled adsorption that are feasible. Both electrically charged metal surfaces and charged species in solution are necessary for physisorption to occur. The electric field’s metal surface charge as it appears at the metal/solution interface. On the other hand, in a chemisorption process, inhibitor molecules may transfer or charge-charge to the metal surface in order to form a coordination bond. It is possible to accomplish this when both positive and negative charges are present on the metal surface. Two different types of inhibitory mechanisms have frequently been proposed. Kind 1: the point where Cl− ions have previously adsorbed on the metal surface and extract cations are adsorbed there. In the presence of Cl− ions, extract cations may be able to adsorb due to the creation of an intermediate bridge and the negative ends of the halide metal dipoles facing the solution, which increase the adsorption of the extract cations onto the dipoles64,65. This would have the beneficial synergistic effect. This is an example of physisorption for the extract cations on the surface of the chloride bridge produced on the CS. kind 2: A large extract cations molecules is present at large extract dose. As a result, Cl− ions may face competition from cationic species for adsorption sites on the CS surface”. In this case, inhibitor species are adsorbed as a result of interactions between donors (π-electrons delocalized within the rings) and recipients (Vacant low-energy d-orbitals of the surface atoms of Fe). The electron density of the rings is greatly increased by the oxygen groups’ capacity to donate electrons66. Figure 14 represents the mechanism of inhibition.
Conclusions
The following conclusions can be drawn from the previous discussion:
-
1.
1. The results of the study showed that the inhibition process rose with increasing both the concentrations of the extract and the temperature of the medium.
-
2.
2.A maximum inhibition performance of 95.2% was shown by the extract at concentration 300 ppm in 1 M HCl and at 45 °C.
-
3.
3.“The adsorption of CPS extract on the CS surface was mainly chemical adsorption and obeyed.
by the Temkin adsorption isotherm.”
-
4.
4. According to the results of the PDP tests, the CPS extract is categorized as a mixed type inhibitor.
-
5.
5. The results of EIS showed that the decrease of the Cdl values and the increase of the Rct when the concentrations of the CPS was added. That is due to the adsorption of CPS molecules on the metal surface.
-
6.
6. FTIR spectra provide evidence of the interaction of the interaction between Fe2+ and the CPS tested.
-
7.
7. AFM and SEM-EDX analysis surface studies showed that a protective layer of CPS extract covers the CS surface.
-
8.
8. What was interesting was that the results in all the methods used were quite consistent with each other.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhang, M. et al. Akebia trifoliate Koiaz peels extract as environmentally benign corrosion inhibitor for mild steel in HCl solutions: integrated experimental and theoretical investigations. J. Ind. Eng. Chem. 101, 227–236 (2021).
Luo, H. et al. A strong and ductile medium-entropy alloy resists hydrogen embrittlement and corrosion. Nat. Commun. 11 (1), 3081 (2020).
Mehdaoui, R. et al. Corrosion Inhibition of carbon steel in hydrochloric acid solution by some synthesized surfactants from petroleum fractions. Res. Chem. Intermed. 42, 5509–5526 (2016).
Eddy, N. O., Momoh-Yahaya, H. & Oguzie, E. E. Theoretical and experimental studies on the corrosion Inhibition potentials of some purines for aluminum in 0.1 M HCl. J. Adv. Res. 6 (2), 203–217 (2015).
Meresht, E. S., Farahani, T. S. & Neshati, J. 2-Butyne-1, 4-diol as a novel corrosion inhibitor for API X65 steel pipeline in carbonate/bicarbonate solution. Corros. Sci. 54, 36–44 (2012).
Gopiraman, M., Selvakumaran, N., Kesavan, D. & Karvembu, R. Adsorption and corrosion Inhibition behaviour of N-(phenylcarbamothioyl) benzamide on mild steel in acidic medium. Prog. Org. Coat. 73 (1), 104–111 (2012).
Ali, S. A., Saeed, M. T. & Rahman, S. U. The isoxazolidines: a new class of corrosion inhibitors of mild steel in acidic medium. Corros. Sci. 45 (2), 253–266 (2003).
Wang, L. Evaluation of 2-mercaptobenzimidazole as corrosion inhibitor for mild steel in phosphoric acid. Corros Sci. 43 (12), 2281–2289 (2001).
Wibowo, A. H., Listiyaningrum, L., Firdaus, M., Widjonarko, D. M. & Storz, H. An improvement of polypropylene Itaconate base coating by simultaneous condensation of tetraethyl orthosilicate. Prog. Org. Coat. 125, 119–127 (2018).
Hembram, K. P. S. S. & Rao, G. M. Properties of zirconia thin films prepared by reactive Magnetron sputtering. Mater. Lett. 61 (2), 502–505 (2007).
