Abstract
Despite the importance of oxygen evolution reaction (OER) catalysts in energy conversion applications, the time-resolved dynamics of their amorphous phases remain elusive. Understanding the structural evolution of OER catalysts is pivotal to improve their design and sustainability and increase the energy conversion efficiency. SrIrO3 is a promising OER catalyst for achieving high energy conversion efficiencies, but it has not been sufficiently characterized in terms of its phase dynamics during operation. Herein, in-situ total X-ray scattering measurements and pair distribution function analysis are used to probe time-resolved structural changes in an IrOx/SrIrO3 catalyst during OER, revealing the occurrence of persistent Ir–O bond shortening and dynamic structural interconversions between multiple crystalline phases and an amorphous component. The relative contents of the main crystalline phases—3C-SrIrO3 (Pnma) and 6H-SrIrO3 (C2/c)—exhibited synchronized changes, whereas those of an additional C2/c phase and the amorphous component showed opposite trends, suggesting active structural interplay among the phases during the reaction. Furthermore, the observed Ir–O bond shortening under applied potential was attributed to a structural change associated with increased oxidation state of Ir, which is closely related to the local environment at the rate-determining step of OER. Our findings underscore the importance of precisely controlling bond distances and structural dynamics at the atomic scale for enhancing the activity of OER catalysts and provide valuable insights for future catalyst design.
Similar content being viewed by others
Introduction
The decarbonization of the global energy system hinges on the development of efficient and sustainable energy conversion and storage technologies, such as electrochemical water splitting, which affords green hydrogen as a clean and versatile energy carrier. However, the anodic half-reaction of this process—the oxygen evolution reaction (OER)—involves complex proton-coupled electron transfer steps and high-energy intermediates, therefore featuring sluggish kinetics and remaining a critical bottleneck. Thus, highly active and durable OER catalysts are required to improve the overall energy conversion efficiency of electrochemical water splitting. Ir-based oxides, particularly IrOx and IrOx/SrIrO3 heterostructures, have emerged as state-of-the-art acidic OER electrocatalysts because of their high activity and corrosion resistance and are therefore indispensable for proton exchange membrane electrolyzers. The SrIrO3 perovskite serves as a conductive and structurally dynamic support that undergoes surface reconstruction upon anodic polarization to form catalytically active amorphous IrOx layers1,2,3,4,5. However, the scarcity and high cost of Ir necessitate the reduction of Ir content, development of alternative materials, and enhancement of the oxygen evolution efficiency.
To achieve these goals, one should understand the mechanisms of the processes occurring on the catalyst surface during the OER. In particular, the direct observation of atomic configurations and structural changes under operating conditions can provide valuable insights for rational catalyst design. Recent advances in operando X-ray absorption spectroscopy (XAS) have enabled the real-time monitoring of the electronic and structural evolution of OER catalysts. These techniques provide critical insights into oxidation state dynamics, coordination environments, and interatomic distances. For Ir-based catalysts, operando XAS has revealed dynamic changes in Ir–O and Ir–Ir interactions, oxidation state fluctuations, and the formation of reactive oxyhydroxide species under applied potentials. Although XAS is highly sensitive to the electronic state of atoms, the interpretation of the corresponding data often depends on structural models and averages, which can limit detailed structural insights6,7,8.
To overcome these limitations, we herein combine in-situ total synchrotron X-ray scattering measurements and pair distribution function (PDF) analysis to study IrOx/SrIrO3, a recently developed highly active OER catalyst9,10,11. Our approach enables the model-independent analysis of atomic pair correlations, as PDF analysis resolves short-range structural changes (particularly in amorphous or disordered phases often invisible to conventional diffraction techniques) and enables the direct observation of Ir–O bond length variations, providing insights into lattice distortion, oxygen incorporation, and the formation of catalytically relevant motifs. Therefore, our study investigates time-resolved changes in Ir–O bond distances obtained using total X-ray scattering measurements. These changes are especially valuable for studying the dynamic restructuring of IrOx/SrIrO3 during the OER, as the interplay between crystalline and amorphous domains plays a critical role in determining catalytic performance. Recent developments in data analysis methods facilitate the separation and extraction of structural information on crystalline and amorphous components, enabling the detailed investigation of dynamic changes in the catalyst surface.
Several mechanisms have been proposed for the OER, including the adsorbate evolution (AEM), lattice oxygen (LOM), and dual-site ones12,13,14. Distinguishing between oxygen evolved from water versus that derived from the lattice requires isotopic labeling with 18O15, direct structural information is still important and the consistency with isotopic analysis leads to the better understanding of the OER mechanism.
The OER activities of perovskite- and delafossite-type oxides are closely related to the M–O bond length16,17, and the rate-determining steps, such as the adsorption/desorption of oxygen species on/from metal oxide surfaces, are probably influenced by the M–O bond strength. This suggests that the effects of the M–O bond length may depend on the OER mechanism, which could be used to identify or quantify the contribution of each mechanism.
