Abstract
Chimeras form by the fusion of at least two distinct genets. Such genetic heterogeneity has been hypothesized to increase resilience of clonal invertebrates, and, given the changing environment of coral reefs, may provide important benefits in coral restoration. Intra- and interspecific pairs of six- and 18-month-old Orbicella faveolata and O. annularis recruits were actively staged in aquaria. Successful fusion was observed in intraspecific pairs, especially in the younger recruits of O. faveolata (70%). Fusion success between other intraspecific pairings ranged from 20 to 40%. Survival and growth of the fused chimeras were evaluated over two years following outplanting alongside large (> 1 cm diameter) and small (< 1 cm diameter) singleton recruits selected from the same cohorts. Chimeras showed 43–57% mortality over the first year, but no subsequent mortality and positive growth rates were maintained, even during the 6 months of the 2023 severe heatwave. Singletons of both size classes suffered continuous whole-colony mortality over the study period and negative growth rates during the heatwave. No chimeras formed between interspecific pairings over six months. This study provides important evidence of fusion success rates in Caribbean boulder corals and of the hypothesized greater resilience of chimeras in a restoration setting independent of colony size.
Similar content being viewed by others
Introduction
Chimerism, a phenomenon in which an organism is formed by the fusion of two or more distinct genets, is widely documented in nature in unicellular eukaryotes, invertebrates, plants, algae as well as vertebrates such as fish, mammals, and humans1,2. It occurs naturally in modular invertebrates via colony fusion3 and, in reef corals, colony fusion often results from gregarious larval settlement4. Corals with such genetic heterogeneity have been readily observed in wild populations5,6,7, though the required genetic sampling (i.e., analyzing multiple tissue samples from locations spread across a colony to compare genotypes) likely underestimates prevalence8. Colony fusion has been repeatedly shown to benefit corals via the simple mechanism of size; larger chimeric juvenile sizes resulting from the fusion of young corals can show enhanced survivorship in this vulnerable life phase9,10,11 and may thus be leveraged in a restoration context to help overcome persistent post-settlement mortality bottlenecks that impair the efficiency of coral seeding and population enhancement efforts12.
The likelihood of colony fusion is expected to be affected by age and genetic relatedness. The fusion of allogenic colonies is believed to be facilitated in young colonies due to the immaturity of histocompatibility and immune responses, and higher genetic relatedness provides less contrast to trigger histocompatibility systems. Several studies in corals have suggested that four months of age is a threshold for successful fusion13,14, though opportunistic fusion has been observed in juvenile and adult corals at older ages15.
In the Anthropocene era, rapidly changing climate as well as local environmental impacts present existential threats to coral populations and coral reef ecosystems. There is considerable debate as to whether reef-building corals have adequate adaptive capacity that can be expressed rapidly enough to persist under such threats16. It has been more recently hypothesized that chimerism may provide an added source of both genetic and phenotypic variability as well as enhanced resilience in specific environmental conditions, through an enhanced ‘genetic repertoire’. This advantage may arise from both drawing on genetic variants of multiple individual and from the potential adjustment of somatic composition among the constituent genets17. Partial, short-term tests of this hypothesis have involved comparing the performance of chimeric and singleton recruits of brooding coral species under ~ one month laboratory thermal stress exposures18 and 48 h field stress exposures by moving recruits to shallow depths (10 m to 2 m)19. The latter study compared gene expression between chimeras and singletons, concluding that chimeras show greater constitutive expression of stress response genes, suggesting that ‘frontloading’ of transcriptional stress response may be a genetic mechanism contributing to greater chimeric resilience19. Non-silent intracolony Single Nucleotide Polymorphisms (SNPs) have also been reported in Pocillopora acuta7, validating the concept of an enhanced ‘genetic repertoire’ in chimeric corals. Notably, no prior studies have addressed this hypothesis of increase stress resilience of chimeras in the context of Caribbean corals, broadcast spawning species, nor long-term performance under field conditions.