Zakeri, A., Bahmani, E. & Aghdam, A. S. R. Plant extracts as sustainable and green corrosion inhibitors for protection of ferrous metals in corrosive media: A mini review. Corros. Commun. 5, 25–38 (2022).
Ayuba, A. M., Auta, M. A. & Shehu, N. U. Experimental and computational studies of vitellaria paradoxa extract as al corrosion inhibitor in acidic and alkaline media. RHAZES: Green. Appl. Chem. 13, 66–86 (2021).
Muhammad, A. A., Auta, M. A. & Shehu, N. U. Comparative study of the inhibitive properties of ethanolic extract of Gmelina Arboreaon corrosion of al in different media. Applied J. Environ. Eng. Science, 6(4),6 – 4(2020).
Jyothi, S., Rao, Y. S. & Ratnakumar, P. S. Natural product as corrosion inhibitors in various corrosive media: a review. Rasayan J. Chem. 12 (2), 537–544 (2019).
Eddy, N. O., Awe, F. & Ebenso, E. E. Adsorption and inhibitive properties of ethanol extracts of leaves of solanum melongena for the corrosion of mild steel in 0.1 M HCl. Int. J. Electrochem. Sci. 5 (12), 1996–2011 (2010).
Lamaka, S. V., Zheludkevich, M. L., Yasakau, K. A., Montemor, M. F. & Ferreira, M. G. High effective organic corrosion inhibitors for 2024 al alloy. Electrochim. Acta. 52 (25), 7231–7247 (2007).
Oguzie, E. E. March. Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. NACE Corros. NACE-10149, NACE (2010).
Umoren, S. A., Gasem, Z. M. & Obot, I. B. Natural products for material protection: Inhibition of mild steel corrosion by date palm seed extracts in acidic media. Ind. Eng. Chem. Res. 52 (42), 14855–14865 (2013).
Alrefaee, S. H., Rhee, K. Y., Verma, C., Quraishi, M. A. & Ebenso, E. E. Challenges and advantages of using plant extract as inhibitors in modern corrosion Inhibition systems: recent advancements. J. Mol. Liq. 321, 114666 (2021).
González-Parra, J. R. & Di Turo, F. The Use of Plant Extracts as Sustainable Corrosion Inhibitors for Cultural Heritage Alloys: A Mini-Review. Sustainability, 16(5), 868 (2024). (2024).
Dehghani, A., Ghahremani, P., Mostafatabar, A. H. & Ramezanzadeh, B. Plant extracts: probable alternatives for traditional inhibitors for controlling alloys corrosion against acidic media—A review. Biomass Convers. Biorefinery. 14 (6), 7467–7486 (2024).
Pourmohseni, M., Rashidi, A. & Karimkhani, M. Preparation of corrosion inhibitor from natural plant for mild stil immersed in an acidic environmental: experimental and theoretical study. Sci. Rep. 14 (1), 7937 (2024).
Badreah, A., Al Jahdaly Badriah, A., Al & Jahdaly Action of the capparis spinoza leaves extract as a green corrosion inhibitor for high carbon steel in acidic solution. Research Square, (2023).
Fadel edian, A., Al-Qudah, Ghassab, M. & Al-Mazaideh Corrosion Inhibition of copper by capparis spinosa L. Extract in strong acidic medium: experimental and density functional theory. Int. J. Electrochem. Sci. 12, 4664–4676 (2017).
Mouden, O. I. E. et al. Inhibitive action of capparis spinosa extract on the corrosion of carbon steel in aqueous medium of hydrochloric acid. J. Mineral. Metal Mater. Eng. 1, 1–7 (2015).
Fadel Wedian, Mahmoud, A., Al-Qudaha, Amad, N. & Abu-Baker The Effect of Capparis spinosa L. Extract as a Green Inhibitor on the Corrosion Rate of Copper in a Strong Alkaline (2016).
Chraka, A., Hadri, E. & Mustapha, Raissouni, I. Lignin (G/S type) as a sustainable biopolymer corrosion inhibitor for Cu-Zn alloy in sodium chloride: Extraction, characterization, electrochemical analysis, Ab initio calculations, and molecular dynamics simulations. Int. J. Biolog Macromole. 317 (1), 144822 (2025).
Mounir Manssouri, A. et al. Anti-Corrosion performance of Ruta graveolens essential oil as A green inhibitor for mild steel in 1 M hcl: evaluations of Electrochemical, DFT and Monte Carlo. Anal. Bioanal Electrochem. 16 (6), 507–536 (2024).
Chu, T. S., Mai, W. J., Li, H. Z., Wei, B. X. & Xu, Y. Q. Bo-Kai Liao, insights into the corrosion Inhibition performance of plant extracts of different genera in the Asteraceae family for Q235 steel in H2SO4 medium. Int. J. Mol. Sci. 26 (2), 561 (2025).
