Abstract
To explore the structure and assembly of the rare bacterial community within sediment samples, as well as their responses their responses to environmental influencing factors, we collected surface sediment and overlying water samples from Sancha Lake across four seasons. MiSeq high-throughput sequencing was applied to the V3-V4 hypervariable regions of the 16 S rRNA genes, and the β - Nearest Taxon index (βNTI) was utilized to analyze the bacterial community assembly in the sediment samples. Our findings uncovered abundant bacterial diversity within the sediment samples of Sancha Lake, with 9314 operational taxonomic units (OTUs) identified, encompassing 59 phyla, 198 classes, 279 orders, 447 families, and 758 genera of bacteria. Proteobacteria and Chloroflexi were the dominant rare bacteria at the phylum level, whereas Coxiella and hgcl_clade were the principal rare bacteria at the genus level. The variety index of rare communities across diverse seasons was notably higher than that of abundant ones (P < 0.01). Bacterial community structure differed between spring and other seasons, and the rare bacterial community exhibited substantial seasonal alterations during non-spring periods. pH, dissolved oxygen (DO), total phosphorus (TP), and soluble reactive phosphorus (SRP) were the predominant environmental factors, exerting an even greater influence on rare bacteria. Within the co-occurrence network, rare bacteria constituted the majority of nodes and connections and were the dominant key species throughout all seasons. The assembly of their community was chiefly deterministic in autumn and random in other seasons. This study indicated that rare bacteria in Sancha Lake were diverse. They were keystone taxa for maintaining community interactions and stable operation, and their assembly process was influenced by both stochastic and deterministic factors.
Similar content being viewed by others
Introduction
Eutrophication of water bodies occurs when there is a substantial rise in nutrients like nitrogen and phosphorus, which can have an impact on aquatic ecosystems, human health, and economic activities1. Eutrophication includes both exogenous and endogenous eutrophication. The main source of nutrients for endogenous eutrophication is surface sediments (1–5 cm)2.
In lake ecosystems, the main locations for the microbial community and the storage of biogenic substances are surface sediments. Microorganisms can affect the distribution, transformation, and utilization of nutrients and heavy metals in sediments through metabolic activities such as assimilation and alienation. In recent years, research on the relationship between microorganisms and endogenous eutrophication in sediments has become hot topic. In terms of the composition and structure of bacterial communities in sediments, Jin et al.3 found that the structure of bacterial communities in Poyang Lake exhibited spatial heterogeneity. Zhong et al.4 investigated the spatiotemporal distribution characteristics of bacterial community composition and its correlation with nutrients in the sediments of Balihe Lake. Regarding the bacterial community diversity, Shao et al.5 conducted 16 S rRNA gene sequence analysis to estimate the vertical diversity of the Taihu Lake in two different trophic states, of which Proteobacteria was the most abundant. Xue et al.6 found that the nitrate nitrogen and water temperature in sediments of Taihu Lake in winter were the main environmental factors of Microcystis. These studies mainly focus on shallow lakes sediments, exploring the composition, structure, and diversity of bacterial communities under eutrophic factors.
The community assembly of bacterial communities is the core of aquatic microbial ecology research, which primarily contains two theoretical schools. The niche theory posits that the biogeographical patterns of microorganisms are determined by deterministic factors including environmental filtration and biological interactions7. In contrast, the neutral theory contends that the structure of microbial communities is shaped by stochastic processes like historical contingency, ecological drift, community formation and extinction8. Multiple tools are employed to explain these theories. For instance, the Neutral Community Model (NCM) proposed by Sloan et al.9, is considered to accurately simulate the assembly process of prokaryotic communities and effectively quantify neutral processes. Currently, the null model is the most prevalent analytical tool. Qi et al.10 clarified the community assembly of bacterial communities in the three main ecological niches of the Ili River sediments, revealing that stochastic processes predominate. Zeng et al.11 compared the bacterial communities of the sediments and the overlying water among ten shallow lakes in Nanjing and discovered that eutrophication strengthened the control of stochastic processes on community assembly. These results mainly explain how the distribution and assembly processes of bacterial communities responded to environmental factors in different habitats.
An ecological community typically consist of a small number of species with high abundance and numerous species with low abundance. The latter are defined as rare species12. There are variable standards for defining rare species, such as relative abundance cut-off values, sequence counts for generating datasets, and empirical thresholds. Numerous scholars have published studies on rare species in sediments, such as Qiu et al.13, who found that the community structure of rare species in alkaline lake sediments in western and north - western China remained unaffected by regional differences, and they were also core species for community interaction and stability. Ren et al.14 studied rare bacterial sub - communities in sediments of hot karst lakes on the Qinghai - Tibet Plateau and found that rare sub - communities were dominated by both deterministic and stochastic processes.
However, most of the studies on bacterial community in sediments are concentrated in northern lakes (Baiyangdian Lake15, southern lakes (Poyang Lake3, plateau lakes (hot karst lakes14, shallow lakes (the Taihu Lake5 and deep lakes (Erken Lake16. The core difference between sub-deep lakes, shallow lakes, and deep lakes stems from the stratification stability driven by water depth, which in turn leads to variations in the vertical distribution patterns of light, dissolved oxygen, and nutrients. Specifically, sub-deep lakes exhibit seasonal stratification with distinct upper and lower layers; shallow lakes are characterized by whole-lake homogeneity, high oxygen levels, and high nutrient levels; while deep lakes show permanent stratification, with anaerobic conditions and oligotrophic status in the bottom layer. These habitat differences may select for microbial communities that differ completely in terms of metabolic types, nutrient requirements, and survival strategies17. However, in current research, reports on sediment bacterial communities in sub-deep lakes of Southwest China remain relatively scarce. This scarcity is particularly pronounced in studies investigating the community composition and assembly mechanisms of rare bacterial taxa within the sediments of such lakes, where relevant research is even more limited.
A core characteristic of sub-deep lakes is their “transitional nature”—hydrologically, they lie between “non-stratified” and “stable stratified” states; ecologically, they accommodate both shallow-water and mid-deep-water habitats; and in terms of material cycling, they balance internal nutrient loading and environmental buffering capacity. This trait also makes them a type of lake that is relatively sensitive to the effects of climate change and human activities. During thermal stratification, the water column can be vertically divided from top to bottom into the mixed layer, thermocline, and stagnant layer18. However, with the abrupt seasonal drop in air temperature, the vertical temperature difference in the water column diminishes, leading to the gradual disappearance of thermal stratification. For a certain period thereafter, vertical convective mixing occurs between the upper and lower water layers, and this period is referred to as the water mixing period19. Sub-deep lakes exhibit moderate water quality and productivity, which enable them to not only meet human needs such as fishery support and drinking water supply but also maintain ecological service functions. Research on these lakes can provide guidance for striking a balance between conservation and utilization, thereby preventing functional degradation of lakes caused by over-exploitation or neglect of protection.