Let us now construct a more robust logical framework linking “M–O bond length” to “OER mechanism selectivity”. Because each OER pathway responds differently to the M–O bond strength, the potential-dependent evolution of the M–O bond length during reaction acts as a dynamic fingerprint of mechanism switching. Therefore, real-time total scattering measurements can reveal which mechanism is favored under specific reaction conditions. First, we assume that short or long bond length can be associated with the abstract concept of “M–O bond strength”. When considering OER in a given catalyst system, multiple pathways coexist rather than a single unified mechanism. Consequently, the central quantity to be determined is the contribution ratio of each pathway, and the M–O bond strength serves as a structural descriptor that reflects mechanism preference.
Examination of each mechanism shows that M–O bond strength does not monotonically accelerate all pathways. Excessively strong or weak bonding can hinder specific reaction steps, implying that each mechanism possesses an optimal descriptor value. For AEM, stronger bonding may facilitate adsorption but slow desorption, reducing overall turnover9,17,18. For LOM, stronger bonding stabilizes the oxygen lattice and promotes reversible lattice oxygen participation, but overly strong bonding suppresses oxygen-exchange kinetics19,20. Usually, the optimal M–O bond strength differs between AEM and LOM. Since the potential-dependent bond-length evolution reflects how close the system is to each optimal bonding region, these structural dynamics can be directly mapped onto the mechanism preference under operating conditions21,22. This conceptual framework naturally leads to a quantitative approach. By applying a two-path parallel rate model and fitting it to the structural descriptor, the mixed contribution of the coexisting mechanisms can be quantitatively determined.
Despite the abundance of operando XAS studies on IrOx-based catalysts, including those on IrOx·nH2O23, BaIrO3 and SrIrO311, and time-resolved local structure of IrOx under acidic and basic conditions24, this technique is inherently sensitive to electronic states. However, total X-ray scattering measurements provide direct structural information and shed light on the dynamics of structural changes, complementing conventional XAS measurements.
Recently, a study combining operand X-ray absorption spectroscopy (XAS), operand surface-enhanced infrared absorption spectroscopy (SEIRAS), ex-situ pair distribution function (PDF) analysis, and density functional theory (DFT) calculations has been reported for IrOx25. Notably, in SEIRAS measurements, an increase in intermediate absorption with rising voltage was observed in sample SA3.5, and operand XAS revealed a shortening of the Ir–O bond by approximately 0.8 Å.
Considering the above, we herein conduct in-situ total X-ray scattering measurements using a custom-designed electrochemical flow cell to monitor structural changes in IrOx/SrIrO3 during the OER and thus provide new insights into the OER mechanism and deepen our understanding of the catalyst structure–function relationship.
Methods
In-situ cell design
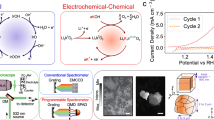
Figure 1 shows the design of the in-situ cell used for total synchrotron X-ray scattering measurements. The cell body was constructed from fluoropolymers resistant to the employed electrolyte (0.1 M HClO4). Kapton film was used as a window material because of its higher mechanical strength and lower background scattering compared with those of glass. The windows were sealed using O-rings to prevent electrolyte leakage. A carbon-printed SiO2 plate (Product number 002083, BAS Inc., Japan) coated with the SrIrO3 catalyst using a Nafion binder served as the working electrode. A smoothflow pump (Model Q, Tacmina Corporation, Japan) was connected to the cell to remove gas bubbles and maintain a uniform electrolyte distribution. To stabilize the Kapton film during operation and reduce background noise, the electrolyte level was carefully controlled to allow for a small headspace for pressure equalization. Measurements were conducted at the SPring-8 BL08W (115.03 keV) beamline using a two-dimensional area detector, enabling rapid data collection during time-resolved measurements26.
Together, the low-background in-situ cell and the simultaneous co-refinement of crystalline and amorphous components constitute a portable and reproducible workflow. This approach ensures scientific rigor and reproducibility through transparent procedures and explicit reporting of uncertainties and sensitivity analyses.
3C-SrIrO3 synthesis
3C-SrIrO3 catalyst was synthesized using the ethylenediaminetetraacetic acid-citric acid (EDTA-CA) complex sol-gel method. 47.61 mg of Sr(NO3)2 and 77.3 mg of H2IrCl6·6H2O were dissolved in a mixed solution of 20.0 mL deionized water and 2.0 mL ethylene glycol (EG). Subsequently, 292.0 mg of EDTA and 10.0 g of citric acid were added as complexing agents, maintaining a molar ratio of total metal ions: EDTA: citric acid = 1:1:3. The pH of the solution was adjusted to 6–7 using aqueous ammonia to ensure complete complexation. The mixture was then heated to 200 °C under continuous stirring until gelation occurred. To obtain the precursor, the resulting gel was dried at 350 °C for 8–9 h. The dried product was finely ground and calcined in air at 700 °C for 5 h. The resulting powder was immersed in 0.1 M HCl with sonication for 3 h to remove residual SrCO3 impurities and to develop the desired surface structure. The final product was obtained by filtering and drying the acid-treated sample.
