Abstract
Hyperspectral imaging in the mid-infrared (MIR) spectral range provides unique molecular specificity by probing fundamental vibrational modes of molecular bonds, making it highly valuable for biomedical and biochemical applications. However, conventional MIR imaging techniques often rely on high-intensity illumination that can induce photodamage in sensitive biological tissues. Single-photon MIR imaging offers a label-free, non-invasive alternative, yet its broader adoption is hindered by the lack of efficient, room-temperature MIR single-photon detectors. We present a single-photon hyperspectral imaging platform that combines cavity-enhanced spontaneous parametric down-conversion (SPDC) with nonlinear frequency up-conversion. This approach enables MIR spectral imaging using cost-effective, visible-wavelength silicon single-photon avalanche diodes (Si-SPADs), supporting room-temperature, low-noise, and high-efficiency operation. By leveraging time gating and intensity correlations of photon pairs generated via SPDC, we can effectively suppress classical background noise and enhance the signal-to-noise ratio, approaching the shot-noise limit. We demonstrate chemically specific single-photon imaging across the 2.9–3.6 \({\upmu }\)m range on biological (egg yolk, yeast) and polymeric (polystyrene, polyethylene) samples. This platform paves the way toward scalable, quantum-enabled MIR imaging for applications in molecular diagnostics, environmental sensing, and biomedical research.
Similar content being viewed by others
Introduction
The development of label-free bioimaging techniques with high chemical specificity and minimal perturbation is important for advancing both fundamental biomedical research and clinical diagnostics1,2,3. Mid-infrared (MIR) light that covers the molecular fingerprint region from approximately 2.5–20 \(\mu\)m is uniquely positioned to address this need due to their ability to directly probe fundamental vibrational modes of molecular bonds4,5,6,7. These intrinsic vibrational signatures provide unambiguous chemical contrast for key biomolecular components such as proteins, lipids, and nucleic acids, allowing detailed analysis of tissue composition, microbial presence, metabolic states, and disease-related biochemical changes8,9. Methods such as near-infrared Raman spectroscopy and Fourier transform infrared (FTIR) spectroscopy have demonstrated considerable potential10,11,12. However, biological tissues can be highly sensitive to optical exposure, and conventional high-intensity illumination can cause photodamage, induce molecular alterations, or disrupt physiological functions13,14. To address these limitations, single-photon MIR imaging techniques have emerged as a promising alternative, providing label-free chemical contrast while substantially minimizing optical perturbation. This advancement offers a viable pathway to truly non-invasive molecular imaging in biological systems.
Traditional single-photon detection in the MIR region faces several critical limitations that restrict its performance and practical use15. A major challenge is the requirement for cryogenic cooling to suppress dark counts and achieve acceptable sensitivity16,17. Conventional MIR detectors such as mercury cadmium telluride (MCT) and blocked impurity band (BIB) detectors exhibit significantly lower performance compared to their visible and near-infrared counterparts. MCT detectors are limited by thermal noise and dark current, resulting in dark count rates on the order of \(10^4\)–\(10^6\) cps at 77–200 K, which makes true single-photon MIR operation impractical. BIB detectors, while offering excellent low-noise photoconductive performance, are not inherently single-photon sensitive and require multiple photons to exceed the noise floor, typically achieving 10–\(10^3\) cps at 4–20 K 18. Emerging superconducting technologies, including superconducting nanowire single-photon detectors (SNSPDs) and transition-edge sensors (TESs), have demonstrated the potential for genuine single-photon sensitivity in the MIR, with SNSPDs already showing high internal detection efficiency up to \(\sim 4~{{{\upmu }m}}\) and sensitivity extended to \(29~{{{\upmu }m}}\), effectively covering the entire molecular fingerprint region19,20,21,22,23,24,25. One limiting factor is that they require deep cryogenic operation (SNSPDs: 0.3–3 K; TESs: 50–100 mK), making them bulky and costly15. SNSPDs achieve dark count rates of 0.1–100 cps, timing jitter of 10–100 ps, and quantum efficiencies up to 95% in the near-IR (dropping below 40% in the MIR). TESs operate calorimetrically with dark counts \(<0.01\) cps, energy resolution of a few eV, and relative slow recovery times of microseconds to milliseconds. In direct MIR detection, SNSPDs typically exhibit hundreds of dark counts per second; however, achieving high system detection efficiency at 3 \({\upmu }\)m—including optimized optical absorption in the nanowire layer - remains to be explored19,22,23,26,27. A persistent obstacle for all direct MIR detectors is blackbody radiation from room-temperature optics, filters, and fibers, which can dominate the background count rate by many orders of magnitude. Achieving true single-photon operation therefore requires cold optics, narrow étendue, ultra-narrowband spectral filtering, and/or time gating to drastically suppress thermal background photons.