Sancha Lake is situated in Sancha Town, Eastern New District, Chengdu City, Sichuan Province (E104° 11′ 16″ ~ E104° 17′ 16″ ~ N30° 13′ 08″ ~ N30° 19′ 56″). It is a sub - deep lake in the southwestern part of China. It lies upstream of the Jiangxi River, which is a tributary of the Tuojiang River in the Yangtze River system. The lake area of Sancha Lake amounts to 27 km2, with a drainage area of 161.25 km2 above the dam site. Its average depth is 8.3 m, and the maximum depth reaches 29.5 m. In Sancha Lake Reservoir, thermal stratification is nearly absent in winter, with the temperature difference between the surface and bottom water being less than 0.1 °C. In summer, thermal stratification is distinct, with the maximum temperature difference reaching 12.7 °C; stratification also occurs in spring and autumn, with a typical temperature difference ranging from 1.4 to 9.0 °C. Thermal stratification in Sancha Lake Reservoir tends to promote the occurrence of water eutrophication: it reduces dissolved oxygen in the bottom water, which in turn favors the release of phosphorus from the sediment. During spring and autumn, the vertical mixing of water further facilitates the diffusion of nutrients released from the bottom to the upper water layers.Sancha Lake serves as a critical drinking water source for the urban area of Eastern New District in Chengdu and is also one of the reservoirs included in Chengdu’s “Four Reservoirs, One Spot, Two Lines” planning initiative. It plays a vital role in advancing the joint protection and governance of the ecological environment and consolidating the ecological barriers of Longquan Mountain and the Tuojiang River. In recent years, with the gradual advancement and implementation of water pollution control projects in Sancha Lake, the input of external pollutants into the basin has been effectively intercepted and controlled, and the overall water quality of the lake has shown a certain improvement trend. However, the problem of water eutrophication in Sancha Lake has not yet been fundamentally resolved—this phenomenon indicates that there may be a significant contribution of endogenous pollution within the lake system. Existing studies have confirmed that surface sediment acts as an important “source” and “sink” for endogenous nutrients such as nitrogen and phosphorus in eutrophic water bodies, and the release process of these nutrients from sediment is a key link in maintaining or exacerbating water eutrophication20. Therefore, exploring the role of microorganisms, especially rare bacteria, in endogenous sediments pollution is of great significance for understanding and improving the eutrophication status of Sancha Lake. Therefore, a systematic investigation into the functional roles and mechanisms of microbial communities—especially rare bacterial taxa—in the migration and transformation of endogenous pollutants within sediments not only provides a scientific basis for in-depth analysis of the causes and evolutionary patterns of eutrophication in Sancha Lake, but also lays a theoretical foundation for the subsequent development of targeted eutrophication control and ecological restoration strategies. This research holds significant academic value and practical significance.
Based on the mentioned research background, we assumed that The rare bacterial taxa in the sediments of Sancha Lake exhibit high diversity, and their community structure is significantly influenced by environmental factors driven by seasonal dynamics. There are distinct seasonal differences in the assembly mechanisms of rare bacterial communities in the sediments of this region. Additionally, rare bacterial taxa play a key role in maintaining network stability and dominate the keystone species across all four seasons. To achieve the above - mentioned research targets, this study applied methods such as environmental physicochemical factors determination, DNA extraction, PCR amplification, Illumina Miseq high - throughput sequencing technology, and network analysis on the bacterial community in Sancha Lake sediments. We investigated their diversity, composition, community assembly and relationship with environmental factors. Therefore, this study holds substantial implications for controlling eutrophication and safeguarding water resources in Sancha Lake Reservoir.
Materials and methods
Site description and sample collection
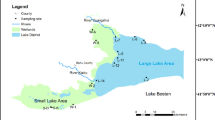
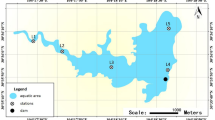
In the study, nine sampling sites were selected in Sancha Lake based on sediment distribution and eutrophication status. No permissions or licences were required to sample in this lake. The sampling sites were: the southern part of the lake centre (L1), the lake’s end (L2, L3), the lake centre (L4), the lake bay (L5, L8), the deep - water zone (L6), the inflow area (L7), and the eastern end of the lake (L9). Overlying water (5–10 cm above the sediment) and surface sediments were collected in spring (April 2017), summer (August 2017), autumn (November 2017), and winter (January 2018). The locations are shown in Fig. 1.
Sampling Locations in Sancha Lake. Nine sites were selected based on sediment distribution and eutrophication status, including areas like the southern part of the lake center, lake’s end, lake center, lake bay, deep - water zone, inflow area, and eastern end of the lake. Sampling of overlying water and surface sediments occurred in four seasons at these sites.
This study monitored DO, permanganate index (CODMn), and SRP in overlying water, mainly based on the standards of the Ministry of Ecology and Environment of the People’s Republic of China, as well as Methods for Monitoring and Analysis of Water and Wastewater (Fourth Edition). DO was assessed by the HQ3OD portable dissolved oxygen meter. CODMn was determined in accordance with the national standard Water Quality—Determination of Permanganate Index (GB 11892-89). For SRP analysis, water samples were filtered through a 0.45-µm filter membrane and directly analyzed for SRP via spectrophotometry.
For the analysis of sediments, TP was extracted using the modified Williams method (SMT)21,22. pH was determined through a glass electrode. TN was measured by the alkaline potassium persulfate digestion UV spectrophotometric method. Total iron (Fe) and total calcium (Ca) measurement were carried out by digestion methods.
DNA extraction and PCR amplification
After centrifugation, the sediments total DNA extraction was carried out in accordance with guidelines provided by the E.Z.N.A.® Soil DNA Kit (Omega Bio - tek, Norcross, GA, U.S.) due to the high moisture content of the sediments. A NanoDrop 2000 spectrophotometre (Thermo Fisher Scientific, Waltham, MA, 02454) was used to determine the concentration and purity of the extracted DNA, and 0.8% agarose gel electrophoresis was employed to evaluate the integrity of DNA. Each sample was analysed in triplicate.