Our two-phased study addressed the formation and resilience of chimeras in the endangered Caribbean reef-building corals, Orbicella annularis and O. faveolata. In phase one, pairs of corals were placed in close contact and evaluated in the lab over six months to document their likelihood of fusing into chimeric colonies and the influence of polyp age (Fig. 1A). In phase two, the resulting fused chimeras of each species were outplanted to a natural reef, alongside singleton colonies of two separate size classes from the same-aged cohorts (Fig. 1B). Their survivorship and growth were followed over an additional two years, during which the record-breaking 2023 heat wave20 provided a unique opportunity to evaluate long-term resilience of chimeras vs. singletons during a severe bleaching event.
Methods
In September 2019 and 2020, spawning of Orbicella annularis and O. faveolata colonies was monitored in Jardines and La Bocana reefs in Puerto Morelos, Quintana Roo, Mexico, between the fourth and ninth night after full moon, according to local spawning predictions for these species (http://crc.reefresilience.org/wp-content/uploads/2020/06/Coral-Spawning-Predictions-Puerto-Morelos-2020.pdf). When spawning occurred, gametes were collected using conical collectors placed over the colonies21.
Gametes were returned to the Reef Systems Academic Unit in Puerto Morelos for fertilization and larval culture. For each cohort, gamete bundles containing both eggs and sperm from four or five parent colonies (details in Suppl. Table 1) were mixed for one hour, after which fertilization was verified via observation of the first division of embryos and quantified (# dividing embryos/ [# dividing embryos + # nondividing eggs])21. The embryos and larvae were reared in an indoor static seawater system under controlled conditions. The seawater was pretreated with ultraviolet C radiation and filtered for sediment and particle retention to 1 micron, with 30% water changes every third day. When the larvae were ready to settle (~ 60 h post fertilization), they were offered cement (type II tetrapod22) and 3-D printed ceramic substrates, previously conditioned in the sea for two months. After settlement, the corals were fed for two weeks with CoralAmino and Koralle-VM liquid supplements (Brightwell Aquatics, USA) following the manufacturer’s recommendations. The substrates with settled corals were then transferred to a closed, recirculating, outdoor aquarium system. Feeding continued three times a week with recently hatched Artemia nauplii. Natural light (350–480 µmol quanta m− 2 s− 1), temperature (27–28 °C), and salinity (35–37 ppt) were continuously monitored and regulated as needed. The seawater in the outdoor system was filtered through 20-micron cartridge filters, and 30% was renewed every third day.
Experimental treatments with Orbicella species recruits from two cohorts born in 2019 and 2020 (indicated by subscripts and size of the polyp icons). (A) Experiment 1: Staged pairs of Orbicella faveolata (blue) and Orbicella annularis (orange) recruits from two cohorts examined for induced chimera formation. The 2019 cohort was 6 mos of age and the 2020 cohort was 18 mos of age at the start of this experiment. The first four treatments (T1-T4) matched the same species and cohorts (intraspecific pairs). The remaining three treatments (T5-T7) combined pairs of recruits from different species or cohorts. (B) Experiment 2: All successfully fused chimeras of each Orbicella species from Expt 1 were outplanted on the reef alongside singleton colonies divided into two size class treatments: Large (L; > 10 mm) and Small (S; < 10 mm). Members of singleton treatments were pooled from colonies of both age cohorts (indicated by subscripts). The shaded box indicates chimeras which were excluded from statistical analysis in order to standardize colony size between chimeras and large singletons. n: number of replicates per treatment; Ø indicates mean diameter (± 1 standard deviation) per treatment.
