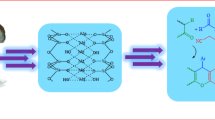
Abstract
This study investigates environmentally friendly and efficient protocols for synthesizing two distinct heterocyclic scaffolds: pyranopyrazoles and dihydropyranochromenes. Dihydropyranochromenes were synthesized using talc in refluxing ethanol, while pyranopyrazoles were obtained from solvent-free under mixing conditions. The advantages of these methods include high yields, operability, and the use of an accessible, inexpensive, and non-toxic basic catalyst. The reaction conditions, including reaction time, molar ratios of reactants, and catalyst amount, were optimized for the synthesis of products, resulting in high yields of 97% for pyranopyrazoles and 95% for dihydropyranochromenes. The synthesized products were characterized using analytical techniques, including FTIR and NMR, which confirmed the successful synthesis. In this study, total energy and band gap energy calculations were performed for dihydropyranochromenes and pyranopyrazoles derivatives. This suggests that talc powder a natural catalyst, can enhance organic synthesis and offer a sustainable and environmentally friendly solution for modern organic chemistry.
Similar content being viewed by others
Introduction
Heterocyclic compounds form the backbone of many pharmaceuticals, agrochemicals, and functional materials1,2. The development of efficient and sustainable synthetic routes for these compounds is of great interest. Multicomponent reactions (MCRs) have emerged as powerful tools in organic synthesis due to their atom economy, high efficiency, and the formation of complex molecules in a single step3,4. This study focuses on the synthesis of two important heterocyclic systems: dihydropyranochromenes and pyranopyrazoles, using talc as a catalyst under both solvent-and solvent-free conditions.
Dihydropyranochromenes are heterocyclic scaffolds with diverse biological activities in terms of their anticancer5, anti-inflammatory6, and antioxidant7 properties. Pyranopyrazoles are another class of heterocyclic compounds with biological activities8 and drug enhancers9 and have been used as antibacterial10,11, anticancer12, analgesic13, and antipyretic agents14. Given their importance, the development of efficient and environmentally friendly methods for their synthesis has become a vital research area.
Traditional methods for synthesizing pyranopyrazoles typically involve the use of solvents, extreme reaction conditions (such as high temperatures and pressures), and complex, costly catalysts, which contribute to significant environmental pollution15,16,17. These challenges underscore the necessity for sustainable and cost-effective alternatives that minimize environmental impacts while achieving high product yields. In response, a mechanochemical process using a mixer mill has been introduced as a green and efficient method for synthesizing pyranopyrazoles. This approach eliminates the need for organic solvents, allows the reactions to occur at ambient temperature and atmospheric pressure, reduces energy consumption, and facilitates easy control over the reaction conditions.
Pyranopyrazole is a synthetic heterocyclic framework composed of pyran and pyrazole moieties. Pyranopyrazoles exist in four isomeric forms (Fig. 1). Among the four isomers, 4H-pyrano [2, 3-c]-pyrazole is the most privileged structuredue to its versatile biological profile.
Previously, several catalysts have been applied for the synthesis of pyranopyrazol such as, Fe3O4@SiO2@(CH2)3NH@CC@Imidazole@SO3H18, CaO@SiO2-SO3H19, RuIII@CMC/Fe3O420, NiFe2O4@SiO2-H14[NaP5W30O110]21, Fe3O4@GO22, Fe3O4@THAM-SO3H23, H14 [NaP5W30O110]24.
Dihydropyranochromenes have been synthesized previously in the presence of sodium acetate25, DABCO26, zinc chloride27, SB-DABCO@eosin28, rGO@ Fe3O429 and Silica-Bonded N-Propylpiperazine Sodium n-Propionate30.
Talс (Mg3Si4O10(OH)2)31, with Lewis base sites, is a green, available, low-cost catalyst. Talc can be used to promote base-catalyzed organic reactions such as tetrahydrobenzo[b]pyrans and benzo[f]chromen31. This study investigates the one-pot multicomponent synthesis of dihydropyranochromenes and pyranopyrazoles using talc powder as the catalyst. The objective of this study is to develop efficient, environmentally friendly, and economically viable synthesis routes for these significant heterocyclic compounds.