Different nonlinear optical techniques have been explored to enable room-temperature MIR bioimaging at the single-photon level, including imaging with undetected MIR photons using nonlinear interferometers28,29,30 and nonlinear up-conversion detection31,32,33,34. While undetected-photon microscopy requires precise interferometric alignment and suffers from low photon conversion efficiency, up-conversion schemes convert MIR photons to the visible range via second-order nonlinear interactions and enable detection with silicon single-photon avalanche diodes (Si-SPADs). Narrow phase-matching, spectral and spatial filtering, and time-gating can suppress nonlinear fluorescence, blackbody radiation, and pump-induced noise, so that only spectrally, spatially, and temporally mode-matched photons are detected, yielding dark counts 3–4 orders of magnitude below cryogenic MCT or BIB detectors18. Compared to direct MIR detection with SNSPDs or TESs, which still experience thermal background unless heavily filtered, up-conversion techniques achieve similar dark count rates (< 200 cps) using simple room-temperature optics. By implementing spontaneous parametric down-conversion (SPDC) and coincidence detection between signal and idler photons, background noise and pump induced noise can further be suppressed, enabling true single-photon sensitivity for low-light imaging and spectroscopy35. Recent advances demonstrate the potential for high-speed imaging and molecular fingerprinting in biomedical research32,34,36, while avoiding the operational complexity and cryogenic requirements of SNSPDs and TESs.
In this work, we present a novel MIR single-photon imaging platform for hyperspectral analysis of polymeric and biological samples. Using a cavity-enhanced SPDC source, we generate time-correlated photon pairs with tunable idler wavelengths from 2.9 to 3.6 µm. This tunability enables chemically specific hyperspectral imaging with single-photon sensitivity, allowing detailed molecular analysis while minimizing photodamage7,37. The signal (near-infrared) and idler (mid-infrared) photons are independently upconverted via second-order nonlinear processes to visible and near-infrared wavelengths and detected using Si-SPADs. Coincidence gating between channels effectively suppresses uncorrelated background, enhancing sensitivity at ultralow photon flux. This dual-path upconversion scheme provides efficient, room-temperature, low-noise detection of MIR single photons. Compared to typical MIR upconversion methods which rely on classical MIR sources and intensity detection31,34,36, our approach achieves high sensitivity, spectral resolution, tunability, and noise rejection-making it suitable for label-free hyperspectral imaging of biological samples under minimally invasive conditions. Our room-temperature detection approach achieves comparable signal-to-noise ratios for MIR spectroscopy through coincidence normalization, mitigating dark noise without the need for cryogenic cooling19,20,27.
Experimental configuration
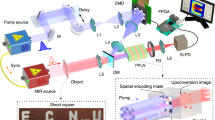
The experimental setup is depicted in Fig. 1 and consists of four main components: a tunable mid-infrared (MIR) single-photon source based on cavity-enhanced SPDC, a single-pass upconversion unit, high-efficiency Si-SPADs (Thorlabs, SPDMH3F) with quantum efficiencies exceeding 65% for the upconverted photons and dark count rates of 250 Hz, and a custom-designed MIR microscope with low-loss optics for MIR light. The photon-pair source is driven by an actively Q-switched nanosecond Nd:YAG laser (Sol compact Q-switched DPSS laser, Bright Solutions) operating at a central wavelength of 1064nm , with a pulse duration of 15 ns and a repetition rate of 40 kHz. The pump pulses are focused into a 40 mm long fan-out structured periodically poled lithium niobate (PPLN) crystal (HC Photonics), which is positioned at the center of a 55 mm long linear optical cavity. This cavity is formed by two identical dielectric mirrors: high-reflectivity (HR) coated for the NIR signal photons and high-transmission (HT) coated for both the idler photons and pump laser. Each mirror has a radius of curvature of 100 mm, resulting in a stable cavity configuration that produces a fundamental Gaussian mode with a beam waist of approximately 120 µm at the center of the crystal (see the inset in Fig. 1). This configuration ensures optimal spatial mode overlap between the interacting waves and efficient nonlinear conversion within the PPLN crystal. The cavity is operated between 10 to 30% of its self-oscillation threshold (300 mW) to ensure stable and low-noise generation of photon pairs via SPDC. The cavity SPDC is single-resonant for NIR signal photons (1400-1700 nm). Spectral tuning of the generated photon pairs is achieved by altering the phase-matching condition through translation of the fan-out structured PPLN crystal along its poling gradient using a high-precision stepper motor. This enables fast continuous wavelength tuning of the MIR idler photon in the 2.9–3.60 \(\mu\)m range. This wavelength range is particularly significant as it encompasses key ro-vibrational absorption bands corresponding to the fundamental stretching modes of biologically relevant functional groups, including O–H, C–H, and N–H bonds. As a result, this MIR region is exceptionally well suited for label-free, chemically selective imaging of biological samples, which we will investigate in the following.
