Abstract
In this study, CoFe2 − xMnxO4 (CMFO-x, x = 0.0, 0.2, 0.5 and 0.8) spinel materials were successfully synthesized using a combustion sol-gel method and used as electrode materials for supercapacitor. X-ray diffraction (XRD) patterns confirmed the successful formation of the spinel structure and the high purity of all samples. In addition, the results of scanning electron microscopy (SEM) showed that the samples doped with Mn cations consisted of smaller nanoparticles than the base sample (CMFO-0.0), which could increase the effective surface area and improve charge transfer. Also, a significant improvement in oxygen vacancy was observed in the CMFO-0.2 sample, which could play a key role in enhancing the electrochemical activity and pseudo-capacitive behavior of this material. In general, it can be said that partial Mnn+ substitution improved the ionic and electronic conductivity of the spinel structure. The electrode based on CMFO-0.2 was able to provide the highest specific capacitance of 433.44 F.g− 1 at a current density of 3 A.g− 1. Based on electrochemical analyses, all synthesized electrode materials exhibited pseudo-capacitive behavior. Furthermore, a symmetrical cell made of CMFO-0.2 material showed an excellent energy density of 438.86 W.h.kg− 1 at a power density of 3800 W.kg− 1 and a constant current density of 3 A.g− 1. Long charge-discharge cycles were used to investigate the life cycle of CMFO-0.2, and the results showed that this sample was able to maintain 92% of its initial capacitance after 5000 cycles.
Similar content being viewed by others
Introduction
Fossil fuel consumption significantly contributes to environmental pollution, carbon dioxide (CO2) emissions and global warming1,2,3,4. To reduce harmful effects on the environment and also to increase requests for green energy, scientists are working to create new, safe and eco-friendly energy sources5,6,7,8,9. Nuclear energy, solar energy, biomass, wind, and hydropower are common forms of alternative energy10,11,12. The absence of a reliable energy storage system to store the produced energy, however, is a serious flaw that they all have in common13. For the energy to be delivered continuously, it must maintain its enormous potential14,15. The portfolio of clean energy must include electrochemical energy16. Supercapacitors (SCs), fuel cells, and batteries are examples of non-conventional energy sources that operate on the electrochemical energy conversion concept17,18. Batteries store energy in the form of chemical energy within the chemical bonds of their electrode’s active materials. This chemical energy is then transformed into electrical energy by redox reactions, such as in a lead-acid battery, or through intercalation, as seen in a lithium-ion battery19,20. A few drawbacks of batteries are their shorter lifespan, lower power density, longer charging times, heating issues, and lack of environmental safety21. Supercapacitors are also a notable energy storage system. These devices act as a bridge between batteries and conventional capacitors in terms of electrochemical properties. In fact, supercapacitors have a high power density compared to conventional capacitors, but a lower energy density than batteries. In addition, compared to batteries, supercapacitors have a longer cycle life, a wider operating temperature range, relatively lower costs, greater safety, and greater environmental compatibility. Supercapacitors are frequently used in applications that call for a sudden surge in energy for a brief period of time, such as grid stabilization, energy harvesting systems like wind turbines and solar arrays, the automotive industry, and uninterruptible power supplies (UPS), because of their high power density22,23,24,25,26,27,28. Supercapacitors are divided into three categories: (i) Electric Double-Layer Capacitors (EDLC) that labor utilizing a non-faradaic process. (ii) Electrochemical pseudo-capacitors that work according to the Faradaic process and (iii) Hybrid supercapacitors that work based on faradic and non-faradic processes. Despite having different energy storage methods, all three supercapacitors have the same design and parts20,29,30,31. By employing activated carbon, carbon nanotubes, and graphene as the electrode material, electrostatic interactions between ions of the liquid electrolyte and electronic charges on the electrode surface allow energy storage in double-layer electric supercapacitors. Pseudocapacitors, on the other hand, store energy by means of charge transfer between metal oxide or polymer electrodes32,33,34. The electrode materials have a significant impact on the supercapacitor’s overall performance, including its specific capacitance, lifespan, power density, and energy density. For the supercapacitor’s charge storage capacitance, the natural properties, the electrode materials’ microstructure design, and the composite fabrication process are crucial35,36. For the electrode material, it is essential to meet two key requirements: (i) enhance the electrochemically active sites by choosing electrode materials with a high specific surface area (SSA); and (ii) adjust the pore size and shape of the electrode material to make it a suitable candidate that facilitates the transmission of electrolyte ions37,38,39,40. The most often used redox pseudo-capacitive materials are transition Metal Oxides (TMOs) and conductive polymers (CPs) because of their ability to exhibit fast-reversible redox reactions, which result in high specific capacitances and lengthy operating cycles39,41. The exceptionally high theoretical specific capacitance of many metal oxides42,43,44,45,46, binary transition metal oxides with a spinel structure (e.g., NiMn2O447, CoMn2O448, ZnMn2O449, MnCo2O450,51, MnFe2O452, CuCo2O453, and other electrode materials have made them important research subjects for supercapacitors51. Among these oxides, manganese-based binary oxides have received the greatest attention as one of the most traditional electrode materials due to their abundance, low toxicity, low cost, and various valence40. On the other hand, as a result of various benefits, including the ability to modify the work function, specific capacitance/capacity, electrical conductivity, and environmental friendliness, spinel-type oxides [A2+] [B3+]2O4 where A and B are transition metal ions are being considered as potential electrochemical energy storage materials54.
Manohar et al.55, synthesized NixMg1−xFe2O4 (x = 0.15, 0.25, and 0.35) nanoparticles by thermal solvent method. At a current density of 1.1 A.g− 1, the electrode composed of this nanostructure exhibited an impressive specific capacitance of 119.04 F.g− 1. This electrode retention 94.51% of its initial capacitance after 5000 cycles. In another study, Zhang et al.40 created a wide range of materials for use as supercapacitor electrode materials by physically milling copper manganese oxide-graphene (CuMn2O4-RGO) composites. CuMn2O4-RGO (1:1) exhibits better energy density and power density and a specific capacitance of 342 F.g− 1 at a current density of 1.0 A. g− 1. Furthermore, it has been shown that another intriguing spinel material for negative electrodes is Fe3O4. In an aqueous K2SO4 electrolyte, asymmetric devices with MnO2 as the positive electrode and Fe3O4 as the negative electrode have an expanded voltage window of 1.8 V. For composite Fe3O4-based electrodes, a capacitance of 50 F.g− 1 has been recorded with a mass loading of 8.8 mg.cm− 256.
In this study, CoFe2 − xMnxO4 (CMFO-x, x = 0.0, 0.2, 0.5 and 0.8) nanostructures were synthesized by hydrothermal method and their electrochemical properties were evaluated in 1 M KOH solution (as an electrolyte). Within the framework of this evaluation, the capacitance behaviors, cyclic stability, and charge transfer characteristics of the samples were analyzed using standard electrochemical tests. The aim of this research is to determine the potential of synthesized nanostructures as efficient electrode materials for energy storage applications in supercapacitors.
