Abstract
This study evaluates the independent correlation between inner cell mass (ICM) and trophectoderm (TE) grading with preimplantation genetic testing for aneuploidy (PGT-A) outcomes in embryos biopsied on Day 5 or Day 6 to determine whether morphology-based selection can serve as an alternative to PGT-A. A retrospective analysis of 1,292 embryos from patients undergoing IVF at a single academic center was conducted between October 2019 and March 2023. Blastocysts were graded using Society for Assisted Reproductive Technology (SART) morphology criteria, and biopsy results were categorized as euploid, aneuploid, mosaic, inconclusive, or low DNA. Maternal age (24–45 years) was analyzed both by SART age groups (< 35, 35–37, 38–40, 41–42, > 42) and in a multivariable logistic regression (euploid vs aneuploid) adjusting for age, biopsy day (Day 5 vs Day 6), inner cell mass (ICM), and trophectoderm (TE). Embryos with mosaic, inconclusive, or low-DNA results were excluded from inferential analyses. Statistical analysis was performed using the chi-square test (p < 0.05). Of the 1,292 embryos, 632 (48.9%) were euploid, 508 (39.3%) aneuploid, and 152 (11.8%) mosaic or inconclusive. Good ICM embryos had significantly higher euploidy rates than aneuploidy on both biopsy days (p < 0.001), while poor ICM embryos had higher aneuploidy rates (p < 0.001). Similarly, good TE embryos were more likely to be euploid than poor TE embryos (p < 0.001). Day 5 embryos had significantly higher euploidy rates than Day 6 embryos across all grading categories (p < 0.05). However, no statistical difference was found between Day 6 good ICM embryos and Day 5 fair ICM embryos (p = 0.1335), while Day 6 good TE embryos had higher euploidy than Day 5 fair TE embryos (p < 0.01). Euploidy declined stepwise across SART age groups. In the adjusted model, each additional year of maternal age after 35 lowered the odds of euploidy by ~ 6%. Both ICM and TE grades predict embryo euploidy, supporting the use of morphological assessment to optimize embryo selection and potentially reduce reliance on PGT-A.
Similar content being viewed by others
Introduction
The use of assisted reproductive technology (ART) has grown significantly over the past two decades, addressing the infertility challenges faced by millions of Americans. In 2021 alone, approximately 2.3% of infants born in the United States were conceived via ART, with in vitro fertilization (IVF) accounting for 99% of these procedures1. While various factors influence the success of IVF, the age of the patient and the quality of the transferred embryo are critical predictors. Poor-quality embryos have lower implantation potential, which is strongly associated with lower pregnancy rates2.
Currently, the standard practice for determining embryo quality involves morphological assessment. In the United States, this is commonly performed using the Society for Assisted Reproductive Technology (SART) grading method3. This method is applied to cleavage-stage embryos, morulae, and blastocysts. For blastocysts, the inner cell mass (ICM) and the outer cell mass, or trophectoderm (TE), are graded as good, fair, or poor based on morphological characteristics. This grading system has been shown to correlate strongly with successful implantation and live births in patients undergoing single blastocyst transfers4.
Chromosomal aneuploidy in embryos has been identified as a key factor contributing to implantation failure. Aneuploid embryos are thought to lack the necessary molecular interactions required for successful implantation into the endometrium5. To improve pregnancy rates per transfer, preimplantation genetic testing for aneuploidy (PGT-A) has become increasingly common in IVF cycles, enabling the selection of euploid embryos for transfer6. However, PGT-A has limitations. The procedure is costly, its effects on embryos remain uncertain, and it is prone to inaccuracies in cases of chromosomal mosaicism without significantly improving pregnancy rates, especially in women under the age of 35.7.