Experimental setup for MIR single-photon hyperspectral imaging based on time-correlated single-photon counting (TCSPC) and cascaded nonlinear optical processes. A nanosecond pulsed 1064 nm laser pumps two fan-out periodically poled lithium niobate (PPLN) crystals. The first crystal is embedded within a linear optical cavity to enable cavity-enhanced spontaneous parametric down-conversion (SPDC), producing time-correlated photon pairs comprising a mid-infrared (MIR) idler photon and a near-infrared (NIR) signal photon. The NIR photon is upconverted intracavity to the visible range via sum-frequency generation (SFG) and detected by a Si-SPAD. The MIR idler photon is directed onto a biological sample mounted on a motorized XYZ translation stage. The transmitted MIR photon is subsequently upconverted to the visible via a second SFG process in the second PPLN crystal and detected by a second Si-SPAD. Arrival times of both photons are recorded using a time tagger. HWP: Half-wave plate. PBS: Polarization beam splitter. DM: Dichroic mirror. GM: Gold mirror. MEO3: 1064 nm mirror. HPF550/750: High-pass filters 550/750 nm. LPF700/950: Low-pass filters 700/950 nm. The top Insets: energy-level diagrams illustrating the cascaded nonlinear interactions involved in intra-cavity SPDC and the sum-frequency upconversion of the signal and idler photons, respectively. The detected upconverted generated wavelengths are 609-650 nm for the signal photons and 780–849 nm for the idler photons in the full spectral scanning range of the SPDC. Bottom insets: Intracavity SPDS and typical hyperspectral images.
Cascaded nonlinear optical processes
In Fig. 1, the insets depict the energy-level diagrams of the cascaded nonlinear optical processes central to our MIR imaging platform. The first stage involves the intra-cavity generation of signal and idler photons via SPDC, where a pump photon at wavelength \(\lambda _p=1064\) nm is converted into a pair of lower-energy photons-a signal photon \(\lambda _s=1510-1683\) nm and an idler photon \(\lambda _i=2900-3600\) nm. The SPDC nonlinear interaction not only conserves energy but also establishes strong time and frequency correlations between the signal and idler photons, potentially enabling quantum-enhanced measurements. Within the same nonlinear crystal, the signal photons remain confined in the cavity and undergo a second nonlinear process-sum-frequency generation (SFG) with the 1064nm pump field. This cascaded interaction results in upconverted signal photons at wavelengths \(\lambda _{\text {up,s}} = (\lambda _s\lambda _{\text {pump}})/(\lambda _s + \lambda _{\text {pump}})\), corresponding to wavelengths in the visible range (609–650 nm). These upconverted signal photons exit the cavity and are detected using Si-SPADs. The SFG process achieves an internal conversion efficiency of approximately 3–5%, depending on the 1064 nm pump power. In parallel, the idler photons generated via intra-cavity SPDC exit the cavity and are directed to the microscope to interrogate the sample. After transmission, the idler photons are coupled into a second PPLN crystal. To perform up-conversion, the idler photons are combined with a separate pump field at 1064nm using a dichroic mirror. The two beams are overlapped and co-propagated in a single-pass through the PPLN crystal, where upconversion occurs, producing upconverted photons with wavelength \(\lambda _{\text {up,i}}\) in the near-infrared range (780–849 nm). With a pump power of \({\approx 100}{mW}\), the upconversion achieves a conversion efficiency of 5–15% depending on the wavelength. These photons are subsequently detected using a second Si-SPAD. The overall efficiency of the upconversion system, accounting for all losses and conversion steps, is approximately 2–6%. The phase-matching (movement) conditions of the upconversion PPLN crystal are synchronized with those of the SPDC crystal using calibrated stepper motors, ensuring spectral alignment across the imaging range. To suppress background noise and prevent contamination from stray light, a series of high-performance optical filters is employed prior to Si-SPADs. These filters are carefully selected to match the upconverted photon bandwidths and ensure efficient rejection of pump leakage, residual fluorescence, and ambient light, thereby enhancing the signal-to-noise ratio in single-photon detection. The arrival times of both the signal and idler photons are recorded using a Time Tagger (Ultra, Swabian Instruments), a time-correlated single-photon counting (TCSPC) module that enables high-resolution temporal gating and correlation measurements.
Results
In the following, we present the characterization results of the MIR photon-pair source, along with demonstrations of low-photon-flux imaging based on time-correlated single-photon detection. The system enables rapid and continuous tuning of the MIR idler photon wavelength across the 2.9–3.6 \(\mu\)m range, enabling hyperspectral analysis of vibrational modes associated with different molecular functional groups. By implementing photon gating and correlation detection, we effectively reduce uncorrelated background noise while suppressing excessive noise of the pump laser.