Experimental section
Materials and synthesis method
Citric acid (CA) with purity of 99.57%, ammonia solution with a volume ratio of 25%, Cobalt nitrate hexahydrate [Co (NO3)2.6H2O, with purity of 99.5%], Manganese nitrate tetrahydrate [Mn (NO3)2.4H2O, with purity of 98%] and Ferric nitrate nonahydrate [Fe (NO3)3.9H2O, with purity of 98%] were ready from the German Merck company. Since none of the compounds needed to be further purified, they were all of analytical purity.
CoFe2 − xMnxO4 nanoparticles synthesized hydrothermally57. In such a way that to create a homogenous solution, a specific quantity of Co (NO3)2.6H2O, Mn (NO3)2.4H2O and Fe (NO3)3.9H2O was dissolved in 50 mL of distilled water and agitated using a magnetic stirrer for a duration of 30 min. The aqueous ammonia solution was then added gradually and dropwise to the mixture while stirring, bringing the pH to 12.0. Next, the cations’ hydroxides were coprecipitated. After that, the mixture was moved to a 100 mL autoclave lined with Teflon and cooked for 24 h at 180 °C. The finished product was separated with a magnet, centrifuged, and repeatedly cleaned with distilled water and ethanol until a pH of neutral was attained once it reached room temperature. After that, the product was baked for 12 h at 70 °C to finish drying. Subsequently, the produced powder was calcined for 3 h at 600 °C.
Structural characterization of CMFO-x
To analyze the crystal structure, the diffraction patterns of the samples were first recorded using an X-ray diffractometer (Tongda TD-3700 (China) equipped with Cu Kα radiation) and were used to determine the phase and lattice order of the synthesized compounds. The structural morphology of the synthesized nanomaterials was studied by field emission scanning electron microscopy (FESEM, MIRA3 FEG-SEM, Tescan), and the elemental distribution was analyzed by energy dispersive X-ray spectroscopy (EDS, MIRA3 FEG-SEM, Tescan). In addition, FTIR spectroscopy of CMFO-x powders was performed at room temperature using a Bruker TENSOR 27 instrument. Powdered samples were mixed with KBr and pressed into tablets. Spectra were collected in the range of 400–4000 cm− 1 with a resolution of 4 cm− 1 and 32 scans for each sample to ensure a high signal-to-noise ratio and reliable detection of vibrational bands present in the samples. Furthermore, Raman testing was performed using a Renishaw Invia system and in order to investigate the chemical states and electronic species present on the surface, X-ray photoelectron spectroscopy (XPS, PHI 5000 Versa Probe, monochrome) was performed.
Electrode preparation
To prepare the electrode active materials, the synthesized spinel materials, activated carbon, and Nafion solution were thoroughly mixed and dispersed in N-methylpyrrolidone (NMP) solvent at a mass ratio of 85:15:5, respectively, to obtain a uniform and stable suspension. In the next step, the prepared suspension was uniformly applied to two copper plates (1 cm2), which were used as current collectors. To achieve 1 mg of active ingredient on the surface of each copper plate, the amount of coating solution was carefully adjusted so that after dropwise application, the determined amount of active ingredient was evenly distributed across the surface of the current collector. After that, these two copper plates were placed in an oven at a temperature of 60 degrees to completely dry the loaded inks. A sheet of ordinary paper soaked in a 1 M KOH electrolyte solution was used as a separator. After the two copper plates were completely dry, the prepared separator was placed between them. To both act as a separator between the plates and to enable the transfer of active ions between the anode and cathode. Finally, the desired electrochemical cell was assembled.
Electrochemical evaluations
To evaluate the electrochemical behavior of the studied samples, cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS) tests were performed using an Autolab potentiostat-galvanostat model PGSTAT30. All measurements were performed at ambient temperature in a two-electrode configuration. Data from the EIS test were modeled and analyzed using ZView® software. In addition, the specific capacitance (F.g− 1) of the samples was calculated from the curves extracted from CV and GCD tests to accurately determine the charge storage performance of the synthesized materials.
Results and discussions
Structure and morphology
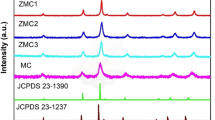
XRD analysis was performed to investigate the phase and crystal structure of synthesized spinel oxides in the 2θ range of 20˚ to 80˚. In order to accurately identify and analyze the crystalline phases of the synthesized spinels, Rietveld refinement was performed using MAUD software. The results of this analysis shown in Fig. 1. The XRD diffraction pattern obtained for CMFO-0.0 is in excellent agreement with the standard sample (Co0.255Fe0.745Co0.745Fe1.255O4; JCPDS 153–3163). The observed peaks around 30˚, 35.5˚, 37˚, 43˚, 53.5˚, 57˚ and 62˚ related to the (111), (220), (311), (222), (400), (331) and (422) plans, respectively. According to the obtained diffraction results, the sample has high purity. This substance has a cubic structure that belongs to the Fd. 3m22 space group58. Furthermore, the diffraction patterns of the Mn-doped species showed a mixture of two phases, CoFe2O4 (JCPDS 153–3163) and CoMn2O4 (JCPDS 153–6781). The CoMn2O4 phase had a tetragonal structure and belonged to the I41/amd:1 space group. Table 1 contains the extracted network parameters for all the studied samples. The diffraction pattern obtained for Mnn+-doped compounds show some shift in the position of the peaks, which is due to the difference in the ionic radius of Mnn+ and Fe2+/3+, as well as the difference in the electronic and valence properties of these cations. The difference in the valence of the cation in the B-site of the spinel structure can also change the oxygen content of the structure; on the other hand, due to the change of the valence in the B-site cations, the valence of the Co cation in the A-site can also be changed. In fact, the presence of Mn3+ in the B-site of the spinel structure causes more Fe cations to be in the Fe2+ state, which induces an oxygen vacancy in the structure59,60. The presence of this cation also affects the oxidation state of Co in the A-site, such that Co cations also tend to be in the Co2+ state in the presence of Mn3+. This situation increases the number of surface active sites for Faradaic redox reactions; on the one hand, cations are in a more reactive state, and on the other hand, the high concentration of oxygen vacancies provides more sites for the adsorption of reactive ions. However, when Mn2+ ions are located at the B site, the above trend is reversed. It is only at high concentrations of Mn at this site that the number of surface active sites and the degree of reactivity of the structure increase, because under such conditions, Mn2+ cations act as the main species participating in the redox processes61,62. For this reason, the electrochemical response of the material is significantly influenced by the phase composition and the way the cations are distributed in the spinel framework.