Results and discussion
Characterization of talk powder
FESEM shows the average particle size of the catalyst (506–759 nm).TGA analysis prove that talk is in thermally stable state. The percentage composition of O, Si, Mg, elements in talk is 54.10, 25.38 and 18.85 respectively which determined by EDX. as, BET, Vp and pore diameter were 5.9994 m2 g− 1, 0.026818 cm3 g− 1, and 17.686 nm respectively31.
Synthesis and characterization of Pyranopyrazoles under mixer milling condition
To evaluate the catalytic role of talc powder in the synthesis of pyranopyrazoles, the reaction was carried out in ethanol under reflux conditions as well as using a mixer mill at room temperature, both with and without the catalyst. The yield of pyranopyrazoles was 19% after 20 min for the reaction without a catalyst, while the talc powder-catalyzed experiment using a mixer mill achieved a 97% yield after just 5 min. This significant difference indicates the importance of a catalyst in the reaction steps. The proposed roles of the talc powder catalyst in the reaction mechanism are shown in Fig. 2.
After the characterization of the basic talc powder, it was used for the synthesis of pyranopyrazoles. To optimize the reaction conditions, 4-nitrobenzaldehyde, hydrazine hydrate, ethyl acetoacetate, and malononitrile were used under different conditions, such as catalyst amount, temperature, and solvents (Fig. 3; Table 1).
Also, the model reaction was carried out in a stainless-steel vial and was conducted with two stainless-steel balls with a diameter of 0.8 mm at frequencies of 10, 15, and 20 Hz at room temperature using a mixer mill. At the end of the reaction, hot ethanol was added, and the entire reaction mixture was scraped; then the catalyst was separated. The progress of the reaction was monitored using thin-layer chromatography (TLC) and n-hexane, ethyl acetate (4:1) eluent. With the optimized reaction conditions (Table 1, entry 11), the best result was obtained using a mixer mill (frequency 20 Hz) and 0.04 g of catalyst without any solvent. The reaction conditions, including the molar ratio of the reactants, amount of catalyst, and milling time, were optimized to achieve the maximum yields (Fig. 4; Table 2).
The reusability of catalyst for synthesis of pyranopyrazole, was investigated in model reaction for six runs. After each run, the catalyst was separated from the reaction mixture and washed with ethanol, dried in room temperature and reused in another run (Fig. 5).
Synthesis and characterization of dihydropyrano[3,2-c]chromenes
To evaluate the catalytic role of talc powder in the synthesis of dihydropyranochromanes, the reaction was conducted in ethanol under reflux conditions, both with and without the catalyst. The results showed a yield of only 10% for dihydropyranochromanes after 90 min without the catalyst, whereas a remarkable 95% yield was achieved in the presence of talc powder after the same duration. These findings underscore the necessity of a catalyst in the reaction. The proposed roles of talc powder as a catalyst in the reaction mechanism are illustrated in Fig. 6.
The talc catalyst was employed under various conditions for the synthesis of the dihydropyranochromenes (Fig. 7). To optimize the reaction parameters, 4-nitrobenzaldehyde (1 mmol), 4-hydroxy coumarin (1 mmol), and malononitrile (1 mmol) were subjected to different experimental conditions, including varying amounts of catalyst and the usage of a mixer mill operating at frequencies of 10, 15, and 20 Hz at room temperature (Table 3). In addition, the reactions were conducted using a thermal stirrer with various solvents. The progress of the reaction was monitored using TLC in a solvent system of n-hexane and ethyl acetate (4:1). At the conclusion of the reaction, the catalyst was separated, and the product was worked up by adding water. Under the optimized reaction conditions (as detailed in Table 3, entry 11), the best results were achieved using ethanol as the solvent and 0.06 g of catalyst (Fig. 8; Table 4).
The reusability of catalyst in synthesis of dihydropyranochromene was studied in model reaction for six runs. After each run, the catalyst was separated from the reaction mixture by filtration and washed with ethanol, dried at room temperature and reused in next run (Fig. 9).