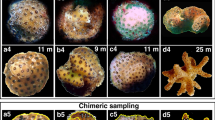
Experiment 1 was initiated in March 2021 to analyze the potential for inducing chimera formation in O. annularis and O. faveolata colonies of different ages (18 months old from the 2019 cohort, mean colony diameter 2.4 ± 1 mm and 6 months of age from the 2020 cohort, mean colony diameter 1 ± 0.5 mm). Ten replicates of each of seven treatment combinations (Fig. 1A) were constructed by carefully detaching each colony from its substrate using a Dremel 3000 rotary tool (Bosch Power Tools BV, The Netherlands) along with ~ 3 mm of the substrate and adhering each to a new substrate with Apoxie sculp (Aves Studio, LLC, USA). Each replicate consisted of two colonies placed at a 3 to 5 mm distance between them on a square tile (3 cm x 3 cm) composed of limestone encrusted with fragments of shells and corals. To identify the species throughout the study, a mark was placed in the lower right corner of every tile with Colony 1 glued to the left side and Colony 2 glued to the right side. A replicate number was marked under the substrate. The colony pairs were reared in the lab in an outdoor aquarium system under the previously mentioned conditions for 6 months. Fusion and survival were evaluated monthly using images of the substrates to identify fusion or rejection between colonies. Specifically, five types of interaction were distinguished (Fig. 2). No Rejection or Fusion (NRF) was observed when each colony grew away from the other without their tissues coming into contact. Fusion (F) of tissue between colonies was determined when the tissue of each colony was uniformly united and the boundary between the colonies could not be visually distinguished; Rejection (R) was determined when colonies remained in contact but the boundary between the colonies could be distinguished; Rejection Mortality (RM) was distinguished by observing tissue mortality of one or both colonies in the contact area between the colonies, as opposed to Natural Mortality (NM) which was designated when mortality of one of the colonies occurred prior to them growing into contact, primarily at the beginning of the experiment (and likely attributable to the initial handling when attaching the colonies to the tiles). The proportion of interaction types between age treatments for each species (i.e. T1 versus T2, and T3 vs. T4, Fig. 1A) was compared using the X2 test in JASP v 0.19 software23 to determine if the likelihood of Fusion and Rejection responses depended on age. For this analysis, Natural Mortality and No Rejection or Fusion interaction types were excluded (as no interaction between these colony pairs in fact occurred) and Rejection Mortality and Rejection categories were pooled.
Interaction responses during chimera formation that were recorded in Orbicella faveolata and O. annularis. NRF: No rejection or fusion, F: Fusion, R: Rejection, RM: Rejection mortality and NM: Natural mortality. Monthly interactions are shown in Supplementary Fig. S1.
In Experiment 2, fused chimeras formed from both species were outplanted on Jardines reef in September 2021 to evaluate their relative performance alongside singletons of different sizes. The overall density of outplants was five colonies per m2. Small (S; <1 cm diameter) and large (L; >1 cm diameter) singleton colonies were selected from a pooled population of the same cohorts (~ 1 year and ~ 2 years of age) that had been cultured under the same conditions (that is, single colonies in each size class were of mixed ages). Sample sizes for each treatment (n) are given in Fig. 1B. Surveys were conducted at 1, 3, 6, 13, and 17 months after outplanting to assess colony survival (by visual inspection) and size (diameter, by in situ measurement using a millimeter-graduated ruler).
Due to the unprecedented and unexpected heatwave that affected the region during May-December of 202320 (months 20–27 of Expt 2), additional surveys were conducted in months 24 (survival and size; at 17.6 DHW), 26 (survival; at 9.9 DHW) and 28 (survival; after return to 0 DHW). Kaplan-Meyer survival analyses followed by Log-rank (Mantel-Haenszel) tests were performed separately for each species to compare survival curves across the three treatments (Large singletons, Small singletons and Chimeras). The Kaplan-Meyer survival analysis with Log-rank test was repeated for O. faveolata, excluding the two larger chimeras from the 2019 cohort (resulting in a total of n = 7 chimeras) to control for size between the chimera and large singleton treatments (see sizes in Fig. 1B). Due to the small sample size of chimeras and the consequent unbalanced nature of their comparison to singletons, we also performed nonparametric bootstrapping using n = 1000 resamplings of the data, refitting the Kaplan-Meier curves for each iteration and visualized these with 95% confidence intervals.
For each colony that survived 24 months, growth was calculated as the change in diameter (cm) per month during the pre-heatwave interval (i.e. months 0–17) and during the heatwave (interval from months 17–24). A nonparametric Wilcoxon paired signed-ranks test was performed within each colony-type treatment to compare growth before vs. during the heatwave. All statistical analyses were performed in JASP v 0.1923 or Rstudio 2025.09.2 (RStudio 2025.09.2 + 418 “Cucumberleaf Sunflower” Release (12f6d5e22720bd78dbd926bb344efe12d0dce83d, 2025-10-20) for Windows).