The calculation of the average crystallite size using the Scherrer equation show that the substitution of Mn ion at the B site of the spinel structure has a significant effect on the crystallinity and the size of the crystal coherence domain. The CMFO-0.0 shows the largest crystallite size with a value of approximately 30.9 nm, indicating higher crystallinity and freer growth of crystals. By adding small to medium amounts of Mn, the crystallite size undergoes significant changes; so that samples with substitution values of x = 0.2, x = 0.5, and x = 0.8 have sizes of about 10.3 nm, 18.5 nm, and 15.5 nm, respectively. The significant decrease in crystallite size in the CMFO-0.2 can be attributed to the increase in nucleation centers as well as lattice strain due to the difference in ionic size and the change in Mn oxidation states, both of which lead to the limitation of crystal growth. With further increase in Mn content, the crystallite size increases to some extent, which could be due to changes in growth dynamics, local accumulation of Mn ions, or the formation of regions with higher crystallinity.
Fourier transform infrared spectroscopy (FTIR) was investigated to study the structural properties, cation distribution of nanoparticles and functional groups in the synthesized spinel compounds in the range of 400 to 4000 cm− 1 (Fig. 2). According to previous studies, CoFe2O4 has two strong absorption peaks in the range of 400 to 600 cm− 1, which are evident in the spectra obtained for all the samples studied. These two peaks at around 430 and 560 cm− 1 shows the metal oxides bands are in spinel structure63,64,65, where the higher band represents the cation stretching vibrations at the tetrahedral lattice sites (A-site) and the lower band related to the cationic stretching vibrations at the B-site (octahedral)66,67. In addition, in the obtained spectrums, the peak observed at around 3445 cm− 1 was related to O-H stretching band due to H2O adsorption on the surface of spinel materials. On the other hand, several peaks observed in the range of 1000 to 3000 cm− 1 were related to carbon stretching bonds with elements such as O, C and H63,68,69,70. The FTIR spectra obtained for the synthesized materials showed the successful and pure formation of products in the spinel phase.
Raman spectroscopy is a very valuable tool for identifying the crystal structure of various materials. Figure 3 shows the Raman spectra of all the studied materials measured at ambient temperature. The obtained Raman spectra show different peaks in the range of 150–750 cm− 1.
According to the previous studies, the phonon states predicted from functional group analysis for the spinel structure include A1g, T1g, Eg, 2A2u, 3T2g, 2Eu, 2T2u and 4T1u71. Among these states, 3T2g, Eg and A1g, are more active and present clear Raman peaks in the mentioned range72,73. The cationic redistribution in the A and B sites of the spinel structure is able to change the symmetry of the crystal structure from its conventional state for spinel (Fd3m) to the I41/amd space group, while there will be a larger number of active vibrational modes in the Raman spectrum. The main peaks observed at frequencies above 600 cm− 1 centered at 620 and 685 cm− 1 are due to the symmetrical stretching of the A-site cations in the tetragonal structure, which was attributed to the A1g phonon state. Other phonon modes observed at frequencies lower than 600 cm− 1 are due to the symmetric and antisymmetric bending of the metal ion in the octahedral space with the oxygen (BO6), namely Eg and 3T2g.
SEM images of the synthesized spinel oxides at 500 nm and 2 μm magnification are presented in Fig. 4. Based on these images, it was found that the studied materials mainly consisted of aggregated nanoparticles with sizes ranging from 200 to 800 nm. The morphology observed for the synthesized materials is consistent with previous studies74,75. Since spinel oxides are composed of smaller nanoparticles, they provide a higher surface area, which allows for greater surface contact with the electrolyte solution.
As is clear from the SEM results, samples doped with Mn cations are composed of smaller nanoparticles than the base sample (BSMFO-0.0). However, it is anticipated that the presence of Mnn+ in the spinel structure, increase the surface area and improve the wetting of spinel surface75,76, which will benefit the surface Faradaic redox reactions. On the other hand, the porous morphology of electrode materials can involve more active surface area during sequential charge-discharge processes in supercapacitors, which results in enhanced specific capacitance of this device.
In addition, the distribution of elements in the synthesized spinel materials was performed using EDS mapping analysis. The results of this analysis are shown in Fig. 5(a-d). As is clear from the obtained images, the elements are uniformly distributed in the material structure. Furthermore, using energy dispersive X-ray (EDX) analysis (Fig. 5), the molar ratios of elements in the synthesized spinel materials were in good agreement with the theoretical values.
The surface chemical composition and electronic structures of the studied spinel materials were investigated using XPS analysis. The results of this analysis are shown in Figs. 6 and 7. Figure 6a, d and g show the high-resolution XPS spectra for Co 2p corresponding to CMFO-0.2, CMFO-0.5 and CMFO-0.8. As can be seen, the spectra obtained for Co 2p have two main spins centered at 780 eV and 796 eV, which correspond to Co 2p3/2 and Co 2p1/2, respectively. Two intense satellite peaks are also clearly observed alongside the Co 2p3/2 and Co 2p1/2 peaks centered at 790 eV and 805 eV. The position of these two satellite peaks and the position of the main peaks (2p3/2 and 2p1/2) indicate that Co is in the Co2+ oxidation state77. The difference between the two spin orbitals, Co 2p3/2 and Co 2p1/2, is caused by the difference in spin orbital gap energy78.
Furthermore, the asymmetric form of the deconvoluted peaks for the Co 2p3/2 spin orbital indicates that the Co element exists in two oxidation states, Co2+/3+, in the spinel structure79. It is well known that metal ions in the spinel structure can concurrently occupy the tetrahedral A-site and the octahedral B-site. As is clearly seen, the content of Co3+ relative to Co2+ in CMFO-0.2 is higher compared to other samples. In other words, more Co in this sample is located in the B-site with higher valence (+ 3). Figure 6b, e and h show the high-resolution XPS spectra of Fe 2p for CMFO-0.2, CMFO-0.5 and CMFO-0.8.