The ABI Applied Biosystems® 2720 thermal cycler (Applied Biosystems, Foster City, CA, USA) was applied for PCR amplification. The 16 S rRNA genes’ V3 ~ V4 hypervariable region was targeted for amplification, and the primers were 338 F (5’-ACTCCTACGGGAGGCAGCA-3’) and 806R (5’-GG ACTACHVGGGTWTCTAAT-3’)23. The amplification reaction mixture (25 µL) consisted of: 0.25 µL Q5® High-Fidelity DNA Polymerase (5 U·µL− 1), 5 µL high GC buffer (5×), 5 µL reaction buffer (5×), 2 µL dNTPs (10 mM), 2 µL template DNA (approximately 10 ng/µL), 1 µL forward primer (10 µM), 1 µL reverse primer (10 µM), and 8.75 µL ddH2O. The amplification protocol consisted an initial 30 s denaturation at 98 °C, 27 cycles of denaturation at 98 °C for 15 s, the 30 s annealing at 50 °C, and a 30 s extension at 72 °C, with a final extension at 72 °C for 5 min, and storage at 4 °C. Each sample was run in triplicate.
Illumina miseq sequencing and data processing and analysis
PCR products were recovered from a 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). The QuantiFluor™ - ST (Promega, Madison, Wisconsin, USA) fluorometer was used to quantify all purified PCR products. The purified amplicons were then employed for library preparation with the TruSeq Nano DNA LT Library Prep Kit (Illumina, San Diego, CA, USA).
The Illumina MiSeq PE300 platform (Shanghai Meiji Biotechnology Co., Ltd.) was utilised for the sequencing process. The NCBI SRA database received the raw data uploaded with the BioProject accession number PRJNA623159.
The raw sequencing data were quality-controlled by Trimmomatic software and assembled with the FLASH software (version 2.7, http://ccb.jhu.edu/software/FLASH/, Centre for Bioinformatics and Computational Biology, Iowa City, IA, USA). The UPARSE software (version 7.1, http://drive5.com/uparse/, Edgar, R.C., Tiburon, CA, USA) was performed for OTUs clustering, based on 97% similarity (the 16 S rRNA and gcd genes24, with singleton sequences and chimeras removed during the clustering process. Species classification and annotation of the most abundant sequence in each OTU were conducted using the RDP classifier (http://rdp.cme.msu.edu/), with the Silva database (Release 115, http://www.arb-silva.de)25 as the reference and a 70% similarity threshold.
Statistical analysis
We used the statistical product and service solutions (SPSS) statistical software (version 20.6, IBM, Armonk, NY, USA) to perform statistical analysis of bacterial community composition and correlation analysis of physicochemical factors. The community contained dominant species with high abundance and rare species with low abundance14. In all samples, the abundant taxa (AT) were defined to have an average relative abundance over 0.1%, and the rare taxa (RT) were defined to have an average relative abundance under 0.01%26.
The vegan package in the statistical software R (v.4.4.1) was applied to reveal the α-diversity. Kruskal - Wallis test and Wilcoxon rank - sum test were employed to evaluate its seasonal differences, and also differences between AT and RT. Spearman correlation was conducted to assess the correlation between the diversity and environmental factors. Based on the Bray-Curtis distance matrix, non-metric multidimensional scaling (NMDS) was used to examine the similarity of community structures among samples.
To reduce the effects of multicollinearity, We screened physicochemical properties of sediments by variance inflation factor (VIF) analysis27. Any physicochemical factors with VIF > 10 were excluded. Initially, we collected species sample data (the OTUs table with 97% similarity) for detrending correspondence analysis (DCA) to compute the gradient length. If the gradient length of the first axis was 3.5 and above, canonical correspondence analysis (CCA) was adopted, otherwise redundancy analysis (RDA) would be employed to evaluated the influence of physicochemical factors on microbial communities.
Files of edges and nodes for network diagrams were generated via the MENA Cloud Platform (http://ieg4.rccc.ou.edu/mena/) and visualized with Gephi (version 0.10.1, Gephi Consortium, Paris, France). To characterise the network topology, key metrics including nodes, edges, clustering coefficients and modularity were computed. The network modules were detected using a greedy modularity maximisation approach28. Nodes were assigned topological roles based on the thresholds of Zi and Pi, and classified into four types: peripheral (Zi < 2.5, Pi < 0.62), connectors (Zi < 2.5, Pi > 0.62), network hubs (Zi > 2.5, Pi > 0.62), and module hubs (Zi > 2.5, Pi < 0.62)29.
The seasonal community assembly of bacterial community in sediments was analysed through βNTI. When |βNTI| < 2, stochastic processes were identified as the predominant drivers of community assembly, otherwise the deterministic processes turned to dominate.
The significance level of this article is set as P < 0.05, and the highly significant level is set as P < 0.01.
Results and discussion
The sediments and overlying water physicochemical properties
Table 1 displayed the physicochemical characteristics of sediments and overlying water. The pH of sediments ranged from 6.06 to 8.24, with weak acidity in spring, weak alkalinity in summer and autumn, and neutrality in winter. DO concentrations (4.10–10.40 mg·L− 1), peaked in spring, followed by winter, with summer and autumn showing the lowest values. TN levels (0.48–2.22 mg·L− 1) were elevated in spring compared to summer, with autumn and winter displaying intermediate concentrations. TP exhibited the concentration from 0.23 to 3.48 mg·g− 1, with the maximum value in winter, moderated levels in spring and autumn, and the minimum record in summer. SRP ranged from 4.00 to 85.80 mg·kL− 1, peaking in spring, decreasing in winter, and reaching minima in summer and autumn. CODMn concentrations ranged from 1.90 to 6.46 mg·L− 1, peaking in winter, decreasing in spring, and reaching minima in summer and autumn. Fe levels varied from 2.30 to 85.00 mg·g− 1, with the highest concentrations in autumn, moderate levels in spring and winter, and lowest in summer. Ca exhibited a range of 25.00–69.70 mg·g− 1, showing maximal values in winter, minimal values in summer, and intermediate concentrations in spring and autumn.