Results
Experiment 1: interactions among intra- and interspecific pairs
The full time course of interactions among colony pairs in the seven treatments is given in Suppl Fig. 1. The fusion success of Orbicella faveolata after six months was dependent on age (X2 = 5.051; p = 0.025 for T1 versus T2; Fig. 1A), with 20% of older colony pairs and 70% of younger pairs forming integrated chimeras (and all remaining replicates showing rejection; Fig. 3A). Orbicella annularis pairs showed no influence of age (X2 = 0.00; p = 1.00 for T3 versus T4; Fig. 1A), with the proportion of Fusion: Rejection of 40%:60% in both age treatments (Fig. 3B). Meanwhile, no fused chimeras were formed in any ‘mismatched’ pair treatments (i.e., neither inter-species T6 − 7 nor different aged pairs, T5, Fig. 1A) with active rejection observed in at least 80% of these treatment pairs (Fig. 3C).
Frequency of interaction responses (see Fig. 2 for definitions) in colony pairs for each treatment (described in Fig. 1A) after 6 months of rearing under laboratory conditions. (A) Orbicella faveolata (OFAV) and (B) Orbicella annularis (OANN) treatments with matched pairs from 2019 and 2020 cohorts. (C) ‘Mis-matched’ treatments with pairs combining different species or age-cohorts. NM + NRF: Natural Mortality plus No Rejection or Fusion, F: Fusion, R: Rejection (including Rejection Mortality, RM). n = 10 replicate pairs for each treatment.
Experiment 2: chimeras vs. singleton performance on the reef
Survival
Chimeras of both Orbicella faveolata and O. annularis outplanted on the reef had a higher survival probability than singletons. For O. faveolata survival curves (Fig. 4A), the Log-rank (Mantel-Haenszel) test indicated significant differences among all treatments (Log-rank test X2 = 16.17; p < 0.001). Chimera survival at the end of the experiment (57%) was 12 times higher than the large singleton colonies (4.5%). Although these large O. faveolata singletons had higher survivorship than the small singletons throughout most of the study, they ended slightly lower than smalls (at 6.4%). These results (Fig. 4A) are for the comparison in which the larger chimeras (from the 2019 cohort; see Fig. 1B) are excluded to control for size differences between chimeras (cohort 2020; Ø: 12.5 ± 4 mm) and large singletons treatments (Ø: 14.5 ± 3 mm). A similar pattern and statistical result occurs when all the O. faveolata chimeras are included (n = 9; Suppl. Figure 2). For O. annularis, the Log-rank test also indicated significant variation in survival patterns among treatments (Log-rank test X2 = 7.7; p = 0.018) (Fig. 4B). Chimeras again showed the highest survival (43%) followed by large (25%) and small (18%) singletons, though these three curves have overlapping confidence intervals. For both species, the survival probability of the chimeras remained constant after an initial post-outplant decline (six months in O. faveolata and one year in O. annularis), even including the heatwave period, whereas singletons of both species showed continued mortality throughout the 28 months of post-outplant observations. Bootstrapping the Kaplan-Meier curves for each species showed an overall similar pattern with clear differentiation of confidence intervals between chimeras and singletons for O. faveolata, and somewhat greater overlap for O. annularis (Suppl. Figure 3).
Kaplan-Meier survival plots (with shaded 95% confidence intervals) of chimeras and singletons of Orbicella faveolata (A; OFAV) and O. annularis (B; OANN) outplanted on the reef. (A) Survival probability plot of OFAV chimeras from 2020 cohort only (larger, 2019 chimeras were excluded to better match the size between chimeras (for this subset, Ø: 12.5 ± 4 mm (mean ± SD) and the large singletons (Ø: 14.5 ± 3 mm)). The red band indicates the duration of the severe heatwave during 2023 (20–27 months after outplanting).
Growth
For both Orbicella species (combined due to low numbers of surviving colonies), the monthly change in colony diameter was highly variable among individual colonies of all treatments. It was positive on average for the period prior to the heatwave event (up to 17 months after outplanting) for all treatments (Fig. 5). During the heatwave, growth was negative on average for both large and small singletons (Fig. 5B-C), whereas chimeras maintained positive growth (Fig. 5A). Results of the nonparametric Wilcoxon Signed-Ranks test indicated that chimeras showed no change in diameter (W = 11.0; p = 0.500), whereas singletons showed significant declines in growth rate (to negative means) after the heatwave event for both small (W = 100.0; p < 0.001) and large (W = 174; p = 0.004) treatments (Fig. 5).