In these spectra, there are also two main spin-orbit peaks, which belong to Fe 2p3/2 and Fe 2p1/2, located at around 711 eV and 725 eV. In addition, two satellite peaks at around 719 eV and 735.5 eV are observed in the spectra of CMFO-0.2 and CMFO-0.8, while in the Fe 2p spectrum of CMFO-0.8, which has a lower Fe content, only one satellite peak at around 718.5 eV is observed80,81. These satellite peaks show that Fe is present in the structure of the produced spinel materials in both Fe2+ and Fe3+ oxidation states. Similarly, for the Mn 2p spectrum, have two main peaks are centered at 641 and 653 eV, which correspond to the spin-orbits of Mn 2p3/2 and Mn 2p1/2, respectively82,83. As can be seen, the deconvoluted spectrum for both spin-orbits revealed that Mn is present in two oxidation states, Mn2+ and Mn3+, in the structure of the synthesized spinel materials84. The high quantity of Mn3+ in the structure of the synthesized spinel materials enables Fe cations to be present mostly in the Fe2+ state. Fe2+ helps to stimulate and further increase oxygen vacancies and as a result enhances the active surface points. In addition, the presence of Mn2+ species induces oxygen vacancies in the structure of spinel oxides. During Faradaic redox processes, the surface are adsorbed OH− species of the electrolyte from the oxygen side to the oxygen vacancy sites and energy is stored in the structure by the diffusion of O2−85. This diffusion also depends on the oxidation states of the cations of the spinel structure; in fact, the diffusion of oxygen ions is carried out by successive cationic oxidations and reductions in the spinel structure. The more oxygen vacancies in the structure, the more paths are provided for ion penetration into the structure, resulting in the use of bulk electrode material. On the other hand, O2− ions in spinel oxides play an important role in charge transfer and facilitating Faradaic reactions between the two electrodes In fact, the O2− ion acts as the main current carrier between the two electrodes. The higher mass transfer potential of this ion between the electrodes (which depends on the energy and ease of its adsorption or desorption process at the electrode surface), the greater the amount of charge that can be stored in the system. The deconvoluted O 1s spectra for the three-doped samples are shown in Figs. 7a-c. As can be seen, the deconvoluted spectra identify three oxygen species, including lattice oxygen (OLat.) associated with Mn-O, Fe-O and Co-O bonds (~ 529.5 eV), oxygen vacancy or weak structural oxygen bonds (OVac, ~ 532 eV) and surface adsorbed oxygen (OChem., ~ 534 eV)86. From the obtained spectra, it is evident that the two oxygen species OVac and OChem are more abundant in the two samples CMFO-0.2 and CMFO-0.8 than in the other sample, especially CMFO-0.2 showed a higher OVac value. This means that these two samples have a greater tendency to adsorb oxygen ions. This property is completely dependent on the distribution of different cationic species with different valences. Therefore, Mn doping in the CMFO-0.0 structure is able to regulated the oxidation states of Fe and Co and consequently change the oxygen vacancy content.
Electrochemical performance
The electrochemical performance of all synthesized spinel materials was carried out using CV, GCD and EIS analyses in a two-electrode cell and 1 M KOH. Figure 8a shows the CV curves of all prepared electrode materials at a scan rate of 10 mV.s− 1. As can be seen from Fig. 8a, the potential window for all electrode materials is in the range of -1.5 to + 1.2 V. From the shape of the obtained curves, the pseudo-capacitance behavior of the synthesized electrode materials is evident87. The peaks observed in the cathodic scan are the product of the oxidation of cations in the spinel structure, which have the ability to adsorb oxygen ions and diffuse them into their structure88.
(a) The comparison of CV curves of synthesis samples at a scan rate of 10 mV∙s− 1 in a potential window of -1.5–1.2 V in 1 M KOH electrolyte; (b) CV curve of CMFO-0.2 at different scan rates; (c) GCD curves of all electrode materials at 3 A.g− 1 (d) Specific capacitance of CMFO-x as a function of current density.
The presence of Mn2+, Fe2+ and Co2+ cations has a greater ability to be oxidized by OH− species of the electrolyte and according to the XPS results, these species increase the oxygen vacancy content and in fact, the sites related to these cations are the anion defect sites and the starting point of the surface Faradaic redox reaction89,90. According to Eq. 1, the area of the CV curve is directly related to the specific capacitance of the synthesized electrode materials. As is clear from the obtained curves, the CV curve corresponding to CMFO-0.2 has a larger area than the other curves. Also, this curve has stronger redox peaks than other studied materials, indicating excellent surface redox reaction kinetics for the adsorption of OH− species. In addition, Fig. 8b shows the CV curves of CMFO-0.2 at different scan rates. It is clearly evident from the obtained curves that with increasing scan rate, the current response obtained from applying potential also increased, which is probably due to the increase in partial polarization of oxygen at the electrode surface, which is also consistent with the Randles–Sevcik equation91. Furthermore, as can be seen, the redox peaks are still clearly visible at high scan rates, indicating the excellent dynamics of the surface redox reaction in this sample.
The polarization of oxygen on the surface of spinel oxides during the redox process is able to change the oxidation states of transition metal cations at both A and B sites. This change in oxidation states in cations near the spinel surface throws the spinel structure out of electrical equilibrium; as a result, other cations further from the surface also change oxidation states to stabilize the structure. The change in the valence of cations due to their oxidation causes the creation of electron-hole sites. The creation of electron holes in the spinel structure enhances their electronic conductivity, and by enhancing this property, the surface redox reaction improves2,6. The electrochemical reactions also require ion transport to occur. Therefore, the ion and electron conductivity of supercapacitor electrode materials is an important characteristic for achieving the best performance. The ion transport in the spinel structures caused by oxygen vacancy and consecutive oxidize and reduce of cations. In fact, the OH− species of the electrolyte is adsorbed to the surface oxygen vacancy sites, at these sites, the adjacent cations are capable of being oxidized and are oxidized immediately after adsorption. With the oxidation of surface cations, the negative charge of the surface increases, and as a result, the internal regions of the electrode material will be more positive than the surface. Therefore, the negative surface charge (O2− species) gradually penetrates from the surface of the electrode material and into the interior regions through oxygen vacancy sites. This ionic penetration continues until the electrode material has no capacity to accept additional ions, or in other words, the electrode material becomes saturated with O2− ions. O2− in this process as a charge carrier, therefore with increase of O2− adsorption enhance the specific capacitance of supercapacitor. The structure of spinel oxides has unique properties that facilitate ion and electron conduction92,93. In particular, since the spinel structure has two cationic sites, both of which allow the presence of multivalence cations, these cations can improve the oxygen ion adsorption capacity in the supercapacitor cell. As shown from the CV results, doping of Mn cation at B-site, especially in the CMFO-0.2 structure, has improved the surface redox reaction. This performance improvement is due to the increase in oxygen vacancy content as a result of inverse spinel effects and the change in the oxidation state of the cations of the structure.
In addition, to further study the electrochemical properties of spinel oxides, GCD analysis was performed at room temperature and 1 M KOH electrolyte, the results of which are shown in Fig. 8c at a constant current density of 3 A.g− 1. The asymmetric form of the GCD curves indicates the pseudo-capacitive nature of the spinel oxides6, which is consistent with the CV results. In addition, Fig. 8d shows the specific capacitances at the different current density for the studied electrode materials at different current densities. As can be seen, at the all current densities, CMFO-0.2 has a higher specific capacitance than other samples. Whereas, CMFO-0.8, CMFO-0.0 and CMFO-0.5 are in second to fourth place respectively in terms of specific capacitance. Therefore, the trend of specific capacitance obtained from GCD is consistent with the CV results. Therefore, the results from CV and GCD show the improvement of functional properties as a result of the increase in oxygen vacancy content and the enhancement of cationic redox properties of the spinel structure. The CV and GCD results showed that Mn doping at the B site of the CMFO-0.0 spinel structure is able to introduce new pathways for ion and electron diffusion in the spinel structure and, by exciting Fe ions, is able to keep them in the Fe2+ state longer (in agreement with the XPS results). The Fe2+ ions present on the spinel surface are oxidized to Fe3+ by absorbing oxygen, and the Mn cations, since they have different oxidation states, act as electron-hole sites at the B site and facilitate the electron conduction resulting from the surface redox process93,94. As a result, the surface redox reaction is significantly improved due to the constructive interaction of the two cations Fe2+ and Mnn+, which is evident in the CV and GCD results. Figure 8c shows that, in contrast to the increase in current density, the specific capacitance of the synthesized samples decreases.