Given the invasive nature and unclear benefits of PGT-A, many clinics have reverted to relying on embryo morphology for selecting suitable embryos for transfer8. Presently, several studies have explored the relationship between TE morphology and euploidy, with results consistently indicating a higher rate of euploidy among embryos with superior morphological scores of the TE9,10. However, there is a notable lack of data specifically examining the correlation between ICM grading and euploid status.
The objective of this study is to evaluate whether an association exists between the grading of the ICM and TE and the PGT results of embryos biopsied on Day 5 or Day 6, and if this association could help in selecting embryos for transfer without resorting to PGT-A.
Material and methods
This retrospective cohort study analyzed embryos from patients undergoing IVF at a single academic center between October 2019 and March 2023. All patients underwent a standard ovarian stimulation protocol using the antagonist protocol. Oocyte retrieval was performed 34 to 36 h after the administration of a trigger using either hCG or Lupron. Mature oocytes were subjected to intracytoplasmic sperm injection (ICSI). Fertilized oocytes were cultured in benchtop incubators with single-step media until the blastocyst stage.
Embryo biopsy was performed on expanded blastocysts on Day 5 or Day 6. Only embryos that underwent PGT-A were included in the study. The age of the patients included in the study ranged from 24 to 45 years old. Patient information was anonymized before analysis.
Blastocysts were graded according to the SART embryo morphology grading criteria. Two experienced embryologists assessed the grades of the ICM and TE, classifying them into three groups: good, fair, and poor. TE biopsy for PGT-A was performed on Day 5 or Day 6 embryos. Biopsy results were categorized into five groups: euploid, aneuploid, mosaic, inconclusive, or low DNA.
Statistical analysis was conducted using the chi-square goodness-of-fit test. A p-value of < 0.05 was considered statistically significant. We analyzed PGT-A outcomes in two steps. First, results were summarized by SART maternal age groups (< 35, 35–37, 38–40, 41–42, > 42), both overall and separately for Day 5 and Day 6 biopsies. Second, we used multivariable logistic regression with euploid status (euploid vs aneuploid) as the outcome. Predictors in this model included maternal age (continuous), biopsy day (Day 6 vs Day 5), ICM grade, and TE grade. To explore whether age influenced embryo quality, we also performed ordinal logistic regression with morphology grade (poor < fair < good) as the outcome and age as the predictor. Embryos with mosaic, inconclusive, or low-DNA results were excluded from these inferential analyses but reported descriptively. Finally, we assessed the strength of the association between morphology and ploidy (euploid vs aneuploid) using chi-square tests and effect size (Cohen’s w). Given the large overall sample size, these associations were well powered despite smaller subgroup counts. Due to the retrospective nature of the study, the informed consent was waived by the University of Miami Institutional Review Board (IRB).
Results
A total of 1,292 embryos were biopsied during the study period, with 852 (65.9%) biopsied on Day 5 and 440 (34.1%) on Day 6. Among these biopsied embryos, 632 (48.9%) were euploid, 508 (39.3%) were aneuploid, and 152 (11.8%) were categorized as mosaic, inconclusive, or low DNA (Table 1). Age-stratified tables showed a stepwise decline in euploidy with increasing maternal age overall (Table 2) and within each biopsy day. This trend remained consistent when analyzed separately for Day 5 (Table 3) and Day 6 (Table 4) biopsies.
Inner cell mass (ICM) grading and PGT-A results
Table 5 highlights the PGT-A results stratified by ICM grade and biopsy day. For Day 5 biopsies, a statistically significant association was observed between ICM grade and PGT-A results. Embryos with a good ICM grade were more likely to be euploid (60.6%) compared to aneuploid (29.9%) (p-value < 0.001). Conversely, embryos with a poor ICM grade were more likely to be aneuploid (61.5%) compared to euploid (34.6%) (p-value 0.002). This trend was even more pronounced in embryos biopsied on Day 6. Embryos with good ICM grades exhibited 50.9% euploidy compared to 30.2% aneuploidy (p-value < 0.001), while poor ICM grades were associated with 63.8% aneuploidy compared to 18.8% euploidy (p-value < 0.001).