Electrochemical setup
OER performance was evaluated in 0.1 M HClO4 using a standard three-electrode electrochemical setup connected to an HZ-7000 electrochemical workstation (Hokuto Denko Ltd., Japan). The catalyst-coated substrate served as the working electrode, while a Pt wire and 3 M NaCl Ag/AgCl electrode were used as the counter and reference electrodes, respectively. A constant potential of 1.5 V was applied during the OER.
PDF analysis
The reduced pair distribution function G(r) has the following relationship with the X-ray structure factor S(Q)26:
where r is the interatomic distance, q is the scattering vector, and S(Q) is the X-ray structure factor. The PDF from total X-ray scattering is given by
where ρ is the atomic number density.
When comparing data from X-ray total X-ray scattering experiments, using G(r), it allows for evaluation without considering variations in density. We used the method developed by Hiroi et al.27 to analyze the PDF data obtained at the BL08W beamline with the possibility of separating crystalline phases. Assuming 10 types of crystalline phases, we monitored the time evolution of each and analyzed the dynamic changes in the amorphous phase obtained as the residual. The crystalline phases and analysis procedure are described in Sect. “Results and discussion”.
Results and discussion
We investigated the structural evolution of the Sr–Ir–O electrocatalyst under OER conditions, focusing on local structural changes during the initial activation of the catalytic surface layer. In-situ X-ray total scattering measurements were conducted to capture real-time structural changes, and complementary ex situ measurements were performed to analyze the effects of varying acid treatment durations in the catalyst preparation process.
Total X-ray scattering provides structural information on both crystalline and amorphous phases. The employed Sr–Ir–O catalyst was assumed to have a crystalline bulk and an amorphous surface, with the surface structure playing a critical role in determining catalytic performance. To enable the in-situ characterization of structural changes during the OER, we used the custom-designed electrochemical cell described above.
Identification of crystalline phases
Structural changes were analyzed based on Bragg peak assignments to identify crystalline phases. The main crystalline phase was 3C-SrIrO3 (Pnma) (Fig. S1). However, discrepancies in the positions and intensities of certain peaks suggested the presence of additional phases, which were tentatively identified as 6H-BaIrO3 (with Ba substituted by Sr), 8H-SrIrO3, 9R-SrIrO3, 9R-BaIrO3 (with Ba substituted by Sr), 10H-Sr5Ir5O21, BCC-Sr0.86IrO3, SrIrO3 (Materials Project #17097), SrIrO3 (Materials Project #1016848), and SrIrO3 (Materials Project #1193907) and belonged to diverse space groups, including Pnma, C2/c, P63/mmc, C2/m, and Im-3. Figure S1 reveals the concurrent presence of the major Pnma phase and C2/c phase. Seven other phases were also detected in small amounts, and their combined content was comparable with that of the major phase. The presence of several closely related crystalline structures was ascribed to the dynamic transformation processes associated with high catalytic activity and could influence the surface activation behavior of the catalyst during the OER.
We therefore treat 3C, 6H, and the additional C2/c polymorphs as structural bases of the IrO₆‑octahedral network; using them in combination functions as a dictionary to linearly approximate reconstructed surface regions, reducing model bias and improving the description of medium‑range order (5–10 Å).
Structural evolution during the OER
In-situ total X-ray scattering measurements enabled the first-time direct observation of the structural evolution of the catalyst surface during the OER. In particular, structural changes during the first 30 min after applying the bias voltage were analyzed in detail. Preliminary tests revealed current flow before the measurement, as demonstrated by the temporal evolution of the current density (Fig. S2). The measurement window corresponded to the time required for current stabilization, reflecting catalyst activation. The total scattering data were collected at 10 min intervals, and their relevance to catalyst activation was investigated.
The collected data were primarily analyzed using G(r), which is suitable for quantifying atomic distances and coordination numbers. The G(r) is obtained with corresponding S(Q) (Fig. S3) which is in relation described by Eq. (1). Figure 2 shows the temporal evolution of G(r), highlighting changes in the local structure accompanying catalyst surface activation under electrochemical conditions.
Reduced pair distribution function (G(r)) of the reference 3C-SrIrO3 (Pnma) catalyst (top). G(r) evolution during in-situ measurements, with the color gradient from dark blue to light blue indicating a transition from the initial state (before current application) to later time points (bottom). The corresponding distances between pairs of elements taken from 3C-SrIrO3 are represented by rectangles.
Our 10-min sampling is intentionally optimized for minute-scale restructuring rather than millisecond–second adsorption/charge-transfer events. A representative timescale τ ≈ L²/D (L ≈ a few nm; D ≈ 10⁻¹⁸–10⁻¹⁶ m² s⁻¹, which is possible as the oxygen diffusion in a fuel cell compound28 naturally falls in the seconds-to-minutes regime, justifying this window for tracking physiological structural change within our resolution.