This phenomenon is because as the current density increases, the effective use of the electrochemically active surface is limited to the outer surface and consequently less surface area of the electrode material is scanned. Therefore, by reducing the surface area involved in the Faradaic redox process, less oxygen is adsorbed on the surface and the specific capacitance decreases. The specific capacitance obtained for CMFO-0.0, CMFO-0.2, CMFO-0.5 and CMFO-0.8 at a current density of 3 A.g− 1 was 352.77, 433.44, 309.78 and 388.78 F.g− 1, respectively. Also, an extraordinary energy density of 438.86 W.h.kg− 1 at a power density of 3800 W.kg− 1 was obtained for CMFO-0.2 at a current density of 3 A.g− 1. The results of this research compared to previous work are presented in Table 2. As shown in this table, the spinel material introduced in this study has performance that is on par with or superior to most similar materials reported in the literature. The increase in specific capacitance, appropriate energy density and power density, and stable pseudo-capacitive behavior confirm the relative superiority of the CMFO-0.2 sample over spinel compounds in previous studies. These results indicate that the synthetic approach used in this study is not only capable of improving the performance of spinel electrodes, but also can be used as an efficient and generalizable strategy for the design and development of a new generation of electrode materials with high capacitance, fast redox kinetics, and favorable cyclic stability.
Electrochemical impedance spectroscopy (EIS) was studied at open circuit potential (OCP) and in the frequency range of 100 kHz to 10 mHz for all spinel materials. Figure 9a presents the Nyquist plots obtained from this analysis. An equivalent circuit was used to obtain parameters related to the system’s resistance to ion and electron diffusion, which originated from the physical chemistry of the structure of the studied supercapacitors. In this equivalent circuit, Rs represents the bulk resistance of the electrode material, the electrolyte solution, and all the intrinsic resistances of the supercapacitor. Rct also represents the resistance to charge transfer at the electrode/electrolyte interface. The diameter of the semicircle in the high frequency region represents Rct and the distance of this semicircle from the origin corresponds to Rs95,96. In addition, ion diffusion at low frequencies is characterized as Open Warburg (WO) in the obtained Nyquist curves. Based on the results of EIS analysis, the Rct obtained for CMFO-0.0, CMFO-0.2, CMFO-0.5 and CMFO-0.8 corresponded to the values of 23.45, 10.38, 24.38 and 14.32 Ω cm2, respectively. As is clear from the EIS results, the CMFO-0.2 sample has a lower resistance to ion and electron diffusion; therefore, the kinetics of the Faradaic redox reaction in this sample will be lower and the surface electrochemical redox reaction will proceed more rapidly. As a result, the charge-discharge speed in this sample will be higher, or in other words, the power density of this sample will be higher. Therefore, the results from EIS are consistent with the results from CV and GCD analyses.
The Bode plot (Fig. 9b) illustrates that the phase angle at low frequencies approaches 45 degrees, indicating the Warburg phenomenon and affirming the presence of diffusion. A phase angle below 90 degrees signifies pseudo-capacitive behavior (in accordance with what was obtained from the CV and GCD results). A low phase angle distinctly indicates enhanced energy storage efficiency and accelerated ion diffusion97. Table 3 presents the parameters associated with electrochemical impedance spectroscopy, including the time constant (τ) and diffusion coefficient (D) for all samples. Table 3 indicates that the diffusion coefficient for the CMFO-0.2 was determined to be 1.21 × 10− 10 m2.s− 1, surpassing that of the other samples. The computed time constant for this sample was 159 µs, which is inferior to the other samples. The results obtained were completely consistent with previous results.
Based on the results of electrochemical analyses, it was determined that CMFO-0.2 has more favorable electrochemical performance than other samples, which originates from the extraordinary structural properties of this compound. Therefore, the life cycle stability for this material was performed using long charge-discharge cycles. The results of this analysis, shown in Fig. 10a, revealed that this sample was able to retain 92% of its initial capacitance after 5,000 consecutive charge-discharge cycles. This stability is impressive considering the electrodes prepared using spinel structure and demonstrates good performances when compared to the other studies. For example, a composite ZnCo2O4-based electrode was able to retain its initial capacitance of about 91.5% after 5,000 cycles98. While ZnCo2O4-based composite materials were only able to retain 72% of the initial capacity after 2000 cycles99. Therefore, the 92% stability achieved in this study demonstrates acceptable structural strength, preservation of the spinel framework during cycling, and effective ion transport capability during long-term operation. In addition, as is clear from the results, the specific capacitance gradually decreases with increasing number of charge-discharge cycles, this phenomenon probably occurs due to cationic leaching during the charge-discharge process. This phenomenon can cause serious damage to the structure of the electrode material and change the diffusion paths of ions and electrons, causing problems, as a result of which the performance of the supercapacitor is disrupted and the specific capacitance is reduced. Furthermore, as presented in Fig. 10b, the red LED with an operating voltage of 2.0 V was successfully illuminated using two assembled supercapacitor cells connected in series. This demonstration confirms the capability of the cells to store and deliver energy effectively in a practical application.
In addition, XRD analysis was performed on the CMFO-0.2 sample after consecutive charge-discharge cycles, and the results are presented in Fig. 11a. As can be seen, the characteristic peaks of the spinel phase remain clearly visible, and the minimal decrease in their intensity indicates the good structural stability of the compound during cycling. Furthermore, the SEM images shown in Fig. 11b, obtained from the cycled sample, demonstrate that the particle morphology and structural integrity are largely preserved even after repeated charge–discharge cycles. Overall, these observations confirm that the CMFO-0.2 compound exhibits excellent structural and morphological stability, making it a promising and efficient electrode material for supercapacitor applications.