Source characterization and data acquisition
A representative measurement of the SPDC source with upconverted photons is shown in Fig. 2a. A 150 ns detection gate is applied in each experimental cycle to suppress background noise and improve detection sensitivity. Detection events are recorded using a time tagger, where the 1064 nm pump synchronization output from the signal generator serves as the ‘start’ signal, and the signal and idler detections are registered as ‘stop’ events on two separate time-tagger channels (see Fig. 1). Figure 2b shows a strong intensity correlation between the gated photon counts at the two detectors, with the observed linear relationship between the signal and idler counts indicating that the upconverted signal and idler photons are strongly correlated due to a common source of intensity fluctuation from the first parametric down-conversion stage. Photon counts at both detectors are integrated for a duration of 2s, and the shaded region in Fig. 2b indicates the shot noise level corresponding to the upconverted idler count. Figure 2c compares the intensity noise of the gated photon counts (orange) with the shot noise limit (black dashed line). In spectral regions where excess intensity noise dominates, the correlation between the two detectors can be exploited to suppress this noise.
This suppression is evident below 3100nm, as indicated by the purple line in Fig. 2c. The rescaled upconverted idler count is computed as:
where \(N_{up,s}^c\) and \(N_{up,i}^c\) are the gated counts of the upconverted signal and idler photons, respectively, and \(\langle N_{up,s}^c \rangle\) is the average upconverted signal count. The effectiveness of this noise mitigation strategy depends on the trade-off between the additional shot noise introduced by the upconverted signal and the excess intensity noise present in the upconverted idler counts. An example of this rescaling applied to data at 3000nm, where excess noise is particularly significant, is shown in Fig. 2d. The counts were time-gated as in Fig. 2a to suppress contributions from dark counts and other background events. Overall the reduction in background noise is 99.4%. These measurements demonstrate that correlated photon detection combined with a rescaling strategy suppresses excess intensity noise in the upconverted idler channel and highlights the potential of correlation-based noise mitigation to improve sensitivity in upconversion-assisted SPDC imaging. At longer wavelengths (\(\ge\) 3100 nm) the increased standard deviation arises from the normalization procedure, where the upconverted idler count is divided by the upconverted signal count and scaled by its average. While effective when excess noise dominates, at longer wavelengths (\(\ge\) 3100 nm) reduced phase-matching efficiency and upconversion gain lower the signal, increasing the relative impact of shot noise and amplifying fluctuations in the correlated data.
Characterization of noise reduction and stability enhancement via signal-idler correlation. (a) shows the raw histograms of photon arrival times from the signal and idler detectors at 2950 nm. A 150 ns gating window (black dashed lines) is applied to both channels to suppress background noise. Arrival times are recorded on a time tagger relative to a trigger signal derived from the pulsed laser. (b) Correlation between detected counts in the signal and idler arms at 3000 nm, with a linear fit (black dashed line) (c) Standard deviation of idler counts before (orange) and after (purple) normalization using signal-idler correlation. Improvement is observed at wavelengths where fluctuations significantly exceed the shot noise level (black dashed line). (d) Histogram of idler counts before and after normalization at 3000 nm. The full width at half maximum (FWHM) is reduced after applying correlation-based rescaling, indicating enhanced stability.
Spectral calibration
The MIR spectra obtained from various sample types exhibited distinct absorption peaks corresponding to O–H and C–H molecular bonds. First the measured spectroscopic traces were normalized by comparing the detected photon counts with and without a polystyrene sheet, as shown in Fig. 3a. The counts without the sample were recorded during imaging to account for wavelength-dependent upconversion efficiency and background spontaneous noise. Transmission was then calculated as the ratio of the sample counts to the reference counts. Further, the initial wavelength is determined based on the mean spectrum of the up-converted signal light field, obtained through Gaussian fitting, while the pump light operates above threshold. The idler wavelength is then extrapolated based on the pump laser wavelength, measured on a traceable wavelength meter (HP 86120B). For MIR imaging, the pump laser is tuned below threshold intensity, and the crystal’s motor position is used to correlate with the wavelength determined in the previous step. To ensure more accurate spectral calibration, we employed a polystyrene (PS) standard reference sample. The PS sample was measured using both the single-photon imaging system and a commercial Fourier Transform Infrared (FTIR) spectrometer (Thermo Scientific, Nicolet iS50 FTIR) using a spectral resolution of 8\({\upmu }\)m\(^{-1}\), see Fig. 3b. The spectrum obtained from the single-photon imaging setup, measured with a 7nm step size and comprising 100 data points, was aligned with the FTIR reference spectrum by calibrating the wavelength axis using a fitting function derived from the Beer–Lambert law:
where \(\widetilde{T}_C\) (\(\widetilde{T}_{\text {SP}}\)) denotes the normalized transmission function with (without) rescaled wavelength \(\lambda '=(a\lambda +b)\), where a and b represent the first- and zero-order corrections, respectively; x denotes the thickness of the sample and \(\alpha\) denotes the absorption coefficient as a function of wavelength. The calibration function also includes R, which accounts for the exponential dependence of absorption on the varying sample thickness x across different measurements. We fit \(\widetilde{T}_C (\lambda ',Rx)\) to the FTIR reference spectrum using three free parameters: a,b, and R. This function serves as the baseline spectrum against and will be used for calibrating all acquired single-photon hyperspectral images. Figure 3b illustrates an example of the fitting procedure, with the grey line indicating the raw normalized absorption spectrum and the dashed orange line representing the spectrum after rescaling to match the FTIR reference (blue line). To ensure consistency across measurements and minimize systematic error, the same set of wavelength correction was applied uniformly to all acquired spectra and images during the calibration process.