The peak at ~ 2.0 Å was ascribed to Ir–O bonds in 3C-SrIrO3 (Pnma)29. Over time, this peak lost intensity and slightly shifted (~ 0.2 Å) to shorter distances in the early stage. The broader peak at 2.3–3.0 Å corresponded to Sr–O correlations (including O–O correlations near 2.8 Å) and became sharper with time, which suggested structural rearrangement.
The deepening valley observed around 2.3 Å corresponded to the weakening of the first peak and sharpening of the second one, implying a substantial structural change on the catalyst surface.
The peak at ~ 3.5 Å included Sr–Ir, Sr–O, and O–O correlations, and that at ~ 3.9 Å, corresponded to Sr–Sr and Ir–Ir distances. The evolution of these peaks during the OER was analyzed further, as discussed later in relation to possible reaction mechanisms.
Figure 3 illustrates the decomposition of total scattering data into contributions from multiple crystalline phases and the amorphous component, based on fitting against a curated collection of Sr-Ir-O crystal structures representing different polymorphs. Both the reduced pair distribution function G(r) and the structure factor S(Q) are shown to confirm the reliability of fitting approach.
The structural evolution of the amorphous component (residual phase) was further examined based on the time-dependent changes in the corresponding G(r) (Fig. 4). In the initial state, a distinct peak appeared at ~ 2.0 Å, which corresponds to Ir–O bonding. With time, this peak lost intensity and shifted to shorter distances. This behavior suggests that the Ir–O bond became shorter after OER onset, implying either an increase in the Ir oxidation state or the formation of a denser local structure. Consistent with this interpretation, operando X-ray photoelectron spectroscopy (XPS) studies30 have shown that semi-crystalline IrOx undergoes an increase in Ir oxidation state from + 3 to + 4 (or + 4 + δ) under OER conditions. Although direct operand transmission electron microscopy (TEM) observation under OER is challenging, ex-situ high-angle annular dark field scanning TEM (HAADF-STEM) studies may provide qualitative evidence of structural evolution. For example, Thakur et al.25 observed a gradual reorganization of amorphous IrOx toward rutile IrO2 after extended operation (> 100 h), whereas our study captures short-time changes (10–30 min) during initial OER activation. Both observations consistently indicate that amorphous IrOx is structurally dynamic, although the timescales differ.
We note that the potential-induced Ir–O bond contraction cannot be attributed to bulk lattice shrinkage. This is because the total scattering results show no systematic reduction in the longer-range Ir–Ir or Ir–O–Ir distances that would indicate lattice contraction. The structural changes are therefore localized around the IrOx units rather than involving the bulk framework.
Under applied potential, the Ir–O and Sr–O distributions in the amorphous component narrow toward shorter distances (partial ordering), consistent with transient high‑oxidation/low‑coordination Ir sites. This provides a structural basis for a reversible redox/strain buffer that tunes bond stiffness during operation.
In contrast, the peak at ~ 2.5 Å, attributed to Sr–O correlations, was initially broad and poorly defined but became sharper and more pronounced with time. This finding indicates that the local ordering around Sr–O bonds improved with time, suggesting the development of partial structural order even within the amorphous regions.
The peak at ~ 3.7 Å—corresponding to Sr–Ir, Sr–Sr, and Ir–Ir atomic correlations—behaved differently from the total G(r). The total G(r) exhibited a double-peak structure in this region, whereas the amorphous component showed a single peak, implying a higher degree of randomness. This peak substantially weakened in the early stage of the OER, subsequently recovering some of the lost intensity. These changes were ascribed to the alterations in the local environment around Ir atoms and the partial crystallization or restructuring of the amorphous phase.
Thus, we concluded that the local structure of the catalyst evolved over time during the OER, with this evolution involving both crystalline and amorphous components. The relative contents of the 3C-SrIrO3 and 6H-SrIrO3 phases exhibited synchronized variations, whereas the changes in the 10th phase and amorphous component contents were the opposite of those in the 3C-SrIrO3 and 6H-SrIrO3 contents (Fig. 5). This behavior suggests a potential structural interconversion of these phases, which possibly involved dynamic reconstruction processes at the catalyst surface.
Changes in the relative contents of the 10 phases during the OER. Difference between the sample before current application and that characterized during the first interval (top). Difference between the samples characterized during the first and second intervals (middle). Difference between the samples characterized during the second and third intervals (bottom).
Such structural variations may reflect an adaptive structural response to the reaction conditions and represent a self-reconstruction mechanism for the formation of active catalytic phases. Additionally, the changes in the Ir–O and Sr–O bond lengths in the amorphous phase suggest that surface structural evolution plays a critical role in the emergence of catalytic activity.