Conclusion
In this study, CoFe2 − xMnxO4 (CMFO-x, x = 0.0, 0.2, 0.5 and 0.8) spinel oxides were successfully synthesized using combustion sol-gel and their performance as supercapacitor electrode materials was evaluated. The XPS results showed that doping of Mnn+ cations at the B-site changes the oxygen vacancy content, indicating a strong dependence of this parameter on the B-site cations. SEM images also show that doping this cation improves the morphological structure and better control the size of the particles that are formed in the nucleation process during synthesis. These changes can affect the final properties of the material as a supercapacitor electrode and improve its electrochemical performance. Partial substitution of Mnn+ cation could improve the oxygen vacancy content of the structure in the CMFO-0.2 sample and create more ion diffusion pathways in the spinel structure. With the increase in oxygen vacancy content, the active sites of the surface increased and consequently the kinetics of surface Faradaic redox reactions improved. On the other hand, the CMFO-0.2 allows for faster transport of charged species due to its optimized morphology and reduced intrinsic resistance to ion diffusion. The more uniform structure and proper particle distribution observed in SEM images allow O2− ions, after surface adsorption, to quickly penetrate the electrode bulk volume and free the surface for subsequent oxidation-reduction reactions to occur. This process increases the frequency of surface Faradaic reactions and facilitates the penetration of ions into the spinel structure. As a result, the charge transfer rate and the number of available active sites are increased, leading to improved energy density and power density of the supercapacitor. Therefore, the pseudo-capacitive behavior observed in CMFO-0.2 can be considered a direct result of the appropriate combination of structural-morphological and surface-active species to carry out the Faradaic redox reaction. CMFO-0.2 showed an extraordinary specific capacitance of 433.44 F.g− 1 at a current density of 3 A.g− 1. This sample also presented an energy density of 438.86 W.h.kg− 1 at a power density of 3800 W.kg− 1 and a current density of 3 A.g− 1. In addition, this sample was able to maintain 92% of its initial specific capacitance after 5000 consecutive charge-discharge cycles. The results of this research showed that the CMFO-0.2 sample is a promising compound for use as an electrode material in supercapacitors.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Mostafaei, J., Çoruh, A., Asgari, A., Asghari, E. & Niaei, A. Supercapacitor application of Sr-doped LaCo0.2Fe0.8O3 perovskite oxide: investigation of structural and electrochemical properties. J. Energy Storage. 93, 112394 (2024).
Ahangari, M. et al. Investigation of structural and electrochemical properties of SrFexCo1–xO3–δ perovskite oxides as a supercapacitor electrode material. J. Energy Storage. 63, 107034 (2023).
Hannan, M. A. et al. Hydrogen energy storage integrated battery and supercapacitor based hybrid power system: A statistical analysis towards future research directions. Int. J. Hydrogen Energy. 47, 39523–39548 (2022).
Narimani-Qurtlar, A. et al. Impact of total substitution on the Li+ conductivity and energy storage behavior of LixTiyO3 as a perovskite-type solid electrolyte. Chem Phys. Lett 142247 (2025).
Asadi, F. et al. Manganese doped La0.8Ba0.2FeO3 perovskite oxide as an efficient electrode material for supercapacitor. J Alloys Compd 175801 (2024).
Ahangari, M. et al. Application of SrFeO3 perovskite as electrode material for supercapacitor and investigation of Co-doping effect on the B-site. Turkish J. Chem. 46, 1723–1732 (2022).
Zou, C., Zhao, Q., Zhang, G. & Xiong, B. Energy revolution: from a fossil energy era to a new energy era. Nat. Gas Ind. B. 3, 1–11 (2016).
Aruchamy, K. et al. One-step green route synthesis of spinel ZnMn2O4 nanoparticles decorated on MWCNTs as a novel electrode material for supercapacitor. Mater. Sci. Eng. B. 252, 114481 (2020).
Attia, S. Y., Mohamed, S. G., Barakat, Y. F. & Hassan, H. H. Al Zoubi, W. Supercapacitor electrode materials: addressing challenges in mechanism and charge storage. Rev. Inorg. Chem. 42, 53–88 (2022).
Narimani-Qurtlar, A. et al. Investigating the environmental impacts of lithium-oxygen battery cathode production: A comprehensive assessment of the effects associated with oxygen cathode manufacturing. J Clean. Prod 144199 (2024).
Mahmoudi, E. et al. LaCoO3-BaCoO3 porous composites as efficient electrocatalyst for oxygen evolution reaction. Chem. Eng. J. 473, 144829 (2023).
Sayyah, A., Ahangari, M., Mostafaei, J., Nabavi, S. R. & Niaei, A. Machine learning-based life cycle optimization for the carbon dioxide methanation process: achieving environmental and productivity efficiency. J Clean. Prod 139120 (2023).
Ahangari, M. et al. Effect of Pd doping on the structural properties and supercapacitor performance of La0.8Sr0.2Cu0.7Mn0.3O3 and La0.8Sr0.2Cu0.4Mn0.6O3 as electrode materials. Electrochim Acta 143274 (2023).
Gao, X. et al. Morphology-controllable Preparation of NiFe2O4 as high performance electrode material for supercapacitor. Electrochim. Acta. 296, 181–189 (2019).
Şahin, M. E., Blaabjerg, F. & Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies vol. 15 at (2022). https://doi.org/10.3390/en15030674
Kalantari, N., Delibaş, N. & Niaei, A. Unveiling the potential of additives in optimizing halide perovskite solar cells performance and reliability. Mater Today Sustain 101011 (2024).
Kandalkar, S. G., Dhawale, D. S., Kim, C. K. & Lokhande, C. D. Chemical synthesis of Cobalt oxide thin film electrode for supercapacitor application. Synth. Met. 160, 1299–1302 (2010).
Sharma, K., Arora, A. & Tripathi, S. K. Review of supercapacitors: materials and devices. J. Energy Storage. 21, 801–825 (2019).
Liu, C., Neale, Z. G. & Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today. 19, 109–123 (2016).
Noori, A., El-Kady, M. F., Rahmanifar, M. S., Kaner, R. B. & Mousavi, M. F. Towards Establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 48, 1272–1341 (2019).
Gautham Prasad, G., Shetty, N., Thakur, S. & Bommegowda, K. B. Rakshitha Supercapacitor technology and its applications: a review. in IOP Conference Series: Materials Science and Engineering 561 12105IOP Publishing, (2019).
Luta, D. N. & Raji, A. K. Optimal sizing of hybrid fuel cell-supercapacitor storage system for off-grid renewable applications. Energy 166, 530–540 (2019).
Samage, A. et al. Room temperature and rapid synthesis of ZnMn2O4 nanostructured spinel using deep eutectic solvent for high energy asymmetric supercapacitors. J. Energy Storage. 97, 112934 (2024).
Yun, J. et al. Stretchable array of high-performance micro-supercapacitors charged with solar cells for wireless powering of an integrated strain sensor. Nano Energy. 49, 644–654 (2018).
Castiglia, V. et al. Modeling, simulation, and characterization of a supercapacitor in automotive applications. IEEE Trans. Ind. Appl. 58, 2421–2429 (2022).
Wang, D. G., Liang, Z., Gao, S., Qu, C. & Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 404, 213093 (2020).
Jayananda, D., Kularatna, N., Steyn-Ross, D. A. & Supercapacitor‐assisted LED (SCALED) technique for renewable energy systems: a very low frequency design approach with short‐term DC‐UPS capability eliminating battery banks. IET Renew. Power Gener. 14, 1559–1570 (2020).
Yang, D. et al. A manganese phosphate cathode for long-life aqueous energy storage. Adv. Funct. Mater. 31, 2100477 (2021).
Wu, D. et al. Morphology controlled hierarchical NiS/carbon hexahedrons derived from nitrilotriacetic acid-assembly strategy for high-performance hybrid supercapacitors. Chem. Eng. J. 433, 133673 (2022).
Ma, Y. et al. Recent advances in transition metal oxides with different dimensions as electrodes for high-performance supercapacitors. Adv Compos. Hybrid. Mater 1–19 (2021).