Computational methods
In this article, we computed the stability of pyranopyrazoles and dihydropyranochromene derivatives using density functional theory (DFT). The simulations were carried out using the Becke-3-Lee–Yang–Parr (B3LYP) method, with a 6–311 G (d, p) basis set, in the Gaussian 09 software37,38,39,40,41,42,43. The HOMO-LUMO energy simulations were performed to evaluate the energetic behaviour of the compounds. The optimization and visualization of the charge distribution in the compounds were carried out with the GaussView 05 software.
The calculations of ELUMO, EHOMO, band gap (Eg=ELUMO-EHOMO) and the total energy for all dihydro pyranochromene derivatives and pyranopyrazoles are illustrated in Tables 5 and 6. The results of these tables indicate that the stability of compounds 7a and 7b is greater than that of the other reported compounds, which is consistent with the experimental data44.
Additionally, Figs. 10 and 11 showcase the optimized structures and the frontier molecular orbital diagram related to compounds 7a and 7b, respectively.
Experimental
Materials and methods
All chemicals were purchased from Merck and Sigma-Aldrich without further purification. We have used the exact same talc material from our earlier publication31 without any modification. All yields refer to isolated products, which were characterized by spectral data. FT-IR spectra were run on a Bruker Equinox 55 spectrometer. The nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 or DMSO-d6 on a Bruker Avance NMR 400 MHz. The melting points were determined on a Buchi B-540 apparatus. A mixer mill model Retsch MM 400, which consisted of two stainless steel vials, was used.
Synthesis of pyranopyrazole in the presence of talc powder
In a stainless-steel mixer mill vessel, a mixture of ethylacetoacetate (1 mmol), hydrazine hydrate (1.5 mmol), aldehydes (1 mmol), malononitrile (1 mmol), and talc powder (0.04 g) was milled at 20 Hz. The reaction progress was monitored by TLC (n-hexane: ethyl acetate [4:1]). After completion of the reaction, hot ethanol was added, and the products were easily separated from the catalyst and purified using recrystallization in ethanol.
Synthesis of dihydropyranochromene in the presence of talc powder
For synthesis of the dihydropyranochromene derivatives after determining the optimal conditions, a mixture of malononitrile (1 mmol), 4-hydroxycoumarin (1 mmol), benzaldehyde derivative (1 mmol), and talc (0.06 g) in ethanol (10 mL) was refluxed for an appropriate time. After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature, and the catalyst was filtered. Then, separation was carried out by adding water to obtain the desired dihydropyranochromene derivatives.
Hot filtration for synthesis of dihydropyranochromene
A mixture of malononitrile (1 mmol), 4-hydroxycoumarin (1 mmol), 4-nitrobenzaldehyde derivative (1 mmol), and talc (0.06 g) in ethanol (10 mL) was refluxed for 45 min. The progress of reaction was monitored by TLC. The conversion yield was 60–65%. In this step, the catalyst was removed from reaction mixture by filtration. The filtrate was refluxed for another 45 min. The conversion yield was 65–70%. This evidence shows that the catalyst is heterogeneous ones with no any leaching in reaction m.
edium.
Conclusions
This paper has demonstrated the versatility of talc powder as a catalyst in multicomponent reactions for the synthesis of pyranopyrazoles and dihydropyranochromanes. The use of talc powder in both solution phase and solvent-free mixer milling conditions has provided efficient, environmentally friendly, and economically viable routes for the synthesis of these important heterocyclic compounds. This suggests that talc powder, as a natural catalyst, can enhance organic synthesis and offer a sustainable and environmentally friendly catalyst for modern organic chemistry. The DFT simulations indicate that compound 7 is more stable for both dihydropyranochromenes and pyranopyrazoles than the other reported compounds, aligning with the experimental data.
Data availability
All data generated or analyzed during this study are included in supplementary.
References
Arora, P., Arora, V., Lamba, H. & Wadhwa, D. Importance of heterocyclic chemistry: a review. Int. J. Pharm. Sci. Res. 3, 2947 (2012).
Al-Mull, A. A review: biological importance of heterocyclic compounds. Der Pharm. Chem. 9, 141–147 (2017).