Looking forward, further improvements in the spatial and temporal resolution of in-situ measurements, combined with theoretical modeling, are expected to advance our understanding of the structural mechanisms underlying amorphous-to-crystalline transformations and their chemical implications in the catalytic function.
It should be noted that PDF analysis provides one-dimensional information, which limits the ability to fully resolve three-dimensional atomic arrangements in amorphous domains. While advanced simulations like reverse Monte Carlo modeling could extract 3D structural details, such modeling requires higher-quality data than one available in this present study. Reducing background signal by using thinner flow cell is the possible way to archive this process.
Discussion
Our in-situ measurements revealed structural changes on the catalyst surface during the OER, as reflected by the persistent Ir–O bond shortening. The surface structure formed upon acid treatment was transformed during OER, incorporating H2O molecules and releasing O2 (Fig. 6). The steady decrease in the number of oxygen atoms surrounding Ir observed under an applied potential indicated structural rearrangement on the surface. This observation can be discussed in the framework of AEM, which describes the OER process through the following four elementary steps (“*” denotes an active site on the catalyst surface).
Step 1: H2O(l) + * ⇌ HO* + H+ + e−
Step 2: HO* ⇌ O* + H+ + e−
Step 3: O* + H2O(l) ⇌ HOO* + H+ + e−
Step 4: HOO* ⇌ * + O2(g) + H+ + e−
Man et al.17 used free energy calculations to show that the strength of M–O bonds in AMO3 perovskites determines the rate-limiting step: when the M–O bond is strong, step 3 becomes rate-limiting, whereas weaker M–O bonds shift the limitation to step 2. This relationship is further supported by the volcano plot established by Suntivich et al.18, which correlates OER activity with the eg orbital occupancy of the transition metal and, hence, the M–O bond strength.
The Ir–O bond shortening observed herein under an applied potential corresponds to M–O bond strengthening and, hence, O* stabilization. According to Seitz et al.9, SrIrO3 with a surface IrOx layer lies on the side of the volcano plot where (ΔGO − ΔGOH) is small, which indicates strong M–O bonding and weak interactions between O* and H+.
Assuming that step 3 is rate-limiting in our system, the OER performance can be enhanced by reducing the free energy barrier of this step, which would entail facilitating the O* → HOO* transition. From the perspective of bond strength, this means slightly weakening the M–O bond to optimize intermediate binding. However, our in-situ findings indicate that under operating conditions, the M–O bond becomes stronger, which can be interpreted as a shift toward a configuration less favorable for OER activity.
From a LOM perspective, stronger bonding stabilizes oxygen lattice and promotes lattice oxygen participation; however, excessive strengthening can increase vacancy formation barriers and suppress kinetics. Therefore, Ir-O bond shortening likely biases mechanism selection toward LOM while reducing AEM contribution, but the overall activity depends on achieving an optimal bonding regime rather than monotonic strengthening.
If external perturbations (e.g., applied strain, compositional tuning, or potential control) can systematically modulate the Ir-O bond length, then the resulting changes in OER kinetics would provide a powerful means to estimate the mechanism contributions. By correlating reaction rates with real-time structural descriptors under controlled Ir-O distances, one could fit mixed-mechanism models and quantitatively estimate the relative fraction of AEM and LOM. This approach would enable a descriptor-driven mechanistic map and identify the optimal bonding regime for maximum activity and stability.
This Ir–O bond shortening can also be understood in the context of general chemical behavior. The O–O bond of molecular oxygen is shorter in its activated state (O2+, 2Пg) than in its ground state (3Σg−)31. Therefore, the observed shortening under an applied potential may reflect the catalyst transitioning into an activated state. Shopov et al.32 reported that the Ir–O bond distance in Ir complexes depends on the Ir oxidation states, equaling 2.01–2.08 Å for Ir3+ and 1.93–1.96 Å for Ir4+.
In our case, the Ir–O bond distance under applied current falls within the lower range, corresponding to a higher oxidation state of Ir. This suggests that under electrochemical conditions, the Ir centers at the catalyst surface are oxidized, which results in shorter and stronger Ir–O bonds.
Our in-situ PDF analysis revealed a persistent shortening of the Ir-O bond distance under OER conditions. Rather than attributing this contraction to bulk lattice effects, it is more reasonably explained by increasing contribution of surface Ir-O bonds formed during the catalytic cycle. In a recent multimodal study, Thakur et al.25 performed density functional theory calculations for multiple IrO2 polymorphs with adsorbed OER intermediates (*OH, *O, *OOH).
Additional analysis (Fig. 7) based on their result demonstrated that Ir-O bonds at the surface are significantly shorter than bulk Ir-O bonds. This shortening originates from the oxidation of Ir to higher valence states under OER potentials and from the increased oxygen coordination number associated with intermediate adsorption.
Population of Ir-O as a function of distance. The structure data was provided from Ref20., Fig. S22-24. The surface of IrO2 modified by an O* intermediate. The surface oxygen is highlighted in green, indicating shorter Ir-O distance compared to other oxygen atoms. These shorter distances are illustrated by red arrows in the distribution plot.