Physicochemical properties of sediments and the overlying water exhibited pronounced seasonal dynamics. Apart from TN, Fe, and Ca, the rest of these properties exhibited significant difference across various seasons (P < 0.05) (Table 1). For instance, wintertime TP concentrations were considerably greater than summertime concentrations (P < 0.05). This may be because the water level of Sancha Lake reached its peak in summer, which lowered its TP concentration. In contrast, with less precipitation in winter, the water level of the lake remained stable, enhancing the retention efficiency of P, and then leading to the increase of TP contents in winter. The finding is in line with the findings of Chen et al.30, who studied the variation of TP contents in the surface sediments of Taihu Lake’s algae - rich areas. It showed a gradual decrease in TP concentration from January in winter to summer. The TP concentration in sediments of all four seasons exceeded 1 mg·g− 1. On the basis of the Nutrient Criteria Technical Guidance for Lakes and Reservoirs31, the TP contents in sediment indicated severe pollution (>0.65 mg·g− 1).
It should be noted that this study only focuses on the analysis of seasonal dynamic characteristics of physicochemical factors in sediments and overlying water. It has not yet conducted research on the distribution patterns of physicochemical parameters in the vertical profile of sediments (e.g., stratification at different depths), vertical migration and transformation mechanisms, and interface exchange processes. Therefore, subsequent studies intend to carry out targeted sample collection and research work in the vertical dimension, which is of great significance for analyzing material cycling at the sediment-water interface and the deep-seated driving mechanisms of physicochemical factors on rare bacterial communities.
Bacterial community composition in sediments
In this study, a total of 9,314 OTUs were measured, covering 59 phyla, 198 classes, 279 orders, 447 families, and 758 genera of bacteria. Among them, 129 OTUs were defined as AT, accounting for 43.51% of the total abundance, while 7,787 OTUs were defined as RT, with an abundance of only 23.49% of all OTUs. These results are consistent with the skewed species distribution described in the rare biosphere theory32, characterized by rare groups with low - abundance but rich taxa.
The top 20 bacterial phyla from the overall taxonomy (OT) are shown in Fig. 2. The dominant phyla included Proteobacteria, Actinobacteria, Cyanobacteria, Chloroflexi, Verrucomicrobia, Bacteroidetes, and Firmicutes.
The top 10 bacterial phyla from RT were Proteobacteria, Chloroflexi, Bacteroidetes, Acidobacteria, Firmicutes, Verrucomicrobia, Actinobacteria, Spirochaetae, Parcubacteria, and Cyanobacteria. As shown in Fig. 3, only 7 bacterial communities had significant seasonal differences in abundance. For instance, the abundance of Chloroflexi was significantly skewed towards the non-spring seasons, and significantly lower in spring (P < 0.01). The average abundance of Actinobacteria in spring was significantly higher than that of other bacteria (P < 0.01).
The top 20 bacterial genera from OT are shown in Fig. 4. The dominant genera consisted of hgcl_clade and Candidatus_Xiphinematobacter.
The top 10 bacterial genera from RT are shown in Fig. 5, involving Coxiella, Syntrophus, Spirochaeta 2, Syntrophorhabdus, hgcI_clade, Flavobacterium, Smithella, Geobacter, H16, and Desulfobacca. All the top 10 bacteria at the genus level exhibited significant differences (P < 0.05). The abundance of hgcl_clade was majorly concentrated in spring and was significantly higher than that of other bacteria (P < 0.01). Coxiella displayed the highest abundance in winter (P < 0.01), while the abundance of Syntrophus in spring was considerably lower compared to that in the other three seasons (P < 0.01).
Proteobacteria was the dominant phylum in OT. Actinobacteria, Chloroflexi, Firmicutes, and Bacteroidetes were also dominant species. Li et al.33 discovered that phylum Proteobacteria dominated in Jinzhou Bay sediments. Chu et al.34 employed metagenomics and found that, in all samples, Proteobacteria dominated phylum level, followed by Firmicutes and Bacteroides in wastewater, and Firmicutes, Bacteroides, Cyanobacteria, Nitrospirobacteria, Actinobacteria in sediment samples. These results align with ours. In spring, Actinobacteria and Cyanobacteria presented much higher relative abundance than other seasons, which is consistent with what Yuan et al.35 found in the eutrophic Taihu Lake.
At the genus level, hgcI_clade was dominant in this study. The hgcI_clade of Actinobacteria is frequently detected in surface water, yet rare in sediments. The Candidatus_Xiphinematobacter of OT, considered dominant genus in sediments, however, was reported rare in other studies. For example, Keshri et al.36 found that hgcI_clade was the dominant microbial species in three central French lakes, consistent with our study. But Cheng et al.37 identified different dominant genera in Chaohu Lake sediments, like Exiguobacterium, Citrobacter, and Acinetobacter. Additionally, there were many unclassified bacteria at the genus level, such as otu68107 and otu32153 of RT. This posed difficulties in analysing bacterial community compositio α-Diversityn but demonstrated the rich bacterial diversity in Sancha Lake sediments, suggesting the existence of valuable resources to explore.
Analysis of the of bacterial community in sediments
The α-diversity of AT and RT in the sediments of Sancha Lake was evaluated using the Chao1 index, Shannon index, and Pielou index. The species richness of the community was estimated by Chao1 index. The diversity of the community was represented using the Shannon index, while the evenness of species distribution within the community was reflected by the Pielou index.
Seasonal variations and differences in the diversity of abundant and rare bacterial communities are shown in Fig. 6. For the Chao1 index: the seasonal variation of the Chao1 index in abundant bacteria was not significant (P > 0.05), whereas the Chao1 index in rare bacteria was extremely significantly lower in spring than in summer (P < 0.01) and significantly lower in spring than in autumn (P < 0.05). For the Shannon index: the Shannon index in abundant bacteria was extremely significantly lower in spring than in summer and winter (P < 0.01), and significantly lower in spring than in autumn (P < 0.05); similarly, the Shannon index in rare bacteria was extremely significantly lower in spring than in summer and autumn (P < 0.01), but significantly lower in spring than in winter (P < 0.05). For the Pielou index: the Pielou index in abundant bacteria was extremely significantly lower in spring than in summer and winter (P < 0.01), and significantly lower in spring than in autumn (P < 0.05); in contrast, the Pielou index in rare bacteria was extremely significantly lower in spring than in the other three seasons (P < 0.01). Across all seasons, the Chao1 index, Shannon index, and Pielou index of rare taxa were extremely significantly higher (P < 0.01) than those of abundant taxa.
It can be concluded that the differences in seasonal variations of diversity between abundant and rare taxa are mainly reflected in the Chao1 index: the seasonal variation of abundant taxa is not significant, while rare taxa exhibit significant variations between spring and summer, as well as between spring and autumn. This indicates that rare species are more significantly affected by seasonal changes.