Jiang, B., Rajale, T., Wever, W., Tu, S. J. & Li, G. Multicomponent reactions for the synthesis of heterocycles. Chem. Asian J. 5, 2318–2335 (2010).
Rahmati, A. & Pashmforoush, N. Synthesis of various heterocyclic compounds via multi-component reactions in water. J. Iran. Chem. Soc. 12, 993–1036 (2015).
Bhosle, M., Wahul, D., Bondle, G., Sarkate, A. & Tiwari, S. An efficient multicomponent synthesis and in vitro anticancer activity of dihydropyranochromene and chromenopyrimidine-2, 5-diones. Synth. Commun. 48, 2046–2060 (2018).
Pratap, R. & Ram, V. J. Natural and synthetic Chromenes, fused Chromenes, and versatility of dihydrobenzo [H] Chromenes in organic synthesis. Chem. Rev. 114, 10476–10526 (2014).
Eslaminejad, T., Mirzaei, E. F. & Abaszadeh, M. Synthesis, antioxidant, cytotoxicity, induce apoptosis investigation and Docking study of new halogenated Dihydropyrano [3, 2-b] chromene-3-carbonitrile derivatives on MCF-7 breast cancer cell line. Iran J. Pharm. Res. IJPR. 22, e132932 (2023).
Mamaghani, M. & Hossein Nia, R. A review on the recent multicomponent synthesis of Pyranopyrazoles. Polycycl. Arom Comp. 41, 223–291 (2021).
Sobh, E. A., Kassab, A. E., El-Khouly, E. A. & Hassan, M. S. New Pyranopyrazole based derivatives: design, synthesis, and biological evaluation as potential topoisomerase II inhibitors, apoptotic inducers, and antiproliferative agents. Bioorg. Chem. 144, 107158 (2024).
Ismail, M. M., Khalifa, N. M., Fahmy, H. H., Nossier, E. S. & Abdulla, M. M. Design, docking, and synthesis of some new pyrazoline and Pyranopyrazole derivatives as anti-inflammatory agents. J. Heterocycl. Chem. 51, 450–458 (2014).
Farooq, S. & Ngaini, Z. Recent synthesis of mono-& bis‐pyranopyrazole derivatives. ChemistrySelect 9, e202400028 (2024).
Asif, M. et al. Lewis base-catalyzed synthesis of highly functionalized spirooxindole-pyranopyrazoles and their in vitro anticancer studies. Med. Chem. Res. 32, 1001–1015 (2023).
Priya, D. et al. Structural insights into pyrazoles as agents against anti-inflammatory and related disorders. ChemistrySelect 7, e202104429 (2022).
Ganguly, S. K. & Jacob, S. Therapeutic outlook of pyrazole analogs: a mini review. Mini Rev. Med. Chem. 17, 959–983 (2017).
Al-Amiery, A. A. et al. Novel pyranopyrazoles: synthesis and theoretical studies. Molecules 17, 10377–10389 (2012).
Yadav, A. R. et al. Review on advancements of pyranopyrazole: synthetic routes and their medicinal applications. Mol. Divers. 1, 1–48 (2024).
Noory Fajer, A., Khabt Aboud, H., Al-Bahrani, H. A. & Kazemi, M. Recent advances on multicomponent synthesis of Pyranopyrazoles using magnetically recoverable nanocatalysts. Polycycl. Arom Compd. 44, 4932–4978 (2024).
Akbarpour, T., Yousefi Seyf, J., Khazaei, A. & Sarmasti, N. Synthesis of Pyrano [2, 3-C] pyrazole derivatives using a novel ionic-liquid based nano-magnetic catalyst (Fe3O4@ SiO2@(CH2) 3NH@ CC@ imidazole@ SO3H + Cl–). Polycycl. Arom Compd. 42, 3844–3864 (2022).
Sameri, F., Mobinikhaledi, A. & Bodaghifard, M. A. Preparation of core/shell Cao@ SiO2-SO3H as a novel and recyclable nanocatalyst for one-pot synthesize of Dihydropyrano [2, 3-C] pyrazoles and tetrahydrobenzo [B] Pyrans. Silicon 14, 1395–1406 (2022).