Taken together, these theoretical insights provide a strong mechanic foundation for our experimental observations: the O motifs at active sites, rather than a uniform contraction of bulk Ir-O distances. This interpretation is consistent with the oxidative environment at the anode, the increase in Ir oxidation state, and the higher oxygen coordination number during OER, thereby reinforcing that the structural dynamics we observe are intrinsic to the catalytic mechanism.
Furthermore, Thakur et al.25 reports that operando XAS measurements on SA3.5 reveal a shortening of the Ir–O bond during operation, accompanied by strong absorption of reaction intermediates observed via SEIRAS. These findings suggest that the strengthened Ir–O bond contributes to the stabilization of reaction intermediates. This stabilization is likely to facilitate the initial steps of the OER, namely the adsorption of H₂O (step 1) and the deprotonation of *OH (step 2).
As discussed above, the rate-determining step in this system is considered to be the step 3, the formation of *OOH, which means that a stronger Ir–O bond may be disadvantageous for this step. However, as shown in the computational results of Thakur et al.25 (Fig. S25 of this reference), the difference in ΔG between steps 2 and 3 is relatively small. Therefore, in the present system, the observed shortening of the Ir–O bond may overall be beneficial to the OER process.
Within the scope of the present dataset, the potential dependence of PDF metrics (medium‑range order and peak broadening) allows us to quantitatively delineate the role of the amorphous component without relying on additional measurements.
Here, we note the role of the amorphous phase in OER based on the general property of amorphous phase. Because the amorphous IrOx phase inherently contains locally unstable and coordinatively flexible environments, it can strongly bind oxygen species that are difficult to adsorb on fully ordered crystalline sites. In addition, the spatial mobility of these unstable sites allows the amorphous layer to assist in the removal of residual adsorbed oxygen through local rearrangement and diffusion-like processes. In this sense, the amorphous phase acts both as a reversible reservoir of oxygen species and as a structurally adaptable surface that supports catalytic turnover. The “Remaining” phase identified in our in-situ analysis corresponds to the amorphous IrOx component, and that the observed Ir–O bond shortening occurs primarily within this phase. This supports the conclusion that the amorphous phase contributes directly to OER activity.
To better understand the structural changes observed during the OER process, it is instructive to compare them with those induced by acid treatment during catalyst synthesis. The supplementary information provides ex situ G(r) analyses at various acid treatment durations, including surface-sensitive data analogous to the in-situ analysis (Fig. S8). Notably, the increased intensity of the Ir–O peak after acid treatment (Fig. S5), along with the narrowing of distributions in the residual phase (Fig. S8 and S9), suggests a transition toward more defined local environments. This may reflect an increase in the average coordination number or a reduction in structural disorder. These features stand in contrast to the low-coordination Ir sites formed under in-situ conditions, indicating that the structural evolution during OER is not a simple extension of the acid treatment effects, but rather a distinct electrochemical transformation.
In summary, our results demonstrate the occurrence of dynamic structural transformations on the catalyst surface during the OER and reveal that these changes impact the reaction energetics and catalytic performance. Moving forward, combining high-resolution time- and space-resolved in-situ techniques with theoretical calculations will be essential for establishing design principles aimed at optimizing bond strength and controlling the rate-limiting steps in catalyst systems.
Conclusion
In-situ total X-ray scattering measurements were performed on an IrOx/SrIrO3 catalyst to investigate its structural evolution during the OER. Time-resolved analysis revealed a persistent Ir–O bond shortening and dynamic structural interconversions among the multiple crystalline phases and amorphous component. The major phases—3C-SrIrO3 (Pnma) and 6H-SrIrO3 (C2/c)—exhibited synchronized relative content fluctuations, whereas another C2/c phase and the amorphous component exhibited fluctuations in the opposite direction. This behavior suggests active structural exchange among these phases under the employed reaction conditions.
The Ir–O bond shortening was ascribed to an increase in the oxidation state of Ir under an applied potential, reflecting the local electronic and structural changes relevant to the rate-limiting step of the OER. These findings highlight the critical role of M–O bond dynamics and phase interactions in dictating catalytic behavior.
Overall, our results emphasize the importance of precise bond distance and structural dynamics control for enhancing OER activity, providing a valuable foundation for future catalyst design strategies aimed at optimizing performance by modulating atomic-scale structural features.
Data availability
Data are available from the authors upon reasonable request from the corresponding authors: Koji Ohara < ohara@mat.shimane-u.ac.jp > and Kentaro Kobayashi < kobayashi@mat.shimane-u.ac.jp >.