Based on statistics of four seasons, Table 2. displays the findings of the Spearman correlation among the diversity indices, the overlying water and sediments physicochemical factors. For AT, the Shannon index demonstrated extremely significantly correlation with pH, DO, SRP, and CODMn (P < 0.01). The Pielou index showed highly significant correlation with pH, DO, and SRP (P < 0.01), significantly correlated with CODMn (P < 0.05). For RT, the Chao1 index exhibited highly significant correlation with pH (P < 0.01) and significant correlation with DO and Fe (P < 0.05). The Shannon index displayed strong pH / DO dependencies (P < 0.01). The Pielou index maintained highly significant correlation with pH and DO (P < 0.01), and significant correlation with SRP (P < 0.05).
In the sediments of Sancha Lake, RT accounted for only 23.49% of the relative abundance, yet their diversity index remained significantly higher than that of AT across different seasons. This disparity may arise from the fact that AT can thrive and reproduce across a broader range of resources, while the proliferation of RT is constrained by specific substrate conditions. Thus, although the presence of various RT can be detected, it is not reflected in their abundance38. Jiao et al.39 observed that in freshwater lakes of the Zhongshan Scenic Area in Nanjing, RT represented only 4.5% of the relative abundance, but their Chao1, Shannon, and PD indices were significantly higher than those of AT within the same lake. These findings suggested that although RT constituted a smaller proportion of abundance, they were key contributors to α-diversity.
In the sediments of Sancha Lake, the bacterial diversity index was influenced by the aquatic environment, yet the environmental factors limiting AT and RT were not entirely the same. This observation aligns with the results of studies by Jiao et al.40. One interpretation of this outcome is that AT and RT may play different functional roles within aquatic ecosystems and occupy distinct ecological niches41. The two communities likely coexist through niche partitioning, with distinct resource requirements and environmental preferences enabling their sustained survival within the ecosystem.
Differences in bacterial communities structure
The NMDS analysis is shown in Fig. 7. Regarding the OT, the bacterial communities from 9 sampling locations during spring were evenly distributed in the 2nd and 3rd quadrants, situated on the left side of the vertical axis. In non-spring seasons, the bacterial communities of the 27 samples from 9 sampling locations were scattered in the 1st and 4th quadrants, positioned on the right side of the vertical axis. They exhibited far distances from the area of the bacterial communities at the nine sampling locations in spring. There was a considerable separation between the spring and non-spring bacterial communities, indicating distinct seasonal differences.
The AT and RT showed similar patterns to the OT, with considerable differences between spring and non-spring communities. In spring, OT, AT and RT had similar community compositions, suggesting that different taxa had little effect on the community structure. In contrast, during non-spring seasons, the spatial distribution of RT was more dispersed than that of AT, which indicated that seasonal changes in non-spring seasons had a greater impact on RT than AT. This phenomenon could relate to the special ecological niche of RT. RT were more vulnerable to environmental changes, leading to strong variability in community structure during non-spring seasons. To the contrary, AT were more stable due to their stronger environmental adaptability.
This research revealed that the bacterial communities in Sancha Lake sediments were remarkably influenced by seasonal changes. OT, AT and RT all exhibited a clear separation between spring and non-spring seasons. However, among non-spring periods, RT were more sensitive to seasonal changes than AT, demonstrating higher complexity in community dynamics. This may reflect the crucial role that RT played in the ecosystem. Dang et al.42 investigated multiple bacterial communities in Danjiangkou Reservoir and found that rare sub - communities of planktonic bacteria showed more distinct spatial separation than abundant ones across the four seasons. Yu et al.43 studied the prokaryotic microbial community structure in Zhangjiayan Reservoir, and discovered considerable seasonal differences in its structure, with clear separation between spring - summer and autumn - winter. Wan et al.44 explored bacterial community structure in Taihu Lake sediments and found that the main bacterial categories varied significantly with nutrient status and seasons. These studies illustrate that the structure of RT change dramatically with seasonal and spatial variations.
Correlations of bacterial communities and environmental factors
Based on the results of Detrended Correspondence Analysis (DCA) of species-sample data (operational taxonomic unit [OTU] table with 97% similarity), Canonical Correspondence Analysis (CCA) was employed to evaluate the effects of sediment physicochemical factors on the total bacterial community and abundant bacterial community, as the gradient length of the first axis for these two communities was ≥ 3.5. In contrast, since the gradient length of the first axis for the rare bacterial community was < 3.5, Redundancy Analysis (RDA) was used to assess the relevant effects.The RDA and CCA analysis are presented in Fig. 8. The length of the arrows indicated the extent of influence. In the OT, CCA1 and CCA2 explained 22.67% and 5.61% of the community variation. In the AT, CCA1 and CCA2 accounted for 28.39% and 5.64% of the community variation. In the RT, RDA1 and RDA2 explained 13.28% and 5.21% of the community variation. Overall, pH, DO, and SRP had a highly considerable impact on OT, AT and RT. The influences of environmental factors on the OT and AT were quite similar: pH, DO, and SRP had a dominant influence on the bacterial communities, while Fe, Ca, TN, TP, and CODMn had less impact. RT, on the other hand, were affected greatly by pH, DO, CODMn, SRP, TN, and Fe. Evidently, compared to OT and AT, RT were more sensitive to environmental factors.
RDA analysis of bacteria and physicochemical factors in Sancha Lake sediments. Note: (a) To enrich bacterial colonies (b) as the total bacterial population (c) as rare bacterial colonies. (a) and (b) Canonical Correspondence Analysis (CCA) illustrating the effects of environmental factors on the structure of microbial communities. (c) Redundancy Analysis (RDA) depicting the associations between environmental factors and microbial communities. RDA analysis of bacteria and physicochemical factors in Sancha Lake sediments. pH, DO, and SRP strongly influenced all communities. RT responded additionally to CODMn, TN, and Fe, indicating broader environmental niche constraints.
On the whole, the dominant environmental factors for all three bacterial communities included pH, DO, and SRP. The impact of SRP overweighted TP in sediments. Contrary to our results, Yin et al.45 found that in the sediments of the Danjiangkou Reservoir area, pH, TP, organic matter (OM), and NH4+-N could have a significantly impact on the composition of bacterial communities. Meanwhile, Winters et al.46 revealed that environmental factors including nitrates, ammonium salts, and phosphates were able to influence the composition of the microbial community in the five Great Lakes sediments of the Laurentian region. This is consistent with our results, where TN became a major environmental factor in RT.