Normalizing and calibrating the spectroscopy. (a) Comparison of detected photons counts with and without a polystyrene sheet. The counts without the sample are measured during imaging to account for wavelength-dependent up-conversion efficiency. Transmission is calculated as the ratio of these counts. (b) Calibration of the MIR wavelength axis was performed using FTIR spectral data from a reference polystyrene sheet. The solid blue trace represents the reference FTIR spectrum, acquired under controlled conditions to ensure accurate spectral positioning of characteristic vibrational modes. The solid green trace corresponds to the raw MIR single-photon spectrum obtained using our imaging system, prior to any spectral correction. The Spectra are measured using a wavelength step size of 7nm. The dashed trace shows the MIR single-photon spectrum after wavelength calibration, aligned to match the FTIR reference. This calibration procedure ensures accurate spectral assignment and consistency across subsequent hyperspectral imaging measurements.
Hyperspectral imaging of polymer samples
The results of the spectroscopic and hyperspectral imaging of the polymer samples are presented in Fig. 4. For each sample, we measure a series of spatial images between 2900 and 3600 nm with a 50 nm wavelength spacing (See the Supplementary Material for all acquired images). Each spatial image has an area of 775 \({\upmu }\)m by 775 \({\upmu }\)m with a step size of 25 \({\upmu }\)m, which is approximately the size of the MIR beam focused on the sample. Each measurement point takes 2s, resulting in a total scan time of approximately 12 hours per sample. Due to the wavelength-varying phase-matching condition at the non-linear crystal, we scan outside of the sample region to obtain a reference photon level, mitigating the nonuniform photon conversion efficiency across different wavelengths. The reference photon scan occurs before and after each spatial scan to mitigate, to the first order, the drift of photon level during imaging. The MIR optical power used for these measurements was approximately 2 fW, corresponding to an average photon flux of 40k photons per second, or roughly one MIR photon per pulse. Figure 4a shows transmission dips in aromatic C-H stretches and asymmetric aliphatic C-H stretches in polystyerene, while Fig. 4b shows asymmetric aliphatic C-H stretches and symmetric aliphatic C-H stretches in low density polyethylene (LDPE). The transmission spectra in Fig. 4a,b are measured with a \(\approx\) \({8}{cm^{-1}}\) spectral resolution. The curved dashed line indicates the fitted spectra obtained from FTIR, with one free parameter that accounts for the sample thickness variation. The vertical dashed lines in Fig. 4a,b indicate the selected spectral positions for spatial imaging shown in Fig. 4c, d, respectively. The high-contrast images from Fig. 4e-h indicate excellent material-specific absorptions in both polymer samples. These results demonstrate the capability of our MIR single-photon imaging system to resolve distinct vibrational absorption features in polymer samples with high spectral and spatial resolution. The strong agreement with the FTIR-fitted spectra and the high-contrast images at selected wavelengths confirm material-specific absorption signatures in both polystyrene and LDPE, highlighting the potential of the system for label-free chemical imaging.
Mid-infrared (MIR) absorption analysis of polymers: (a) polystyrene sheet and (b) low-density polyethylene (LDPE). In both figures, the dashed curved lines represent rescaled FTIR spectroscopy results for comparison. The vertical dashed lines in (a) and (b) mark the wavelengths corresponding to the three spatial images shown in (c) and (d), respectively. The contrast image between selected wavelengths is presented in (e-h), demonstrating good sensitivity to the relevant C–H vibrational transitions. Each spatial image has an area of 775 \(\mu\)m by 775 \(\upmu\)m with 25 \(\upmu\)m step size.
Hyperspectral imaging of egg yolk and yeast cells
In addition, measurements of egg yolk and yeast samples are presented in Fig. 5. Figure 5a shows transmission dips corresponding to O–H stretching (3030 nm) and asymmetric aliphatic C–H stretching (3430 nm) vibrations in egg yolk, while Fig. 5b displays similar features for the yeast sample. The curved dashed lines represent FTIR reference spectra, which show good agreement with the single-photon spectra. To accommodate the semi-solid nature of biological samples, the FTIR measurements were performed in attenuated total reflectance(ATR) mode, which may account for the relatively shallow O–H dip observed in the egg yolk spectrum. The contrast images in Fig. 5e–h, derived from the spatial maps in Fig. 5c, d at the indicated spectral positions (vertical dashed lines in Fig. 5a, b clearly reveal material-specific absorption features at key vibrational wavelengths. The single-photon spectra show good agreement with FTIR reference data, and the contrast images highlight material-specific absorption at key wavelengths.