Trophectoderm (TE) grading and PGT-A results
Table 6 provides the PGT-A outcomes stratified by TE grade and biopsy day. A significant relationship was also identified between TE grade and PGT-A results. On Day 5, embryos with good TE grades were more likely to be euploid (62.1%) compared to aneuploid (27.7%) (p-value < 0.001), while embryos with poor TE grades had a 51.3% chance of aneuploidy and 46.2% chance of euploidy (p < 0.001). For embryos biopsied on Day 6, this association was even stronger. Embryos with good TE grades exhibited 56.8% euploidy compared to 28.4% aneuploidy (p-value < 0.001). In contrast, poor TE grades showed 67% aneuploidy compared to 20.9% euploidy (p < 0.001).
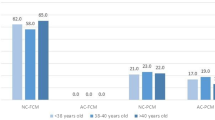
Day 5 vs. Day 6, euploid and aneuploid embryos, based on inner cell mass grade and trophectoderm grade
Within the same day of embryo biopsy, the percentage of euploid embryos declined as the ICM and TE grading moved from good to poor (p-value < 0.05), except for Day 5 fair vs. Day 5 poor ICM and TE grade embryos, where while there was a decline, it did not reach statistical difference threshold. This trend is visualized in Figs. 1 and 2, which illustrate the progressive decrease in euploidy rates as both ICM and TE quality decline across both biopsy days.
There was a statistical difference in the rate of euploidy between Day 6 embryos with a good TE grade compared to Day 5 embryos with a fair TE grade (p-value < 0.01). However, there was no statistical difference in the rate of euploidy between Day 6 good ICM grade embryos and Day 5 embryos with fair ICM grades (p = 0.1335). In addition, there was no statistical difference in euploid embryo percentage when comparing Day 5 embryos with poor ICM or TE grading and Day 6 embryos with fair ICM or TE grading (p = 0.6702 and p = 0.5943, respectively).
Multivariable model
In the adjusted logistic regression, maternal age after the age of 35 independently lowered the odds of euploidy (≈6% per additional year). In addition, embryo morphology declined with age using an ordinal logistic regression. The results showed that embryos were increasingly likely to have lower morphology grades for both ICM and TE by about 5–6% per year after the age of 35. This trend is also clearly seen in Table 7, where the proportion of “good” embryos declines and “fair” or “poor” grades increase across SART age groups.
Discussion
Assisted reproductive technology has become a cornerstone in addressing infertility, with IVF as its most utilized modality1. Embryo quality, assessed through morphological grading, remains a critical determinant of IVF outcomes3. As the integration of PGT-A becomes more prevalent, questions arise regarding its cost–benefit ratio, accuracy, and overall impact on clinical outcomes. This study addresses a gap in the literature by assessing the association between ICM and TE grading and PGT-A outcomes, a novel approach that dissects their individual predictive values.
Several studies have demonstrated a strong correlation between overall embryo grade and PGT-A results9,10. However, no prior research has analyzed the predictive value of ICM and TE grades separately.
The current findings highlight that both ICM and TE grades are equally predictive of euploidy, supporting the clinical utility of detailed morphological assessment. Embryos with good grades in either ICM or TE were significantly more likely to be euploid, while poor grades in either were associated with a higher prevalence of aneuploidy. This highlights that the quality of the ICM is as effective as the quality of the TE in predicting PGT outcomes.
Results from this study further emphasize that Day 5 embryos consistently had higher euploid rates across both ICM and TE grades compared to Day 6 embryos. Within the same day, significant differences were observed between good vs. fair grades and fair vs. poor grades (p < 0.05), except for Day 5 fair vs. Day 5 poor ICM and TE grades, which were not statistically significant. The absence of a significant difference could indicate that Day 5 embryos with “fair” and “poor” grades may share similar intrinsic developmental and chromosomal characteristics, as well as dynamic changes limiting their predictive distinction. Maternal age was strongly associated with both ploidy and morphology: euploidy fell progressively across SART age groups, and older age was linked to lower ICM/TE grades.