Co-occurrence network analysis of bacterial communities in sediments
The co - occurrence network diagrams at the phylum level for spring, summer, autumn and winter are illustrated in Fig. 9. In spring, the sediment bacteria network was composed of 670 nodes and 891 edges (635 positive, 256 negative). RT had 443 nodes and 519 edges (350 positive, 169 negative). The clustering coefficient of the spring network was 0.209, and the modularity was 1.379. In summer, there were 1577 nodes and 1605 edges (1280 positive, 325 negative) in the sediment bacteria network. The RT had 1176 nodes and 1174 edges (932 positive and 238 negative). The summer network’s clustering coefficient was 0.624, and the modularity was 1.322. In autumn, the sediment bacteria network contained 1136 nodes and 1996 edges (1251 positive,745 negative) The RT had 719 nodes and 990 edges (550 positive, 426 negative). The clustering coefficient of the autumn network was 0.213, and the modularity was 0.797. In winter, the sediment bacteria network was composed of 1235 nodes and 1582 edges (973 positive, 609 negative). The RT had 813 nodes and 898 edges (468 positive, 431 negative). The clustering coefficient of the winter network was 0.187, and the modularity was 0.938. For all seasons, it appeared that RT occupied the majority of nodes in the network, supporting its stable operation. Proteobacteria was the node with the highest proportion. In the sediment bacteria networks, the number of positive correlation edges was greater than that of negative correlation edges, indicating that the synergy among bacterial communities was strong while the antagonism is weak. The synergy was the most prominent in the spring sediment bacteria network, with the positive correlation proportion reaching 71.3%.
The co - occurrence network of bacteria communities in Sancha Lake sediments. Note: Nodes represent bacterial phyla, and edges denote pairwise correlations between phyla—red edges indicate positive correlations, while green edges indicate negative correlations. RT constituted most nodes and edges, maintaining network complexity. Positive correlations dominated (71% in spring), suggesting cooperative interactions under eutrophic conditions.
As presented in Fig. 10, the topological analysis at the genus level showed that there were 5 key species that could be identified in spring, among which the RT were Cellvibrio, Acidovorax, Caulobacter and hgcI_clade. In summer, 11 key species were identified. Among them, Syntrophus, BSV13, Haliangium, Ferritrophicum, CandidatusRenichlamydia and Novosphingobium were RT. In autumn, only 1 key species, the rare species Peptoclostridium, was detected. In winter, 5 key species were discovered, including Nitrospira, Pseudomonas, Coxiella, Nocardioides as well as H16, which were RT. Obviously, RT dominated the key species in all four seasons.
In the sediments of Sancha Lake, the bacteria in spring and summer exhibited high modularity. Notably, in spring, the synergy among bacteria reached its peak, so the spring network demonstrated the highest stability. Actinobacteria and Cyanobacteria, which contributed significantly to the spring network, also made the eutrophication pattern in Sancha Lake less likely to be disrupted. The reasons for these results are as follows: the co - occurrence network analysis principle measures interaction relationships among different microbial taxa in various samples through abundance correlations. Then, it abstracts simple patterns from intricate interactions and identifies cooperation (resource acquisition, cooperative exchange or niche overlap) or competition (competition for resources or space, niche separation) relationships among species to infer community assembly and evolution mechanisms47. Microbial networks with a higher degree of modularity are more stable than those with a lower degree, since diverse and complex microbial communities can better resist environmental stress than simple ones. High modularity stabilizes the network structure, as it prevents the microbial changes from spreading throughout the network, making the network less likely to be disturbed48.
The topology of co - occurrence network compared the nodes and edges of AT and RT in the ecological network. It suggested that RT might make a substantial contribution to the stability and operation of the network. The past microbiology studies focused mainly on AT, for their high abundance and ease of monitoring. However, Lynch et al.49 discovered that RT could rapidly adjust the ecosystem by their sensitive response to the environment, so as to concentrate key species on RT, rather than newly imported microbes. As a result, even with small populations, the low - abundance RT were able to impact the ecosystem significantly. Jiao et al.40 demonstrated that AT were more often at the network centre, and also acknowledged the potential of RT to be key species. These studies implies that RT are crucial for ecosystem adjustments, and beneficial for ecosystem stability, which are confirmed by our results.
As depicted in Fig. 10, numerous unidentified RT at the genus level, such as otu36728 and otu42124, had emerged as key species. This indicated that new species among RT also played a vital role in maintaining the stability of the ecological network.
Mechanism of bacterial community assembly in sediments
The assembly mechanisms of OT, AT and RT in sediments, based on the null model, are shown in Fig. 11. The assembly of OT and AT were mainly driven by stochastic processes in all seasons. However, the assembly of RT differed among seasons. Stochastic processes were dominant in spring, summer and winter, while deterministic processes took the lead during autumn. The fact that OT and AT were mainly shaped by stochastic processes in all seasons suggested that the communities were primarily influenced by random factors such as genetic drift, dispersal limitation, and homogenizing dispersal. Seasonal changes in temperature, light, and nutrient content did not significantly alter the assembly mechanism of OT and AT. This indicated that these communities might possess strong environmental adaptability and stability.
βNTI analysis of bacterial communities in different seasons. OT and AT are mainly shaped by stochastic processes year - round. RT, however, have a different trend. They’re mostly influenced by stochastic processes in spring, summer, and winter, but deterministic processes dominate in autumn. Note: (a) shows the OT, (b) shows the AT, and (c) shows the RT. OT: Overall Taxonomy, AT: Abundant Taxa, RT: Rare Taxa. Sp: Spring, Su: Summer, Au: Autumn, Wi: Winter, S: Sediment, L: Sampling location.
For RT, the assembly pattern was driven mainly by stochastic processes in spring, summer, and winter, while deterministic processes prevailed in autumn. Compared to AT, the deterministic processes possessed larger proportion in RT. This shift in assembly mechanisms might result from the higher sensitivity of RT to environmental variations. Unlike AT, RT have smaller population sizes and weaker competitiveness, but often possess strong environmental adaptability or specific ecological functions, enabling them to occupy unique ecological niches. In autumn, changes in environmental conditions (such as nutrient availability, temperature fluctuations, and DO levels) intensified the species competitions and selection pressure for specific ecological niches. As a result, RT were more susceptible to environmental factors and interactions between species, leading to a higher proportion of deterministic processes.