Change in diameter of Orbicella of three colony types, chimeras (A) and two size classes of singletons (B: Small and C: Large) that survived the entire study duration outplanted on the reef. The two species are combined due to the low number of surviving colonies (total n = 6 for Chimeras, n = 14 for Small Singletons; n = 34 for Large Singletons). Growth intervals are depicted before (~ 17 mos) and during (months 17–24 mos) a regional severe heatwave. Bar plots show mean values; error bars represent 95% CI.
Discussion
The results of our current study provide evidence to support the hypothesis proposed by Rinkevich (2019) that chimerism improves coral resilience. Prior literature on fusion in reef-building corals has focused on branching Indo-Pacific species, dominated by Stylophora pistillata, Pocillopora spp., and Acropora spp4,9,10,11,13,18,19,24,25. Here, we extend characterization of formation and field performance of chimeras to the Caribbean reef-building boulder coral Orbicella spp. including during an intense heatwave event.
Bi-chimerism (i.e., chimeras comprised of precisely two genets, as addressed in this current study) confers a substantial benefit to Orbicella spp. during the crucial juvenile life history phase (Fig. 4), which has been identified as the most important bottleneck to efficiently extending coral population enhancement to ecologically and evolutionarily meaningful scales12,26. For O. faveolata, we found a 12x improvement in post-outplant survivorship (Fig. 4A), whereas for O. annularis, though survivorship was improved over singletons, the margin was lower than the 2x (based on the higher survival of singletons; Fig. 4B) that would be required to compensate for the number of initial settling larvae.
Our results also support the hypothesis that chimeras can display increased stress resilience17 and, at least for O. faveolata, this effect is not accounted for simply by increased colony size. The occurrence of the worst marine heatwave to date during the latter portion of our second, field-based experiment (months 20–24)20 provided an important real-world test of climate stress resilience and confirmed that chimeras performed better under long-term (> six months) temperature stress. Chimeras of both species showed no mortality during this heatwave and no effect on their albeit highly variable growth rates, in comparison to continued whole colony mortality (Fig. 4) and reduction to mean negative growth rates of singletons (Fig. 5). The mechanisms by which genetic heterogeneity confer this increased heat resistance warrants further investigation.
The hypothesis that chimerism may confer particular resilience to climate stressors17 has previously had limited empirical support. Jiang et al.25 exposed Acropora austera chimeras resulting from aggregated settlement (hence with up to seven fused polyps per colony) and singletons to short term (two-week) laboratory exposure to elevated temperature and pCO2. The rate of symbiont uptake and overall survivorship were higher for chimeras irrespective of temperature and pCO2 exposure treatments, likely simply attributable to larger size (see below). However, the polyp-specific growth rate was lower for chimeras than singletons, and even more so in the elevated temperature treatment. Vidal-Dupiol et al.19 induced a multifaceted experimental stress (elevated temperature and light) on chimeras and singletons of Stylophora pistillata that had been growing at 10 m depth in a field nursey for one year by raising them to 2 m depth for 48 h to compare their transcriptomes. While the chimeras had displayed higher survivorship over the previous year of growout in the field nursery, no mortality was observed in the short stress exposure. However, the transcriptomic comparison of the stressed and unstressed chimeras versus singletons suggested that the chimeras had fundamental differences in their transcriptomic stress response, with greater constitutive expression of stress genes. This suggests a mechanism that could account for greater stress resistance of chimeras.
Many previous studies have demonstrated enhanced performance of coral chimeras in various settings and response types, such as field survivorship9,10,19, symbiosis establishment or bleaching25,27, or disease resilience28. However, all of these previous examples are confounded by, and often explicitly attributed to, colony size. That is, fused corals are larger, and larger corals have higher survivability in general due to their modular architecture. For example, Williamson et al.28 showed higher survival probability of chimeric brain coral recruits with more component genets during experimental disease exposure. However, the chimeras with more contributing genets were larger, and thus less likely to succumb to gradual disease mortality. We specifically incorporated conspecific singleton colonies from multiple age cohorts to attempt to control for chimera size, with partial success. Although the O. annularis chimeras were still substantially larger than any of the singletons, in O. faveolata, our chimeras from the 2020 cohort had a mean (± SE) diameter of 12.7 ± 4 mm compared to the Large Singleton treatment with a diameter of 14.7 ± 3 mm. These O. faveolata chimeras out-performed these singletons of similar size (Fig. 4A), especially during heatwave conditions (Fig. 5), despite quite low sample sizes.