Zhao, J. & Burke, A. F. Review on supercapacitors: technologies and performance evaluation. J. Energy Chem. 59, 276–291 (2021).
Ray, A. et al. Study on charge storage mechanism in working electrodes fabricated by sol-gel derived spinel NiMn2O4 nanoparticles for supercapacitor application. Appl. Surf. Sci. 463, 513–525 (2019).
Reina, M. et al. Boosting electric double layer capacitance in laser-induced graphene‐based supercapacitors. Adv. Sustain. Syst. 6, 2100228 (2022).
Wang, F. X. et al. Spinel LiMn2O4 nanohybrid as high capacitance positive electrode material for supercapacitors. J. Power Sources. 246, 19–23 (2014).
Wang, Y., Wu, X., Han, Y. & Li, T. Flexible supercapacitor: overview and outlooks. J. Energy Storage. 42, 103053 (2021).
Bhagwan, J., Kumar, N. & Sharma, Y. Spinel-MgMn2O4 nanofibers: an attractive material for high performance aqueous symmetric supercapacitor. J. Energy Storage. 46, 103894 (2022).
Pongprayoon, P. & Chaimanatsakun, A. Revealing the effects of pore size and geometry on the mechanical properties of graphene nanopore using the atomistic finite element method. Acta Mech. Solida Sin. 32, 81–92 (2019).
Dai, G. et al. Multi-scale model for describing the effect of pore structure on carbon-based electric double layer. J. Phys. Chem. C. 124, 3952–3961 (2020).
Forouzandeh, P., Kumaravel, V. & Pillai, S. C. Electrode materials for supercapacitors: a review of recent advances. Catalysts 10, 969 (2020).
Zhang, C. et al. CuMn2O4 spinel anchored on graphene nanosheets as a novel electrode material for supercapacitor. J. Energy Storage. 34, 102181 (2021).
Lokhande, P. E., Chavan, U. S. & Pandey, A. Materials and fabrication methods for electrochemical supercapacitors: overview. Electrochem. Energy Rev. 3, 155–186 (2020).
Sheikhzadeh, M., Sanjabi, S., Gorji, M. & Khabazian, S. Nano composite foam layer of CuO/graphene oxide (GO) for high performance supercapacitor. Synth. Met. 244, 10–14 (2018).
Racik, K. M. et al. Fabrication of manganese oxide decorated copper oxide (MnO2/CuO) nanocomposite electrodes for energy storage supercapacitor devices. Phys. E Low-dimensional Syst. Nanostruct. 119, 114033 (2020).
Wu, H., He, D. & Wang, Y. Facile one-step process synthesized reduced graphene oxide/Mn3O4 nanocomposite for a symmetric supercapacitor. Mater. Lett. 268, 127613 (2020).
Kumar, R. et al. Homogeneous reduced graphene oxide supported NiO-MnO2 ternary hybrids for electrode material with improved capacitive performance. Electrochim. Acta. 303, 246–256 (2019).
Kumar, R., Singh, R. K., Vaz, A. R., Savu, R. & Moshkalev, S. A. Self-assembled and one-step synthesis of interconnected 3D network of Fe3O4/reduced graphene oxide nanosheets hybrid for high-performance supercapacitor electrode. ACS Appl. Mater. Interfaces. 9, 8880–8890 (2017).
Zhang, M., Song, Z., Liu, H. & Ma, T. Biomass-derived highly porous nitrogen-doped graphene orderly supported NiMn2O4 nanocrystals as efficient electrode materials for asymmetric supercapacitors. Appl. Surf. Sci. 507, 145065 (2020).
Feng, Y. et al. Oxygen vacancies enhance supercapacitive performance of CuCo2O4 in high-energy-density asymmetric supercapacitors. J. Power Sources. 458, 228005 (2020).
Ameri, B., Davarani, S. S. H., Moazami, H. R. & Darjazi, H. Cathodic electrosynthesis of ZnMn2O4/Mn3O4 composite nanostructures for high performance supercapacitor applications. J. Alloys Compd. 720, 408–416 (2017).
Shanmugavadivel, M., Dhayabaran, V. V. & Subramanian, M. Fabrication of high energy and high power density supercapacitor based on MnCo2O4 nanomaterial. J. Phys. Chem. Solids. 133, 15–20 (2019).
Guo, X. et al. Hierarchical core-shell electrode with NiWO4 nanoparticles wrapped MnCo2O4 nanowire arrays on Ni foam for high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 563, 405–413 (2020).
Gopi, C. V. V. M. et al. Co9S8-Ni3S2/CuMn2O4-NiMn2O4 and MnFe2O4-ZnFe2O4/graphene as binder-free cathode and anode materials for high energy density supercapacitors. Chem. Eng. J. 381, 122640 (2020).
Zhang, P., Liu, X., He, H., Peng, Y. & Wu, Y. Engineering RuO2 on CuCo2O4/CuO nanoneedles as multifunctional electrodes for the hybrid supercapacitors and water oxidation catalysis. J. Alloys Compd. 832, 154962 (2020).
Kumar, V. et al. Hybrid aqueous supercapacitors based on mesoporous spinel-analogous Zn-Ni-Co-O nanorods: effect of Ni content on the structure and energy storage. J. Alloys Compd. 882, 160712 (2021).
Manohar, A., Vijayakanth, V., Vattikuti, S. V. P. & Kim, K. H. Electrochemical investigation on nickel-doped spinel magnesium ferrite nanoparticles for supercapacitor applications. Mater. Chem. Phys. 301, 127601 (2023).
Nawwar, M., Poon, R., Sahu, R. P., Puri, I. K. & Zhitomirsky, I. Fe3O4 spinel-Mn3O4 spinel supercapacitor prepared using celestine blue as a dispersant, capping agent and charge transfer mediator. Ceram. Int. 46, 18851–18858 (2020).
Ansari, S. M. et al. Eco-friendly synthesis, crystal chemistry, and magnetic properties of manganese-substituted CoFe2O4 nanoparticles. ACS Omega. 5, 19315–19330 (2020).
Saei, J. N. & Asadpour-Zeynali, K. Enhanced electrocatalytic activity of fluorine doped Tin oxide (FTO) by trimetallic spinel ZnMnFeO4/CoMnFeO4 nanoparticles as a hydrazine electrochemical sensor. Sci. Rep. 13, 12188 (2023).
Biswas, P. et al. Morphology-controlled ag modified mixed valent manganites as a supercapacitor electrode material with adequate energy, power density, and large capacity retention. J Alloys Compd 182323 (2025).
Biswas, P. et al. Structural and enhanced dielectric properties of Al-modified lanthanum strontium manganites. Ceram. Int. 50, 30514–30532 (2024).
Dash, B., Routray, K. L., Saha, S., Sarun, P. M. & Sarangi, S. Insights into the effects of Mn substitution in CoFe2O4 nanoferrites involving high-frequency storage device applications. Int. J. Min. Metall. Mater. 32, 1245–1258 (2025).