In summary, the assembly mechanisms of bacterial community in sediments were predominantly dominated by stochastic processes. Meanwhile, the homogeneous and heterogeneous selection accounted for a larger proportion in RT and varied greatly with seasons, which indicated that they had greater sensitivity to environmental changes and were significantly influenced by deterministic processes. Li et al.50 investigated the influencing factors and stochastic processes of microbial community assembly in the water and sediments of Wuchang lakes and discovered that stochastic processes played a more important role. Ren et al.14 studied the abundant and rare bacterial sub - communities in Qinghai - Tibet Plateau thermokarst lake sediments. They found that both abundant and rare sub - communities were dominated by stochastic processes. Nevertheless, deterministic processes (including homogeneous and heterogeneous selection) also contributed significantly to the assembly of the rare sub - communities. The above research findings, together with those of this study, collectively confirm that the overall assembly of sediment bacterial communities is dominated by stochastic processes. However, compared with abundant bacterial communities, the assembly of rare bacterial communities is more susceptible to regulation by deterministic processes. This common characteristic also underscores the close association between the structure of rare bacterial communities and environmental factors.
Conclusion
(1) In this study, a total of 9314 OTUs were detected, representing 59 phyla, 198 classes, 279 orders, 447 families, and 758 genera. At the phylum level, the rare taxa (RT) were dominated by Proteobacteria and Chloroflexi, with significant seasonal differences in Chloroflexi (P < 0.01). At the genus level, RT were primarily Coxiella and hgcl_clade, where Coxiella reached its highest abundance in winter (P < 0.01). (2) In Sancha Lake sediments, the bacterial community was diverse, and RT exhibited greater diversity than AT, making an important contributor to α-diversity. (3) The bacterial community in Sancha Lake sediments was mainly affected by the spring and non-spring seasons. RT were more affected by seasonal changes compared to AT in the non-spring seasons. (4) pH, DO, and SRP were the dominant environmental factors influencing the bacterial community, and RT were more sensitive to environmental factors. (5) RT occupied most nodes in the network, playing a pivotal role in maintaining network stability, and dominating the keystone species across all seasons. (6) In the bacterial community assembly mechanisms in sediments, OT and AT were primarily driven by stochastic processes. However, in autumn, RT were more influenced by deterministic processes, indicating their greater sensitivity to environmental changes and stronger regulation by niche differentiation and environmental selection. (7) In the eutrophic sediments of Sancha Lake, RT played a core role in the biological network despite their lower abundance. They might contribute significantly to the purification of endogenous pollution and degradation of organic matter.
Data availability
The datasets analysed during the current study are available in the NCBI repository (https://www.ncbi.nlm.nih.gov/). The BioProject accession number is PRJNA1336117.
References
Alexander, T. J., Vonlanthen, P. & Seehausen, O. Does eutrophication-driven evolution change aquatic ecosystems? Philos. Trans. R. Soc. B-Biol. Sci. 372 (1712), 20160041 (2017).
Yang, Y., Gao, B., Hao, H., Zhou, H. & Lu, J. Nitrogen and phosphorus in sediments in China: a national-scale assessment and review. Sci. Total Environ. 576, 840–849 (2017).
Jin, X. et al. Environmental factors influencing the spatial distribution of sediment bacterial community structure and function in Poyang lake. Res. Environ. Sci. 30 (4), 529–536 (2017).
Zhong, M. et al. Spatial-temporal distribution of bacterial communities and main nutrients driver of sediment in a shallow lake. Earth Environ. Sci. 51 (4), 377–387 (2023).
Shao, K., Gao, G., Wang, Y., Tang, X. & Qin, B. Vertical diversity of sediment bacterial communities in two different trophic States of the eutrophic lake Taihu, China. J. Environ. Sci. 25 (6), 1186–1194 (2013).
Xue, Y. et al. The diversity of bacterial communities in the sediment of different lake zones of lake Taihu in winter. China Environ. Sci. 38 (2), 719–728 (2018).
Gilbert, J. A. et al. Defining seasonal marine microbial community dynamics. ISME J. 6 (2), 298–308 (2012).
Zhou, J. & Ning, D. Stochastic community assembly: does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 81 (4), 1–32 (2017).
Sloan, W. T. et al. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 8 (4), 732–740 (2006).
Qi, R. et al. Distinct composition and assembly processes of bacterial communities in a river from the arid area: ecotypes or habitat types? Microb. Ecol. 84 (3), 769–779 (2021).
Zeng, J. et al. Patterns and assembly processes of planktonic and sedimentary bacterial community differ along a trophic gradient in freshwater lakes. Ecol. Indic. 106, 105491 (2019).
Jia, X., DiniAndreote, F. & Salles, J. F. Unravelling the interplay of ecological processes structuring the bacterial rare biosphere. ISME Commun. 2 (1), 1–11 (2022).
Qiu, Z. et al. Large scale exploration reveals rare taxa crucially shape microbial assembly in alkaline lake sediments. Npj Biofilms Microbiomes. 10 (1), 62 (2024).
Ren, Z., Luo, W. & Zhang, C. Rare bacterial biosphere is more environmental controlled and deterministically governed than abundant one in sediment of thermokarst lakes across the Qinghai-Tibet plateau. Front. Microbiol. 13, 944646 (2022).
Song, Y. et al. Antibiotic pollution and its effects on the Spatiotemporal variation in microbial community structure and functional genes in sediment of Baiyangdian lake. Environ. Sci. 45 (8), 4904–4914 (2024).
Haglund, A. L., Lantz, P., Törnblom, E. & Tranvik, L. Depth distribution of active bacteria and bacterial activity in lake sediment. FEMS Microbiol. Ecol. 46 (1), 31–38 (2003).
Zhou, T. X. et al. Spatial distribution of composition and diversity of aquatic bacterial communities in lake fuxian during vertical stratification period. J. Lake Sci. 34 (5), 1642–1655 (2022).
Jones, I. D., Winfield, I. J. & Carse, F. Assessment of long-term changes in habitat availability for Arctic charr(Salvelinus alpinus) in a temperate lake using oxygen profiles and hydroacoustic surveys. Freshw. Biol. 53 (2), 393–402 (2008).
Chen, Y. et al. Spatiotemporal variation characteristics of mixed layer depth and hypoxic zone during the thermal stratification decay period in a Southern Chinese reservoir: a case study of Tianshuiku reservoir in Nanning City. J. Lake Sci. 35 (5), 1623–1634 (2023).