We show that the key Caribbean reef-building corals, Orbicella faveolata and Orbicella annularis, both broadcast spawning hermaphroditic boulder corals, are capable of colony fusion up to 18 months of age. Indeed, O. annularis showed no effect of age (i.e., identical fusion rates between 6- and 18-month-old recruits, Fig. 3B) while O. faveolata demonstrated the expected age effect with younger colonies showing 2.5x higher fusion rates than older (Fig. 3A); but all were at least 50% older than the four-month age threshold previously described in Pacific branching corals13,14. Previous work has also suggested different age cutoffs when rejection responses mature to a level that fusion is not successful; e.g., Nozawa & Hirose15 showed successful fusion of coral recruits up to three years of age.
In addition to immaturity of immune and histocompatibility systems, genetic relatedness is also expected to influence allorecognition and hence the likelihood of successful colony fusion in modular invertebrates5,11. The results of our study show that chimeras do not form between O. faveolata and O. annularis thus suggesting that there is some level of non-self-recognition even in early-stage corals29. One prior study suggests that inter-species chimeric tissue is implicated in a pathological condition in Montipora spp. corals30, though earlier work suggested that xenografts could be successful between closely related species of Hydra (reviewed by Campbell and Bibb31. Meanwhile, intraspecific genetic relatedness was not strictly controlled in our fusion experiment as individual colonies were haphazardly selected from fertilization batches of either four or five parent colonies. Hence, it is possible that each pair represented either sibs, half-sibs, or un-related pairs, with the likelihood of related pairs (conferring higher fusion probability) being theoretically higher in the cohorts with fewer contributing parents (i.e., the younger cohort of O. faveolata and the older cohort of O. annularis; see Suppl. Table 1). Thus, we conclude that the observed age effect for O. faveolata fusion success may have been enhanced by potentially greater genetic relatedness in the younger treatment given that there were four contributing parents (compared to five for the older ones).
Our results confirm previous calls that the greater performance of chimeric coral recruits might be leveraged to improve the outcomes of coral restoration10,17 especially because many restoration efforts now consider how corals will respond to the changing conditions of today’s oceans. Chimerism may serve to increase genetic variation of coral populations in the face of global climate change17. Our experiment involved ‘constructing’ two-entity chimera by gluing two young singleton recruits in close proximity on a new substrate and this was a labor-intensive process not suitable for restoration scale application. Nonetheless, corals often settle gregariously and fuse naturally, so chimeras certainly enter restoration pipelines at some frequency. However, we currently lack techniques to control this process, making it challenging to leverage bi-chimerism actively in large scale restoration. It is possible that limiting co-settling larval batches to highly related, full-sib crosses would increase the frequency of polyp fusion4,5, but this confounds (or at best complicates) the intent to maximize genetic diversity in resultant outplanted cohorts32. Studies have also shown that increasing the larval supply (relative to available settlement space) can also increase aggregated settlement33, but gregarious settlement can be overdone, resulting in density-dependent mortality of settlers34,35,36 and likely increased frequency of multi-entity versus bi-chimeras. While not studied here, multi-chimeras (i.e., chimeras formed from a larger contributing number of initial polyps/genets) might have a higher threshold of survival to represent a net, per-larva benefit. For example, Ligson et al.24 showed survivorship to peak at a group size of 6–9 (across different group sizes of aggregated/fused settlers up to 28) but with a maximum survival increment of only about 3x, and Shefy et al.10 showed similar survival probabilities, less than 2x, for so-called bi-chimeras and multi-entity chimeras. Thus, in larval-limited restoration practices, two-entity chimeras may be expected to provide greater net benefit over larger entities. Considerable work remains to develop effective means to control the spatial distribution of gregarious settlement to encourage chimera formation while ensuring net benefit in the per-larva production of surviving corals on the reef.
Data availability
All data generated or analyzed during this study are included in Supplementary Information files accompanying this publication.