Chen, Y. et al. CMC/Fe3O4 hybrid: an efficient, magnetic, retrievable, self-organized nanocatalyst for green synthesis of Pyranopyrazole and polyhydroquinoline derivatives. Mol. Divers. 23, 421–442 (2019).
Maleki, B., Baghayeri, M., Abadi, S. A. J., Tayebee, R. & Khojastehnezhad, A. Ultrasound promoted facile one pot synthesis of highly substituted Pyran derivatives catalyzed by silica-coated magnetic NiFe2O4 nanoparticle-supported H14[NaP5W30O110] under mild conditions. RSC Adv. 6, 96644–96661 (2016).
Khaleghi Abbasabadi, M., Azarifar, D. & Esmaili Zand, A. H. R. Sulfonic acid-functionalized Fe3O4‐supported magnetized graphene oxide quantum dots: a novel organic‐inorganic nanocomposite as an efficient and recyclable nanocatalyst for the synthesis of Dihydropyrano [2, 3‐c] pyrazole and 4H‐chromene derivatives. App Organomet. Chem. 34, e6004 (2020).
Faroughi Niya, H., Hazeri, N. & Maghsoodlou, M. T. Synthesis and characterization of Fe3O4@ THAM-SO3H as a highly reusable nanocatalyst and its application for the synthesis of Dihydropyrano [2,3‐c] pyrazole derivatives. App Organomet. Chem. 34, e5472 (2020).
Heravi, M., Ghods, A., Derikvand, F., Bakhtiari, K. & Bamoharram, F. H14[NaP5W30O110] catalyzed one-pot three-component synthesis of Dihydropyrano [2, 3-c] pyrazole and Pyrano [2, 3-d] pyrimidine derivatives. J. Iran. Chem. Soc. 7, 615–620 (2010).
Maghsoodlou, M. T. et al. A green and novel three-component one-pot synthesis of tetrahydrobenzopyran, Pyrano [2, 3-d] pyrimidine, and 3, 4-dihydropyrano [c] Chromene derivatives using sodium acetate. Iran. J. Org. Chem. 6, 1197–1202 (2014).
Shinde, S., Rashinkar, G. & Salunkhe, R. DABCO entrapped in agar-agar: a heterogeneous gelly catalyst for multi-component synthesis of 2-amino-4H-chromenes. J. Mol. Liq. 178, 122–126 (2013).
Srinivas, V. One-pot synthesis of 2-amino-5, 10-dihydro-5, 10-dioxo-4-phenyl-4 h-benzo [g] Chromene derivatives catalyzed by ZnCl2. Synth. Commun. 41, 806–811 (2011). Rao, V.R.
Jarrahi, M., Maleki, B. & Tayebee, R. Magnetic nanoparticle-supported Eosin Y salt [SB-DABCO@ Eosin] as an efficient heterogeneous photocatalyst for the multi-component synthesis of Chromeno [4, 3-b] Chromene in the presence of visible light. RSC Adv. 12, 28886–28901 (2022).
Bayzidi, M. & Zeynizadeh, B. The immobilized zirconocene chloride on magnetite-reduced graphene oxide: a highly efficient and reusable heterogeneous nanocatalyst for one‐pot three‐component synthesis of tetrahydrobenzo [b] pyrans and dihydropyrano [3, 2‐c] chromenes. ChemistrySelect 7, e202202708 (2022).
Niknam, K. & Jamali, A. Silica-bonded N-propylpiperazine sodium N-propionate as recyclable basic catalyst for synthesis of 3, 4-dihydropyrano [c] Chromene derivatives and biscoumarins. Chin. J. Catal. 33, 1840–1849 (2012).
Hajihasani Bafghi, M., Bamoniri, A. & Mirjalili, B. F. Talc as a natural mineral catalyst for the one pot-three component synthesis of benzo[f]chromene, dihydropyrano[3,2-c]chromene, and tetrahydrobenzo[b]pyran derivatives under different conditions. Sci. Rep. 15, 14167 (2025).
Dehghani Tafti, A., Mirjalili, B. F., Bamoniri, A. & Salehi, N. Rapid four-component synthesis of Dihydropyrano [2, 3-c] pyrazoles using nano-eggshell/Ti (IV) as a highly compatible natural based catalyst. BMC Chem. 15, 6 (2021).