In embryos biopsied on Day 6, there was a higher euploidy rate in embryos with higher grades (good, fair) compared to Day 5 embryos with one grade lower (fair, poor). These differences did not reach statistical significance except for Day 6 embryos with a good TE grade compared to Day 5 embryos with a fair TE grade (p-value 0.011). Given these results, it seems prudent to prioritize transferring Day 5 or Day 6 embryos with good ICM or TE grading, followed by Day 5 fair/poor embryos, and lastly Day 6 embryos with fair/poor grading. This strategy would maximize the likelihood of euploid selection while potentially reducing reliance on invasive procedures like PGT-A. Furthermore, these results reinforce the superior predictive value of good morphology at earlier developmental stages and highlight that delayed development not only reduces the euploidy rate but also diminishes the predictive impact of morphological grading. Although this study did not correlate with pregnancy rates, live birth rates after elective single embryo transfer of a euploid embryo remain high across different age groups, often exceeding 50% (Practice Committee of the ASRM).
The invasiveness of PGT-A has been a point of contention. The biopsy procedure, though minimally invasive, carries risks of damaging embryos and negatively impacting implantation potential7. Additionally, PGT-A is associated with significant costs, often ranging from $4,000 to $7000 per cycle, excluding the baseline IVF expenses8. Considering these factors, it is crucial to evaluate whether the benefits of PGT-A—such as potentially higher live birth rates—justify its routine application.
While some studies claim that PGT-A can reduce the time to achieve a live birth by selecting euploid embryos for transfer6, others challenge this assertion, citing inconsistent evidence8. For instance, a systematic review by Gleicher et al.11 found that the purported benefits of PGT-A in reducing time to pregnancy were not universally observed, particularly in younger patients with a higher likelihood of producing euploid embryos. The debate over whether PGT-A is worth its risks remains unresolved, particularly given the lack of uniform improvements in clinical outcomes.
Emerging evidence points to the risks associated with PGT-A, including the potential for misdiagnosis due to chromosomal mosaicism. A study by Huang et al.12 found that up to 20% of embryos classified as mosaic based on PGT-A results could result in healthy live births if transferred. Furthermore, biopsy-related risks, including the loss of viable embryos, remain a concern13. These findings underscore the need for careful consideration of whether PGT-A should be universally applied.
The results of this study reveal a significant association between embryo morphology and PGT-A outcomes, emphasizing the predictive value of ICM and TE grades for chromosomal euploidy. Embryos graded as “good” for either ICM or TE were significantly more likely to be euploid, while those with “poor” grades showed a higher prevalence of aneuploidy. This association was consistent across both Day 5 and Day 6 biopsies, with Day 5 embryos exhibiting superior euploid rates compared to Day 6 embryos. The finding that ICM and TE grades have equivalent predictive value underscores the importance of evaluating both components separately rather than relying on an overall morphological score. These results contribute to the growing evidence supporting the utility of morphological assessment in embryo selection. Furthermore, the significant trends observed highlight that detailed grading can serve as a non-invasive alternative or complement to PGT-A, particularly in resource-limited or high-risk populations, thereby reducing the dependency on invasive procedures while maintaining high predictive accuracy for successful implantation.
This study’s major strength lies in its focus on the separate evaluation of ICM and TE grades, providing novel insights into their individual roles as predictors of PGT-A outcomes. The robust sample size of 1292 embryos enhances the statistical power of the findings, and the study’s use of the widely accepted SART grading criteria ensures methodological consistency.