Abbreviations
- AEM:
-
Absorbate evolution mechanism
- CA:
-
citric acid
- DFT:
-
density functional theory
- EDTA:
-
ethylenediaminetetraacetic acid
- EG:
-
ethylene glycol
- HAADF-STEM:
-
high-angle annular dark field scanning transmission electron microscopy
- LOM:
-
lattice oxygen mechanism
- OER:
-
oxygen evolution reaction
- PDF:
-
pair distribution function
- SEIRAS:
-
surface-enhanced infrared absorption spectroscopy
- TEM:
-
transmission electron spectroscopy
- XAS:
-
X-ray absorption spectroscopy
- XPS:
-
X-ray photoelectron spectroscopy
References
Wan, G. et al. Amorphization mechanism of SrIrO3 electrocatalyst: how oxygen redox initiates ionic diffusion and structural reorganization. Sci. Adv. 7, 2. https://doi.org/10.1126/sciadv.abc7323 (2025).
Ben-Naim, M. et al. Understanding degradation mechanisms in SrIrO3 oxygen evolution electrocatalysts: chemical and structural microscopy at the nanoscale. Adv. Funct. Mater. 31, 34. https://doi.org/10.1002/adfm.202101542 (2021).
Schweinar, K., Gault, B., Mouton, I. & Kasian, O. Lattice oxygen exchange in rutile IrO2 during the oxygen evolution reaction. J. Phys. Chem. Lett. 11, 5008–5014. https://doi.org/10.1021/acs.jpclett.0c01258 (2020).
Nong, H. N. et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 587, 7834. https://doi.org/10.1038/s41586-020-2908-2 (2020).
Kasian, O. et al. J. Degradation of iridium oxides via oxygen evolution from the lattice: correlating atomic scale structure with reaction mechanisms. Energy Environ. Sci. 12, 3548–3555. https://doi.org/10.1039/C9EE01872G (2019).
Ebner, K. et al. Time-Resolved Potential-Induced changes in Fe/N/C-Catalysts studied by in situ modulation excitation X-Ray absorption spectroscopy. Adv. Energy Mater. 12, 14. https://doi.org/10.1002/aenm.202103699 (2022).
Pasquini, C. et al. Operando tracking of oxidation-state changes by coupling electrochemistry with time-resolved X-ray absorption spectroscopy demonstrated for water oxidation by a cobalt-based catalyst film. Anal. Bioanal Chem. 413, 5395–5408. https://doi.org/10.1007/s00216-021-03515-0 (2021).
Czioska, S. et al. Increased Ir–Ir interaction in iridium oxide during the oxygen evolution reaction at high potentials probed by Operando spectroscopy. ACS Catal. 11, 15. https://doi.org/10.1021/acscatal.1c02074 (2021).
Seitz, L. C. et al. F. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014. https://doi.org/10.1126/science.aaf5050 (2016).
Li, N. et al. Identification of the active-layer structures for acidic oxygen evolution from 9R-BaIrO3 electrocatalyst with enhanced iridium mass activity. J. Am. Chem. Soc. 143, 180001–180009. https://doi.org/10.1021/jacs.1c04087 (2021).
Cao, W. et al. Investigating the factors influencing the activity of iridium-based perovskite oxide electrocatalyst for acidic water oxidation by Operando XAS. Electrochem. Soc. Meet Abstr Prime. 2774. https://doi.org/10.1149/MA2024-02422774mtgabs (2024).
Ma, Q. & Mu, S. Acidic oxygen evolution reaction: Mechanism, catalyst classification, and enhancement strategies. Interdiscip Mater. 53–90. https://doi.org/10.1002/idm2.12059 (2023).
Nielsen, P. Y. & Goddard, R. J. The reaction mechanism with free energy barriers at constant potentials for the oxygen evolution reaction at the IrO2 (110) surface. J. Am. Chem. Soc. 139, 149–155. https://doi.org/10.1021/jacs.6b07557 (2016).
Zhang, W. et al. Strontium doped IrOx triggers direct O – O coupling to boost acid water oxidation electrocatalysis. Angew Chem. 137, e202418456. https://doi.org/10.1002/ange.202418456 (2025).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465. https://doi.org/10.1038/nchem.2695 (2017).
Toyoda, K., Hinogami, R., Miyata, N. & Aizawa, M. Calculated descriptors of catalytic activity for water electrolysis anode: application to delafossite oxides. J. Phys. Chem. C. 119, 6495–6501. https://doi.org/10.1021/jp5092398 (2015).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165. https://doi.org/10.1002/cctc.201000397 (2011).
Suntivich, J. et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385. https://doi.org/10.1126/science.1212858 (2011).
Huang, Z. F. et al. Tuning of lattice oxygen reactivity and scaling relation to construct better oxygen evolution electrocatalyst. Nat. Commun. 12, 1. https://doi.org/10.1038/s41467-021-24182-w (2021).
Wang, F. et al. Activating lattice oxygen in high-entropy LDH for robust and durable water oxidation. Nat. Commun. 14, 1. https://doi.org/10.1038/s41467-023-41706-8 (2023).