Aliabadi, R. S. & Mahmoodi, N. O. Green and efficient synthesis of Pyranopyrazoles using [Bmim][OH–] as an ionic liquid catalyst in water under microwave irradiation and investigation of their antioxidant activity. RSC Adv. 6, 85877–85884 (2016).
Pore, D. et al. Green access to novel Spiro Pyranopyrazole derivatives. Tetrahedron Lett. 54, 5876–5878 (2013).
Kanakaraju, S., Prasanna, B., Basavoju, S. & Chandramouli, G. Ammonium acetate catalyzed an efficient one-pot three component synthesis of Pyrano [3, 2-c] Chromene derivatives. Arab. J. Chem. 10, S2705–S2713 (2017).
Ghorbanipour, F., Nezhad, S. M., Pourmousavi, S. A., Zare, E. N. & Heidari, G. Superparamagnetic polymer nanocomposite as a catalyst for the synthesis of Pyrano [3, 2-c] chromene, Pyrano [2, 3-c] pyrazole, and benzylpyrazolyl coumarin. Inorg. Chem. Commun. 147, 110271 (2023).
Volokhov, V. M. et al. Quantum-chemical calculations of the enthalpy of formation of isomeric 5/6/5 tricyclic Tetrazolotetrazine derivatives annelated with Nitroazoles. Russ J. Phys. Chem. B. 18, 28–36 (2024).
Khakimov, D. V., Svitanko, I. V. & Pivina, T. S. Computational insight into the crystal structures of Cubane and Azacubanes. J. Mol. Model. 30, 93 (2024).
Umar, Y. & El-Rayyes, A. A. Theoretical investigation of the vibrational and electronic properties of Tetraphenylammonium and its boron, aluminum, gallium, carbon, silicon, germane, phosphorus and arsenic analogues. Comput. Theor. Chem. 1231, 114423 (2024).
Mehta, N. & Martin, J. M. L. On the sensitivity of computed partial charges toward basis set and (exchange-) correlation treatment. J. Comput. Chem. 45, 1017–1032 (2024).
Tognetti, V. & Joubert, L. Exchange-correlation effects in interatomic energies for pure density functionals and their application to the molecular energy prediction. J. Comput. Chem. 45, 2270–2283 (2024).
Divya, P., Jeba Reeda, V. S. & Bena Jothy, V. Fungicide compound 2, 3-dichloronaphthalene-1,4-dione: non-covalent interactions (QTAIM, RDG and ELF), combined vibrational spectroscopic investigations using DFT approach with experimental analysis, electronic, molecular Docking scrutiny in-vitro assay and thermodynamic property analysis. J. Mol. Liq. 400, 124544 (2024).
Bissi Nyandou, P. F., Noudem, P., Mveme, C. D. D., Fouejio, D. & Zekeng, S. S. DFT study of the intrinsic electronic, optical, NBO, transport, and thermodynamic properties of 12,12-dimethyl-7-phenyl-7,12-dihydrobenzo[a]acridine-3-carbonitrile (BACN). Chin. J. Phys. 89, 1862–1882 (2024).
Mallah, D., Mirjalili, B. F., Basharnavaz, H. & Bamoniri, A. B(III)-catalyzed synthesis of Spirooxindole and dihydro-2-oxopyrrole under solventless conditions in a ball mill, along with DFT computations. RSC Adv. 15, 25949–25964 (2025).
Acknowledgements
The Research Council of Yazd University is gratefully acknowledged for financial support of this work.
Author information
Authors and Affiliations
Contributions
AD, BFM and HB designed and performed the research, analysed the data, interpreted the results, and prepared the manuscript. AD performed the assay and conducted the optimization, and purification of compounds. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dehghanizadeh, A., Mirjalili, B.B.F. & Basharnavaz, H. Natural talc: a basic, cost-effective, and available catalyst for the one-pot synthesis of dihydropyranochromenes and pyranopyrazoles, along with related DFT calculations. Sci Rep 16, 2464 (2026). https://doi.org/10.1038/s41598-025-32187-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32187-4