MIR transmission analysis of biological samples: (a) Egg yolk and (b) yeast. The dashed curved lines show rescaled FTIR spectra for reference, with vertical dashed lines marking the wavelengths of the spatial images in (c) and (d). Contrast images in (e-h) highlight sensitivity to O–H and C–H vibrational transitions. Each spatial image has an area of 775 \({\upmu }\)m by 775 \({\upmu }\)m with 25 \(\upmu\)m step size.
Discussion and outlook
Single-photon imaging in the MIR region has shown significant promise for label-free biological imaging. This technique operates at sub-picowatt photon flux levels, enabling non-invasive spectral and spatial information from biological tissues and polymer samples without the need for exogenous labeling agents. The demonstration of hyperspectral imaging over the 2.9–3.6 \({\upmu }\)m range on biological samples such as yeast cells and egg yolk samples, as well as polymer samples including polystyrene and polyethylene, highlights the chemical specificity achievable with this platform. The strong agreement with FTIR spectra confirms the fidelity of our measurements, while single-photon sensitivity ensures minimal sample perturbation, an essential feature for applications involving sensitive biological materials or dynamic processes. A key strength of our approach lies in the integration of cavity-enhanced SPDC with nonlinear frequency up-conversion, facilitating the use of standard off-the-shelf available Si-SPADs. This room-temperature operation combined with high quantum efficiency and low dark counts overcomes the primary limitations of conventional MIR detectors, which typically require cryogenic cooling and exhibit inferior performance at the single-photon level. By leveraging time gating and intensity correlations of SPDC-generated photon pairs, we can suppress classical background noise and enhance the signal-to-noise ratio, approaching the shot-noise limit. With its effective noise suppression and high sample-specific sensitivity at ultralow flux, we foresee this approach extending beyond material characterization, offering potential in non-invasive detection in areas such as biomedical diagnostics, environmental sensing, and chemical analysis. These attributes establish it as a versatile and powerful tool for advancing modern mid-IR photonics.
Future work will focus on enhancing imaging resolution, for example, by incorporating near-field scanning techniques that enable sub-diffraction-limited imaging of bacterial cells. The imaging performance, in terms of photon flux and acquisition speed, is constrained by the pump laser repetition rate (40kHz), resulting in a total scan time of approximately 12 hours per sample. Employing a megahertz pump laser could yield an improvement of up to three orders of magnitude, allowing sub-minute hyperspectral imaging speeds, an essential advancement for high-throughput applications. Additionally, nonlinear up-conversion efficiency and phase-matching bandwidth impose spectral constraints and affect overall sensitivity. While the molecular MIR signature spans 2.5–20 \({\upmu }\)m, our present wavelength range (up to 3.6 \({\upmu }\)m) can be extended: PPLN can already reach 4.5 \({\upmu }\)m, and by using alternative nonlinear crystals such as AGS, AGSe, or ZGP together with appropriate pump wavelengths, the coverage can be expanded across the full molecular fingerprint region up to 20 \({\upmu }\)m. Continued optimization of non-linear crystal design, such as custom poling structures or novel materials, could broaden the spectral coverage and improve conversion efficiency. Coupling this imaging modality with advanced data analysis techniques, including neural network–based models for automated spectral classification and denoising, enables the extraction of deeper chemical and structural insights from complex hyperspectral datasets12,38. This integration could accelerate discovery in molecular biology, materials science, and clinical practice. Furthermore, by leveraging quantum correlated photon pairs from SPDC, photon heralding could potentially enhance the signal-to-noise ratio beyond the shot-noise limit39.
In summary, our results demonstrate label-free chemically selective imaging of polymers and biological samples at ultralow photon flux, confirming the system’s potential for noninvasive analysis of sensitive specimens. By overcoming key challenges in MIR single-photon detection and imaging, this approach opens a new avenue for ultrasensitive, chemically specific imaging and, with further improvements in speed, resolution, and spectral coverage, is poised to become a versatile tool in mid-infrared photonics.
Methods
Sample preparation
Polymer samples of polystyrene (PS) and low-density polyethylene (LDPE) were cleaned and mounted on infrared-transparent substrates suitable for mid-infrared transmission measurements. Biological samples, including yeast and egg yolk, were prepared as thin semi-solid layers to ensure sufficient optical transmission in the MIR region.