References
Kapsetaki, S. E. et al. Is chimerism associated with cancer across the tree of life? PLoS One. 18, e0287901 (2023).
Buss, L. W. Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc. Natl. Acad. Sci. U. S. A. 79, 5337–5341 (1982).
Rinkevich, B. Natural chimerism in colonial urochordates. J. Exp. Mar. Bio Ecol. 322, 93–109 (2005).
Puill-Stephan, E., van Oppen, M. J. H. H., Pichavant-Rafini, K. & Willis, B. L. High potential for formation and persistence of chimeras following aggregated larval settlement in the broadcast spawning coral, Acropora millepora. Proc. R. Soc. B: Biol. Sci. 279, 699–708 (2012).
Puill-Stephan, E., Willis, B. L., van Herwerden, L. & van Oppen, M. J. H. Chimerism in wild adult populations of the broadcast spawning coral Acropora Millepora on the great barrier reef. PLoS One. 4, e7751 (2009).
Schweinsberg, M., Weiss, L. C., Striewski, S., Tollrian, R. & Lampert, K. P. More than one genotype: how common is intracolonial genetic variability in scleractinian corals? Mol. Ecol. 24, 2673–2685 (2015).
Oury, N. & Magalon, H. Investigating the potential roles of intra-colonial genetic variability in Pocillopora corals using genomics. Sci. Rep. 14, 6437 (2024).
Guerrini, G. et al. Spatial distribution of conspecific genotypes within chimeras of the branching coral stylophora pistillata. Sci. Rep. 11, 22554 (2021).
Raymundo, L. J. & Maypa, A. P. Getting bigger faster: mediation of size-specific mortality via fusion in juvenile coral transplants. Ecol. Appl. 14, 281–295 (2004).
Shefy, D., Shashar, N. & Rinkevich, B. Exploring traits of engineered coral entities to be employed in reef restoration. J. Mar. Sci. Eng. 8, 1–15 (2020).
Amar, K. O., Chadwick, N. E. & Rinkevich, B. Coral kin aggregations exhibit mixed allogeneic reactions and enhanced fitness during early ontogeny. BMC Evol. Biol. 8, 1–10 (2008).
Banaszak, A. T. et al. Applying coral breeding to reef restoration: best practices, knowledge gaps, and priority actions in a rapidly-evolving field. Restor. Ecol. 31, e13913 (2023).
Frank, U., Oren, U., Loya, Y. & Rinkevich, B. Alloimmune maturation in the coralStylophora pistillatais achieved through three distinctive stages, 4 months post–metamorphosis. Proc. Biol. Sci. 264, 99–104 (1997).
Nozawa, Y. & Loya, Y. Genetic relationship and maturity state of the allorecognition system affect contact reactions in juvenile seriatopora corals. Mar. Ecol. Prog Ser. 286, 115–123 (2005).
Nozawa, Y. & Hirose, M. When does the window close? The onset of allogeneic fusion 2–3 years post-settlement in the scleractinian coral, echinophyllia aspera. Zoological Stud. 50, 396–396 (2011).
Torda, G. et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 7, 627 (2017).
Rinkevich, B. Coral chimerism as an evolutionary rescue mechanism to mitigate global climate change impacts. Glob Chang. Biol. 25, 1198–1206 (2019).
Huffmyer, A. S., Drury, C., Majerová, E., Lemus, J. D. & Gates, R. D. Tissue fusion and enhanced genotypic diversity support the survival of Pocillopora acuta coral recruits under thermal stress. Coral Reefs. 40, 447–458 (2021).
Vidal-Dupiol, J. et al. Frontloading of stress response genes enhances robustness to environmental change in chimeric corals. BMC Biol. 20, 167 (2022).
Hoegh-Guldberg, O. et al. Coral reefs in peril in a record-breaking year. Science 382, 1238–1240 (2023).
Banaszak, A. T., Schutter, M., Guendulain Garcia, S. D., Mendoza Quiroz, S. M. & Gómez Campo, K. Guía Práctica Para La Restauración Con Base En La Producción de Reclutas Sexuales de Corales Con Énfasis En Acropora Palmata (2018). https://www.icriforum.org/coralrestoration/.