Jia, B., Fu, W., Yu, J., Zhang, C. & Tang, Y. Relationship among sediment characteristics, eutrophication process and human activities in the Sancha Lake, Sichuan, Southwestern China. China Environ. Sci. 33 (9), 1638–1644 (2013).
Ruban, V., Brigault, S., Demare, D. & Philippe, A. M. An investigation of the origin and mobility of phosphorus in freshwater sediments from Bort-Les-Orgues Reservoir, France. J. Environ. Monit. 1 (4), 403–407 (1999).
Ruban, V. et al. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments — a synthesis of recent works. Fresenius J. Anal. Chem. 370 (2), 224–228 (2001).
Xu, N., Tan, G. C., Wang, H. Y. & Gai, X. Effect of Biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil. Biol. 74, 1–8 (2016).
Schloss, P. D., Gevers, D. & Westcott, S. L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One 6 (12), e27310 (2011).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 (D1), D590–D596 (2013).
Jiao, S. & Lu, Y. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Chang. Biol. 26 (8), 4657–4668 (2020).
Hair, J. F. Multivariate Data Analysis: An Overview (Springer, 2011).
Wu, P. et al. Unraveling the spatial-temporal distribution patterns of soil abundant and rare bacterial communities in china’s subtropical mountain forest. Front. Microbiol. 15, 1323887 (2024).
Ling, N. et al. Insight into how organic amendments can shape the soil Microbiome in long-term field experiments as revealed by network analysis. Soil. Biol. Biochem. 99, 137–149 (2016).
Chen, C., Shen, Q., Zhong, J., Liu, C. & Fan, C. Distribution characteristics of phosphorus in surface sediments during seasons of algae blooms in bloom-accumulation area in the Taihu lake. Resour. Environ. Yangtze River Basin 23 (9), 1258–1264 (2014).
Zhang, X., Zhao, Y. & Jin, Y. Diversity and phylogenetic analysis of bacteria in sediments of Xiaokou sites in lake Wuliangsuhai. J. Inner Mongolia Agric. Univ. (Nat. Sci. Ed.) 32 (4), 206–212 (2011).
Welch, D. B. M. & Huse, S. M. Microbial diversity in the deep sea and the underexplored rare biosphere. In Handbook of Molecular Microbial Ecology II: Metagenomics in Different Habitats. 243–252 (2011).
Li, Y. et al. Divergent adaptation strategies of abundant and rare bacteria to salinity stress and metal stress in polluted Jinzhou Bay. Environ. Res. 245 (Mar.), 118030 (2024).
Chu, B. T. T. et al. Metagenomics reveals the impact of wastewater treatment plants on the dispersal of microorganisms and genes in aquatic sediments. Appl. Environ. Microbiol. 84 (5), e02168–e02117 (2018).
Yuan, W. et al. The vertical distribution of bacterial and archaeal communities in the water and sediment of lake Taihu. FEMS Microbiol. Ecol. 71 (2), 263–276 (2010).
Keshri, J., Pradeep Ram, A. S. & Sime-Ngando, T. Distinctive patterns in the taxonomical resolution of bacterioplankton in the sediment and pore waters of contrasted freshwater lakes. Microb. Ecol. 75 (3), 662–673 (2017).
Cheng, H. et al. Changes of bacterial communities in response to prolonged hydrodynamic disturbances in the eutrophic water-sediment systems. Int. J. Environ. Res. Public. Health. 16 (20), 3868 (2019).
Liu, L., Yang, J., Yu, Z. & Wilkinson, D. M. The biogeography of abundant and rare bacterioplankton in the lakes and reservoirs of China. ISME J. 9 (9), 2068–2077 (2015).
Jiao, C. et al. Abundant and rare bacterioplankton in freshwater lakes subjected to different levels of tourism disturbances. Water 10 (8), 16 (2018).
Jiao, S., Chen, W. & Wei, G. Biogeography and ecological diversity patterns of rare and abundant bacteria in oil-contaminated soils. Mol. Ecol. 26 (19), 5305–5317 (2017).
Zhang, W. et al. The diversity and biogeography of abundant and rare intertidal marine microeukaryotes explained by environment and dispersal limitation. Environ. Microbiol. 20 (2), 462–476 (2018).
Dang, C. et al. Rare biosphere regulates the planktonic and sedimentary bacteria by disparate ecological processes in a large source water reservoir. Water Res. 216, 118296 (2022).
Yu, X. et al. Seasonal changes of prokaryotic microbial community structure in Zhangjiayan reservoir and its response to environmental factors. Sci. Rep. 14 (1), 1–14 (2024).
Wan, Y., Ruan, X., Zhang, Y. & Li, R. Illumina sequencing-based analysis of sediment bacteria community in different trophic status freshwater lakes. Microbiol. Open 6(4), e00450 (2017).
Yin, X. et al. Composition and predictive functional analysis of bacterial communities in surface sediments of the Danjiangkou reservoir. J. Lake Sci. 30 (4), 1052–1053 (2018).
Winters, A. D., Marsh, T. L., Brenden, T. O. & Faisal, M. Molecular characterization of bacterial communities associated with sediments in the Laurentian great lakes. J. Gt Lakes Res. 40 (3), 640–645 (2014).
Goberna, M. & Verdú, M. Cautionary notes on the use of co-occurrence networks in soil ecology. Soil. Biol. Biochem. 166, 108534 (2022).
De Vries, F. T. et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9 (1), 1–12 (2018).
Lynch, M. D. J. & Neufeld, J. D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 13 (4), 217 (2015).
Li, X. et al. Comparing diversity patterns and processes of microbial community assembly in water column and sediment in lake Wuchang, China. PeerJ 11, e14592 (2023).
Acknowledgements
This research was funded by Student Research Training Program(242005).
Funding
This research was funded by Student Research Training Program(242005).
Author information
Authors and Affiliations
Contributions
Y.L. writing - review & editing, investigation, conceptualization. Y.L. and Y.W. writing - original draft, visualization, project administration. Z.L. and S.L. writing - original draft, visualization. S.G. and Y.L. methodology, investigation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Li, Y., Wu, Y. et al. Structure and community assembly of rare bacterial community in sediments of Sancha Lake. Sci Rep 16, 2162 (2026). https://doi.org/10.1038/s41598-025-31889-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31889-z