Abd El Aziz, S. et al. Carob fruit extract as naturally products corrosion inhibitor for copper-nickel alloys in Brine solutions. Sci. Rep. 14, 29290 (2024).
Aliyazicioglu, R., Eyupoglu, O. E., Sahin, H., Yildiz, O. & Baltas, N. Phenolic components, antioxidant activity, and mineral analysis of capparis spinosa L. African J. Biotechnology. 12 (47), 6643–6649 (2013).
Formica, J. F. & Regelson, W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Tox. 33, 1061–1080 (1995).
BHarborne, J. Williams Ch.A, advances in flavonoid research since 1992. Phytochemistry 55, 481–504 (2000).
Hollman, P. C. H. & Katan, M. B. A brief review of the impact of dietary polyphenols on. Cardiovasc. Disease Biomed. Pharmacother. 51, 305–310 (1997).
Standard Practice for. Laboratory Immersion Corrosion Testing of Metals, G31-72 401 (ASTM, 1990).
Basiony, E. L., Elgendy, N. M., Nady, A., Migahed, H., Zaki, E. G. & M. A. & Adsorption characteristics and Inhibition effect of two schiff base compounds on corrosion of mild steel in 0.5 M HCl solution: experimental, DFT studies, and Monte Carlo simulation. RSC Adv. 9, 10473–10485 (2019).
Barsoukov, E. & Macdonald, J. R. Impedance Spectroscopy, Theory, Experiment and Applications 2nd edn (Wiley Inter-science, 2005).
Chadili, M. et al. Corrosion Inhibition of 3003 al alloy in molar hydrochloric acid solution by Olive oil mill liquid by-product. Int. J. Corros. 7, 62395 (2021).
Kaczerewska, O. et al. Effectiveness of O-bridged cationic gemini surfactants as corrosion inhibitors for stainless steel in 3 M hcl: experimental and theoretical studies. J. Mol. Liq. 249, 1113–1124 (2018).
Lgaz, H., Salghi, R., Subrahmanya, K., Chaouiki, A., Jodeh, S. & Shubhalaxmi & Correlated experimental and theoretical study on Inhibition behavior of novel Quinoline derivatives for the corrosion of mild steel in hydrochloric acid solution. J. Mol. Liq. 244, 154–168 (2017).
Fekry, A. M. & Ameer, M. A. Electrochemical investigation on the corrosion and hydrogen evolution rate of mild steel in sulphuric acid solution. Int J. Hydrogen Energy. 36 (17), 11207–11215 (2011).
Serdaroğlu, G. & Kaya, S. Organic and inorganic corrosion inhibitors, in Organic Corrosion Inhibitors, Wiley, 59–73 (2021).
Fergachi, O. et al. Corrosion Inhibition of ordinary steel in 5.0 M HCl medium by benzimidazole derivatives: electrochemical, UV–visible spectrometry, and DFT calculations. J. Bio- Tribo-Corros. 5, 21–33 (2019).
Yaro, A. S., Wael, R. K. & Khadom, A. A. Reaction kinetics of corrosion of mild steel in phosphoric acid. J. Univ. Chem. Technol. Metall. 45 (4), 433–448 (2021).
Macdonald, J. R. & Johanson, W. B. in Theory in Impedance Spectroscopy. (eds Macdonald, J. R.) (John Wiley& Sons, 1987).
Heakal, F. E., Deyab, M. A., Osman, M. M., Nessimb, M. I. & Elkholy, A. E. Synthesis and assessment of new cationic gemini surfactants as inhibitors for carbon steel corrosion in oilfield water. RSC Adv. 7, 47335–47352 (2017).
Liu, H. et al. Synergistic effect of iodide ion and Methyl Violet on the corrosion Inhibition of carbon steel in phosphoric acid. Int. J. Electrochem. Sci. 13 (11), 10718–10732 (2018).
Samide, A. Tutunaru, quinine sulfate: a pharmaceutical product as effective inhibitor for carbon 908steel in HCl solution. Cent. Eur. Chem. 12 (9), 901–900 (2014).
Ferreira, K. C. R. et al. Corrosion Inhibition of carbon steel in HCl solution by aqueous brown onion Peel extract. Int. J. Electrochem. Sci. 11, 406–418 (2016).
Pardityana, A., Khosfirah, F., Santosa, P. I. & Wan Nik, W. B. Corrosion analysis of renewable inhibitor of citrus aurantifolia peels extract for API 5L grade B steel in acid solution. Int. J. Corros. Scale Inhib. 1 (1), 397–410 (2024).
Amin, M. A. et al. Monitoring corrosion and corrosion control of iron in HCl by non-ionic surfactants of the TRITON-X series – Part III. Immersion time effects and theoretical studies. Corros. Sci. 53 (5), 1895–1909 (2011).