Chamberland, V. F. et al. New seeding approach reduces costs and time to outplant sexually propagated corals for reef restoration. Sci. Rep. 7, 18076 (2017).
JASP Team. JASP (Version 0.19.3) (2024).
Ligson, C. A., Cabaitan, P. C. & Harrison, P. L. Survival and growth of coral recruits in varying group sizes. J. Exp. Mar. Bio Ecol. 556, 151793 (2022).
Jiang, L. et al. Gregarious larval settlement mediates the responses of new recruits of the reef coral Acropora austera to ocean warming and acidification. Front Mar. Sci 9, 236 (2022).
Vardi, T. et al. Six priorities to advance the science and practice of coral reef restoration worldwide. Restor Ecol 29, 1423 (2021).
Hancock, J. R. et al. Coral husbandry for ocean futures: leveraging abiotic factors to increase survivorship, growth, and resilience in juvenile Montipora capitata. Mar. Ecol. Prog Ser. 657, 123–133 (2021).
Williamson, O. M., Dennison, C. E., O’Neil, K. L. & Baker, A. C. Susceptibility of Caribbean brain coral recruits to stony coral tissue loss disease (SCTLD). Front. Mar. Sci. 9, 821165 (2022).
Palmer, C. V., Graham, E. & Baird, A. H. Immunity through early development of coral larvae. Dev. Comp. Immunol. 38, 395–399 (2012).
Work, T. M. et al. Inter-specific coral chimerism: genetically distinct multicellular structures associated with tissue loss in Montipora capitata. PLoS One. 6, e22869 (2011).
Campbell, R. D. & Bibb, C. Transplantation in coelenterates. In Invertebrate Immune Defense Mechanisms 60–69 (MSS Information Corporation, 1972).
Baums, I. B. et al. Considerations for maximizing the adaptive potential of restored coral populations in the Western Atlantic. Ecol. Appl. 29, e01978 (2019).
Sampayo, E. M., Roff, G., Sims, C. A., Rachello-Dolmen, P. G. & Pandolfi, J. M. Patch size drives settlement success and Spatial distribution of coral larvae under space limitation. Coral Reefs. 39, 387–396 (2020).
Doropoulos, C., Evensen, N. R., Gómez-Lemos, L. A. & Babcock, R. C. Density-dependent coral recruitment displays divergent responses during distinct early life-history stages. R. Soc. Open. Sci. 4, 170082 (2017).
Cameron, K. A. & Harrison, P. L. Density of coral larvae can influence settlement, post-settlement colony abundance and coral cover in larval restoration. Sci. Rep. 10, 1–11 (2020).
Suzuki, G., Arakaki, S., Suzuki, K., Iehisa, Y. & Hayashibara, T. What is the optimal density of larval seeding in acropora corals? Fish. Sci. 78, 801–808 (2012).
Acknowledgements
Eduardo Ávila Pech, Tania Doblado Speck, Aurora Claudia Padilla Souza, Coralium lab personnel, volunteers and students as well as UNAM staff who participated in the 2019 and 2020 spawning seasons are acknowledged for their assistance in the field. Spawn collection was conducted under the Permiso de Pesca de Fomento permit number PPF/DGOPA-05122/060919 issued by the Dirección General de Ordenamiento Pesquero y Acuícola to Aurora Claudia Padilla Souza and permit number PPF/DGOPA-070/20 to ATB. Outplanting of coral recruits was conducted under permit number SGPA/DGVS/04402/21 issued by the Subsecretaría de Gestión para la Protección Ambiental of the Dirección General de Vida Silvestre to ATB.
Funding
Funding was provided by The Builders Initiative to SECORE Int. as well as by CONAHCYT through project No. 425888 and by MAR Fund Project No. MX12-034 to ATB.
Author information
Authors and Affiliations
Contributions
SQM, MWM, and ATB conceived the study, SQM, RTR, and GGRT performed the experiments, SQM and MWM analyzed the results, and SQM, MWM, and ATB drafted the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mendoza-Quiroz, S., Miller, M.W., Tecalco-Rentería, R. et al. Chimerism as a strategy to improve the resilience of boulder corals. Sci Rep 16, 1501 (2026). https://doi.org/10.1038/s41598-025-31525-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31525-w