Despite its strengths, the study is limited by its retrospective nature, which may introduce inherent biases related to patient selection and data collection. Additionally, the findings are restricted to a single academic center, which may limit generalizability. The dataset was de-identified and did not include patient-level demographics beyond maternal age and biopsy day. We acknowledge that the small size of some groups could have affected our conclusion. We believe future studies should incorporate multicenter designs and prospectively evaluate the relationship between ICM, TE, and euploidy rates.
Conclusion
The findings of this study highlight the predictive value of both ICM and TE grades for PGT-A results, emphasizing the clinical utility of detailed morphological assessment. While PGT-A offers potential benefits, its costs, risks, and inconsistent impact on time to pregnancy necessitate careful consideration. Prospective, randomized studies are needed to fully elucidate the role of PGT-A in improving IVF outcomes and to guide evidence-based clinical practice.
Data availability
The research data supporting the results of this manuscript are included in the supplementary file submitted alongside the manuscript.
References
Sunderam, S. et al. Assisted Reproductive Technology Surveillance - United States, 2018. MMWR Surveill. Summ. 71(4), 1–19 (2022).
Kirillova, A. et al. Should we transfer poor quality embryos?. Fertility Res. Pract. 6(1), 2 (2020).
Racowsky, C. et al. Standardization of grading embryo morphology. Fertil. Steril. 94(3), 1152–1153 (2010).
Heitmann, R. J. et al. The simplified SART embryo scoring system is highly correlated to implantation and live birth in single blastocyst transfers. J. Assist. Reprod. Genet. 30(4), 563–567 (2013).
Diedrich, K. et al. The role of the endometrium and embryo in human implantation. Hum. Reprod. Update 13(4), 365–377 (2007).
Hipp, H. S. et al. Trends and Outcomes for Preimplantation Genetic Testing in the United States, 2014–2018. JAMA 327(13), 1288–1290 (2022).
Zwingerman, R. & Langlois, S. Committee Opinion No 406: Prenatal testing after IVF with preimplantation genetic testing for aneuploidy. J. Obstet. Gynaecol. Can. 42(11), 1437-1443.e1 (2020).
Simopoulou, M. et al. PGT-A: who and when? Α systematic review and network meta-analysis of RCTs. J. Assist. Reprod. Genet. 38(8), 1939–1957 (2021).
Yoshida, I. H. et al. Can trophectoderm morphology act as a predictor for euploidy?. JBRA Assist. Reprod. 22(2), 113–115 (2018).
Greenwood, E. A. et al. Blastocyst morphology correlates with preimplantation genetic testing for aneuploidy (PGT-A) results and may further predict pregnancy potential after euploid single embryo transfer (SET). Fertil. Steril. 112(3), e152–e153 (2019).
Gleicher, N. et al. The futility of PGT-A for all patients. Reprod. Biomed. Online 42(5), 902–908 (2021).
Huang, J. et al. Mosaic embryos: risk or potential?. Hum. Reprod. 36(8), 2306–2315 (2021).
Minasi, M. G. et al. Aneuploidy testing: should we biopsy embryos?. Hum. Reprod. 31(6), 1270–1280 (2016).
Author information
Authors and Affiliations
Contributions
George Attia and Pasquale Patrizio conceived and designed the study. Patryk Piekos, Suset Rodriguez, and Joelle Mouhanna were responsible for data collection and analysis. Mohammed Ibrahim and Roberta Rubino performed the statistical analyses. Patryk Piekos drafted the manuscript, and all authors contributed to the interpretation of the data, critically revised the manuscript, and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was a retrospective analysis of de-identified patient data and did not involve direct patient interaction or intervention. Therefore, formal Institutional Review Board (IRB) approval was not required. All data were handled in compliance with ethical standards and institutional guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Piekos, P., Rodriguez, S., Mouhanna, J. et al. Independent inner cell mass and trophectoderm morphology as non-invasive predictors of embryo euploidy in IVF. Sci Rep 16, 3077 (2026). https://doi.org/10.1038/s41598-025-31167-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31167-y