Pradityana, A., Sulistijono, Winarto, Widiyono, E., Luwar, B. & Mursid, M. Effect of temperature on the application of Myrmecodia Pendans extract for environmentally friendly corrosion inhibitor. AIP Conf. Proc., 1840, 030001 (2017).
Eissa, M., Etaiw, S. H., El-Waseef, E. E., El-Hossiany, A. & Fouda, A. S. The impact of environmentally friendly supramolecular coordination polymers as carbon steel corrosion inhibitors in HCl solution: synthesis and characterization. Sci. Rep. 14 (1), 2413 (2024).
Laamari, M. R., Benzakour, J., Berrekhis, F., Derja, A. & Villemin, D. Adsorption and corrosion Inhibition of carbon steel in hydrochloric acid medium by hexamethylenediamine tetra (methylene phosphonic acid). Arab. J. Chem. 9 (1), S245–S251 (2016).
Dahdele, J., Danaee, I. & Rashed, G. R. Adsorption isotherm of N,N’-Bis(2,4,6-Trihydroxyacetophenone)-2,2-Dimethylpropandiimine as A corrosion inhibitor on Sa-210 steel in alkaline NaCl Solution.J. Chil. Chem. Soc. 61, 3025–3030 (2016).
Lebrini, M., Robert, F. & Blaninieres, P. A. Corrosion inhibition by Isertia coccinea plant extract in HCl solution, Int. J. Electrochem. Sci., 6 2443–2460 (2011).
Magar, H. S., Hassan, R. Y. A. & Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sens. (Basel). 21 (19), 6578 (2021).
Ali, I. H. & Suleiman, M. H. A. Effect of acid extract of leaves of Juniperus procera on corrosion Inhibition of carbon steel in HCl solutions. Int. J. Electrochem. Sci. 13 (4), 3910–3922 (2018).
Wang, M., Du, Z. & Gao, J. Electrochemical and surface analysis studies on corrosion Inhibition of Q235 steel by Imidazoline derivative against CO2 corrosion. Corros. Sci. 53, 354–368 (2011).
Fouda, A. S., Etaiw, S. E. H., El-Aziz, A., El-Hossiany, D. M., Elbaz, U. A. & A. A. & Experimental and theoretical studies of the efficiency of metal–organic frameworks (MOFs) in preventing aluminum corrosion in hydrochloric acid solution. BMC Chem. 18 (1), 21 (2024).
Ali, M. A., El-Hossiany, A. A., Ouf, A. M. & Fouda, A. S. Assessment of corrosion restraint effect of carbon steel immersed in hydrochloric acid by expired Tilmicosin drug. Sci. Rep. 15 (1), 37621 (2025).
Khaled, M. A., Ismail, M. A., El-Hossiany, A. A. & Fouda, A. S. Novel pyrimidine-bichalcophene derivatives as corrosion inhibitors for copper in 1 M nitric acid solution. RSC Adv. 11, 25314–25333 (2021).
Fouda, A. S., Abdel-Wahed, H. M., Atia, M. F. & El-Hossiany, A. Novel porphyrin derivatives as corrosion inhibitors for stainless steel 304 in acidic environment: synthesis, electrochemical and quantum calculation studies. Sci. Rep. 13, 17593 (2023).
Mo, S., Qin, T. T., Luo, H. Q. & Li, N. B. Insights into the corrosion Inhibition of copper in hydrochloric acid solution by self-assembled films of 4-octylphenol. RSC Adv. 5, 90542–90549 (2015).
Kumari, P. P., Shetty, P. & Rao, S. A. Electrochemical measurements for the corrosion Inhibition of mild steel in 1 M hydrochloric acid by using an aromatic Hydrazide derivative. Arab. J. Chem. 10 (5), 653–663 (2017).
Yadav, M., Gope, L., Kumari, N. & Yadav, P. Corrosion Inhibition performance of Pyranopyrazole derivatives for mild steel in HCl solution: Gravimetric, electrochemical and DFT studies. J. Mol. Liq. 216, 78–86 (2016).
Acknowledgements
All the authors are intended to the Mansoura University for the facilities provided during this research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding is provided by the Science, Technology, and Innovation Funding Authority (STDF) in cooperation with the Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
O. A. E. and M.E. E carried out the experimental part,, A. M. O and A.E prepared the Figures, A. S. F wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elgyar, O.A., Yassen, M.E., Ouf, A.M. et al. Insight into the anti-corrosion performance of capparis spinoza extract as a green corrosion inhibitor for carbon steel in hydrochloric acid environment. Sci Rep 15, 44137 (2025). https://doi.org/10.1038/s41598-025-30968-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-30968-5