Tang, L. et al. Metal–oxygen bonding characteristics dictate activity and stability differences of RuO2 and IrO2 in the acidic oxygen evolution reaction. Phys. Chem. Chem. Phys. 27, 18. https://doi.org/10.1039/d5cp00666j (2025).
Song, J. et al. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49 (7), 2196–2214. https://doi.org/10.1039/C9CS00607A (2020).
Xu, J. et al. IrOx·nH2O with lattice water–assisted oxygen exchange for high-performance proton exchange membrane water electrolyzers. Sci. Adv. 9, eadh1718. https://doi.org/10.1126/sciadv.adh1718 (2023).
Achilli, E. et al. In situ dispersive EXAFS in electrocatalysis: the investigation of the local structure of IrOx in chronoamperometric conditions as a case study. J. Spectrosc. 2014 (480102), 1–7. https://doi.org/10.1155/2014/480102 (2014).
Thakur, N. et al. Identifying active sites of IrOx catalysts for OER: A combined Operando XAS, SEIRAS, and theoretical study. J. Am. Chem. Soc. 147, 30613–30625. https://doi.org/10.1021/jacs.4c18510 (2025).
Ohara, K. et al. Time-resolved pair distribution function analysis of disordered materials on beamlines BL04B2 and BL08W at SPring-8. J. Synchrotron Rad. 25, 1627–1633. https://doi.org/10.1107/S1600577518011232 (2018).
Hiroi, S., Ohara, K. & Sakata, O. Structural characterization of the delithiated noncrystalline phase in a Li-Rich Li2VO2F cathode material. Chem. Mater. 33, 5943–5950. https://doi.org/10.1021/acs.chemmater.1c01466 (2021).
Ji, H. et al. Enhanced oxygen diffusion in epitaxial lanthanum–strontium–cobaltite thin film cathodes for micro solid oxide fuel cells. Ener Env Sci. 6, 116–120. https://doi.org/10.1039/C2EE21647G (2013).
Kronbo, C. H., Nielsen, M. B., Kevy, S. M., Parisiades, P. & Bremholm, M. High pressure structure studies of 6H-SrIrO3 and the octahedral tilting in 3C-SrIrO3 towards A post-perovskite. J. Solid State Chem. 238, 74–82. https://doi.org/10.1016/j.jssc.2016.03.012 (2016).
Mom, R. V. et al. Operando structure–activity–stability relationship of iridium oxides during the oxygen evolution reaction. ACS Catal. 12, 5174–5184. https://doi.org/10.1021/acscatal.1c05951 (2022).
Bassani, P., Montaldi, E. & Fumi, F. G. Electronic States of diatomic molecules: the O2 + molecular ion. Il Nuovo Cimento. 3, 893–901. https://doi.org/10.1007/BF02823491 (1956).
Shopov, D. Y. et al. A full set of iridium (IV) pyridine-alkoxide stereoisomers: highly geometry-dependent redox properties. Chem. Sci. 8, 1642–1652. https://doi.org/10.1039/c6sc03758e (2017).
Acknowledgements
This work was financially supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan from a WE project (JPNP14021). Synchrotron radiation experiments were performed with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2022A1039, 2023B1405, 2024A1028, 2024A1030, 2024B1025, 2024B1030). This work was conducted as an SDGs Research Project of Shimane University.We thank Shimadzu Science West Co., Ltd. and WOWDESIGN Co., Ltd. for the support of developing the operand flow cell. We thank Zhihao Zhang, Nohara Hiroi for their technical supports.
Author information
Authors and Affiliations
Contributions
Kentaro Kobayashi: Writing – original draft, Writing – Review & Editing, Methodology, Investigation, participation in the main measurement at BL08W, Initial experimental trials performed at BL12B2, Formal analysis. Satoshi Hiroi: Investigation, participation in the main measurement at BL08W, Software, Formal analysis. Koji Ohara: Project administration, Methodology, Investigation, Participation in the main measurement at BL08W, Writing – original draft, Writing – Review & Editing. Neha Thakur: Investigation, Experimental supervision. Mukesh Kumar: Investigation, Experimental supervision. Weijie Cao: Material synthesis, Investigation, Participation in the main measurement at BL08W, Experimental supervision. Kengo Nakada: Software, Writing – Review & Editing. Jo-chi Tseng: Software, Formal analysis, Investigation, Set up and participation in the main measurement at BL08W. Hiroki Yamada: Software, Investigation, Setup of measurement instruments at BL08W. Seiya Shimono: Investigation, Setup of measurement instruments at BL08W. Yu-Cheng Shao: Investigation, Setup and participation for initial experimental trials performed at BL12B2. Hirofumi Ishii: Investigation, Setup and participation for initial experimental trials performed at BL12B2. Yoshiharu Uchimoto: Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kobayashi, K., Hiroi, S., Ohara, K. et al. In-situ total X-ray scattering reveals the structural evolution of SrIrO3 during the oxygen evolution reaction. Sci Rep 16, 1740 (2026). https://doi.org/10.1038/s41598-025-31418-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31418-y