Data acquisition and scanning procedure
For each sample, hyperspectral spatial images were recorded by scanning the MIR wavelength in increments of approximately 50nm between 2900nm and 3600 nm. At each wavelength, spatial scanning was performed on a 775 \({\upmu }\)m by 775 \({\upmu }\)m area using a motorized stage with 25 \({\upmu }\)m step size in both lateral directions. Each spatial pixel was integrated for 2 s to accumulate sufficient photon counts for reliable imaging. Due to wavelength-dependent phase-matching conditions in the nonlinear crystals affecting photon conversion efficiency, reference scans were acquired outside the sample area before and after each spatial scan to record baseline photon levels. These reference measurements were used to normalize the spatial images, correcting for nonuniform conversion efficiency and slow drift in photon flux during acquisition.
Data processing and analysis
Time gating between signal and upconverted idler photons was applied to suppress background counts and enhance signal-to-noise ratio. Transmission spectra at each spatial location were constructed by normalizing the detected photon counts at each scanned wavelength individually. Spectral features were compared with reference FTIR spectra obtained from standard laboratory instruments. FTIR measurements on biological samples were performed in ATR mode to accommodate their semi-solid form. The spectral resolution of the hyperspectral images was approximately 8 \({\upmu }\)m\(^{-1}\), limited by the spectral bandwidth of the SPDC source and the up-conversion phase-matching bandwidth. Spatial contrast images were generated at selected spectral bands corresponding to key molecular vibrational modes identified in the transmission spectra. The contrast of the image and the chemical specificity were evaluated to demonstrate the capability of the platform for the differentiation of biological samples and material.
AI assisted copy editing
AI were used solely to enhance the clarity, readability, and stylistic consistency of the manuscript text, ensuring correctness in grammar, spelling, punctuation, and tone. All scientific content, interpretations, and conclusions remain the responsibility of the authors.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Diem, M. et al. Molecular pathology via IR and Raman spectral imaging. J. Biophoton. 6, 855–886 (2013).
Shaked, N. T., Boppart, S. A., Wang, L. V. & Popp, J. Label-free biomedical optical imaging. Nat. Photonics 17, 1031–1041 (2023).
Ghosh, B. & Agarwal, K. Viewing life without labels under optical microscopes. Commun. Biol. 6, 559 (2023).
Shaw, R. A. & Mantsch, H. H. Vibrational biospectroscopy: from plants to animals to humans: a historical perspective. J. Mol. Struct. 480, 1–13 (1999).
Zhao, G.-J. & Han, K.-L. Hydrogen bonding in the electronic excited state. Acc. Chem. Res. 45, 404–413 (2012).
Shi, L. et al. Mid-infrared metabolic imaging with vibrational probes. Nat. Methods 17, 844–851 (2020).
Pilling, M. & Gardner, P. Fundamental developments in infrared spectroscopic imaging for biomedical applications. Chem. Soc. Rev. 45, 1935–1957 (2016).
Carter, E. A., Tam, K. K., Armstrong, R. S. & Lay, P. A. Vibrational spectroscopic mapping and imaging of tissues and cells. Biophys. Rev. 1, 95–103 (2009).
Hackshaw, K. V., Miller, J. S., Aykas, D. P. & Rodriguez-Saona, L. Vibrational spectroscopy for identification of metabolites in biologic samples. Molecules 25, 4725 (2020).
Nicolson, F., Kircher, M. F., Stone, N. & Matousek, P. Spatially offset Raman spectroscopy for biomedical applications. Chem. Soc. Rev. 50, 556–568 (2021).
Fahelelbom, K. M., Saleh, A., Al-Tabakha, M. M. & Ashames, A. A. Recent applications of quantitative analytical FTIR spectroscopy in pharmaceutical, biomedical, and clinical fields: a brief review. Rev. Anal. Chem. 41, 21–33 (2022).
Thomsen, B. L. et al. Accurate and fast identification of minimally prepared bacteria phenotypes using Raman spectroscopy assisted by machine learning. Sci. Rep. 12, 16436 (2022).
Magidson, V. & Khodjakov, A. Circumventing photodamage in live-cell microscopy. Methods Cell Biol. 114, 545–560 (2013).
Wäldchen, S., Lehmann, J., Klein, T., Van De Linde, S. & Sauer, M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 5, 15348 (2015).
Dello Russo, S. et al. Advances in mid-infrared single-photon detection. In Photonics, vol. 9 470 (MDPI, 2022).
Razeghi, M. & Nguyen, B.-M. Advances in mid-infrared detection and imaging: a key issues review. Rep. Prog. Phys. 77, 082401 (2014).
Wang, P. et al. Sensing infrared photons at room temperature: from bulk materials to atomic layers. Small 15, 1904396 (2019).
Sun, X. et al. HgCdTe avalanche photodiode array detectors with single photon sensitivity and integrated detector cooler assemblies for space lidar applications. Opt. Eng. 58, 067103–067103 (2019).
Wollman, E. E. et al. Recent advances in superconducting nanowire single-photon detector technology for exoplanet transit spectroscopy in the mid-infrared. J. Astron. Telescopes Instrum. Syst. 7, 011004 (2021).
Nagler, P. C., Sadleir, J. E. & Wollack, E. J. Transition-edge sensor detectors for the origins space telescope. J. Astron. Telescopes Instrum. Syst. 7, 011005 (2021).
Taylor, G. G. et al. Low-noise single-photon counting superconducting nanowire detectors at infrared wavelengths up to 29 \(\mu\)m. Optica 10, 1672–1678 (2023).
Chang, J. et al. Efficient mid-infrared single-photon detection using superconducting NbTiN nanowires with high time resolution in a Gifford-McMahon cryocooler. Photonics Res. 10, 1063–1070 (2022).
Pan, Y. et al. Mid-infrared Nb4N3-based superconducting nanowire single photon detectors for wavelengths up to 10 \(\mu\)m. Opt. Express 30, 40044–40052 (2022).
Verma, V. et al. Single-photon detection in the mid-infrared up to 10 \(\mu\)m wavelength using tungsten silicide superconducting nanowire detectors. APL Photonics 6, 256 (2021).
Colangelo, M. et al. Large-area superconducting nanowire single-photon detectors for operation at wavelengths up to 7.4 \(\mu\)m. Nano Lett. 22, 5667–5673 (2022).
Azem, A. et al. Mid-infrared characterization of NbTiN superconducting nanowire single-photon detectors on silicon-on-insulator. Appl. Phys. Lett. 125, 253 (2024).
Hampel, B., Mirin, R. P., Nam, S. W. & Verma, V. B. A 64-pixel mid-infrared single-photon imager based on superconducting nanowire detectors. Appl. Phys. Lett. 124, 253 (2024).
Lemos, G. B. et al. Quantum imaging with undetected photons. Nature 512, 409–412 (2014).
Kalashnikov, D. A., Paterova, A. V., Kulik, S. P. & Krivitsky, L. A. Infrared spectroscopy with visible light. Nat. Photonics 10, 98–101 (2016).
Kviatkovsky, I., Chrzanowski, H. M., Avery, E. G., Bartolomaeus, H. & Ramelow, S. Microscopy with undetected photons in the mid-infrared. Sci. Adv. 6, eabd0264 (2020).
Dam, J. S., Tidemand-Lichtenberg, P. & Pedersen, C. Room-temperature mid-infrared single-photon spectral imaging. Nat. Photonics 6, 788–793 (2012).
Huang, K., Fang, J., Yan, M., Wu, E. & Zeng, H. Wide-field mid-infrared single-photon upconversion imaging. Nat. Commun. 13, 1077 (2022).
Ge, Z. et al. Quantum entanglement and interference at 3 \(\mu\)m. Sci. Adv. 10, eadm7565 (2024).
Junaid, S. et al. Video-rate, mid-infrared hyperspectral upconversion imaging. Optica 6, 702–708 (2019).
Mancinelli, M. et al. Mid-infrared coincidence measurements on twin photons at room temperature. Nat. Commun. 8, 15184 (2017).
Zhao, Y. et al. High-speed scanless entire bandwidth mid-infrared chemical imaging. Nat. Commun. 14, 3929 (2023).
Hermes, M. et al. Mid-ir hyperspectral imaging for label-free histopathology and cytology. J. Opt. 20, 023002 (2018).
Ho, C.-S. et al. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat. Commun. 10, 4927 (2019).
England, D. G., Balaji, B. & Sussman, B. J. Quantum-enhanced standoff detection using correlated photon pairs. Phys. Rev. A 99, 023828 (2019).
Acknowledgements
We acknowledge the support from the European Partnership on Metrology: SEQUME (20FUN05) and are grateful for the fruitful discussions with Jan C. Petersen on FTIR.
Funding
This research was funded by the Danish Agency for Institutions and Educational Grants and QuRaman project under QuantEra supported by Innovation Fund Denmark (1116-00003B) and the European Partnership on Metrology (21GRD07 “PlasticTrace” and 20FUN05 “SEQUME”).
Author information
Authors and Affiliations
Contributions
Y.M. and M.L. designed the experiment. Y.M. performed the measurements. Y.L. and M.L. analysed the data. Y.M., A.A.J. and A.N.R. developed the software for XYZ stage control and synchronization of the OPO and upconversion source. L.M. and S.M.M.F. developed the upconversion unit. Y.M. and M.L. wrote the initial manuscript with input from all authors. M.L. acquired funding, conceived the project and supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meng, Y., Jørgensen, A.A., Rasmussen, A.N. et al. Hyper-spectral imaging with up-converted mid-infrared single-photons. Sci Rep 16, 2448 (2026). https://doi.org/10.1038/s41598-025-32361-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32361-8