Rani, B., Ghosh, S. & Sahu, N. K. A synergistic experimental and computational study on cation engineering in Mn-doped CoFe2O4 nanoparticles for high-performance supercapacitors. Electrochim Acta 147122 (2025).
Akhtar, S. et al. Toxicity of PEG-Coated CoFe2O4 nanoparticles with treatment effect of Curcumin. Nanoscale Res. Lett. 13, 1–8 (2018).
Habibi, M. H. & Parhizkar, H. J. FTIR and UV–vis diffuse reflectance spectroscopy studies of the wet chemical (WC) route synthesized nano-structure CoFe2O4 from CoCl2 and FeCl3. Spectrochim Acta Part. Mol. Biomol. Spectrosc. 127, 102–106 (2014).
Slatineanu, T. et al. Synthesis and characterization of nanocrystalline Zn ferrites substituted with Ni. Mater. Res. Bull. 46, 1455–1460 (2011).
Priyadharsini, P., Pradeep, A., Rao, P. S. & Chandrasekaran, G. Structural, spectroscopic and magnetic study of nanocrystalline Ni–Zn ferrites. Mater. Chem. Phys. 116, 207–213 (2009).
Bahlawane, N. et al. Tailoring the properties and the reactivity of the spinel Cobalt oxide. Phys. Chem. Chem. Phys. 11, 9224–9232 (2009).
Farajollahi, A. & Poursattar Marjani, A. Preparation of MWCNT/CoMn2O4 nanocomposite for effectual degradation of Picric acid via peroxymonosulfate activation. Sci. Rep. 14, 11475 (2024).
Sandosh, T. A. & Simi, A. Morphology controlled synthesis of one-dimensional CoMn2O4 nanorods for high-performance supercapacitor electrode application. Chem. Pap. 75, 2295–2304 (2021).
Joseph, A. M., Thangaraj, B., Gomathi, R. S. & Adaikalam, A. A. R. Synthesis and characterization of cobalt ferrite magnetic nanoparticles coated with polyethylene glycol. Adv. Nano Biol. M&D 1, 71–77 (2017).
Yu, T., Shen, Z. X., Shi, Y. & Ding, J. Cation migration and magnetic ordering in spinel CoFe2O4 powder: micro-Raman scattering study. J. Phys. Condens. Matter. 14, L613 (2002).
Bakhshi, H., Shokuhfar, A. & Afghahi, S. S. S. Structural, magnetic and Raman study of CoFe2O4@ C core–shell nanoparticles. Ceram. Int. 41, 10736–10744 (2015).
Chandramohan, P., Srinivasan, M. P., Velmurugan, S. & Narasimhan, S. V. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 184, 89–96 (2011).
Rotjanasuworapong, K., Lerdwijitjarud, W. & Sirivat, A. Synthesis and characterization of Fe0.8Mn0.2Fe2O4 ferrite nanoparticle with high saturation magnetization via the surfactant assisted co-precipitation. Nanomaterials 11, 876 (2021).
Yan, Z. et al. Effect of manganese substitution of ferrite nanoparticles on particle grain structure. Nanoscale Adv. 4, 3957–3965 (2022).
Dippong, T., Levei, E. A., Petean, I., Deac, I. G. & Cadar, O. A strategy for tuning the structure, morphology, and magnetic properties of MnFe2O4/SiO2 ceramic nanocomposites via mono-, di-, and trivalent metal ion doping and annealing. Nanomaterials 13, 2129 (2023).
Reddy, M. P., Mohamed, A. M. A., Zhou, X. B., Du, S. & Huang, Q. A facile hydrothermal synthesis, characterization and magnetic properties of mesoporous CoFe2O4 nanospheres. J. Magn. Magn. Mater. 388, 40–44 (2015).
Subedi, A., Yang, D., Yun, Y., Xu, X. & Dowben, P. A. Surface-to-bulk core level shift in CoFe2O4 thin films. J Vac Sci. Technol. A 40, (2022).
Nappini, S. et al. Surface charge and coating of CoFe2O4 nanoparticles: evidence of preserved magnetic and electronic properties. J. Phys. Chem. C. 119, 25529–25541 (2015).
Zhang, M. et al. High entropy spinel oxide (Ni0.2Co0.2Zn0.2Cu0.2Mg0.2)Fe2O4 nanofibers for efficient oxygen evolution reaction. J. Mater. Chem. A. 13, 1287–1301 (2025).
Xiang, W. et al. 3D atomic-scale imaging of mixed Co-Fe spinel oxide nanoparticles during oxygen evolution reaction. Nat. Commun. 13, 179 (2022).
Sadighi, Z. et al. Positive role of oxygen vacancy in electrochemical performance of CoMn2O4 cathodes for Li-O2 batteries. J. Power Sources. 365, 134–147 (2017).
Garg, N., Mishra, M. & Ganguli, A. K. Electrochemical and magnetic properties of nanostructured CoMn2O4 and Co2MnO4. RSC Adv. 5, 84988–84998 (2015).
Dai, L., Zhou, X., Yang, Y., Hu, P. & Ci, L. Ordered porous Mn – Co spinel oxide (CoMn2O4) with vacancies modulation as efficient electrocatalyst for Li – O2 battery. J. Colloid Interface Sci. 670, 719–728 (2024).
Cao, J., Zhang, D., Ren, B., Song, P. & Xu, W. Tungsten single atoms incorporated in Cobalt spinel oxide for highly efficient electrocatalytic oxygen evolution in acid. Energy Environ. Sci. 17, 5911–5921 (2024).
Li, A. et al. Directed surface reconstruction of Fe modified Co2VO4 spinel oxides for water oxidation catalysts experiencing Self-Terminating surface deterioration. Adv. Mater. 36, 2401818 (2024).
Ahangari, M. et al. Effect of Mn substitution at the B-site of Ba0.2Sr0.8FeO3 as efficient electrode material for supercapacitor devices. Electrochim Acta 146474 (2025).
Mostafaei, J. et al. Supercapacitor performance of the cobalt-substituted Ba0.2Sr0.8MnO3 perovskite oxide in symmetric and asymmetric configurations. Mater. Today Chem. 46, 102779 (2025).
Goudar, J. A. et al. Cobalt-Based materials in supercapacitors and batteries: A review. Adv. Energy Sustain. Res. 6, 2400271 (2025).
Liu, G., Shao, J., Gao, Y., Chen, Z. & Qu, Q. One-pot syntheses of spinel AB2O4 (A = Ni or Co, B = Mn or Fe) microspheres with different Hollow interiors for supercapacitors application. Chin. J. Chem. 35, 67–72 (2017).
El-Latif, E. I. A. et al. Modeling the diffusion coefficient of charge carriers in metal ion batteries using the Randles‐Sevcik equation. Adv Theory Simulations 2500346 (2025).
Rani, B. J., Sivanantham, A. & Cho, I. S. Nanostructured spinel Manganates and their composites for electrochemical energy conversion and storage. Adv. Funct. Mater. 33, 2303002 (2023).
Sivaguru, G. et al. Rational design of asymmetric spinel/defect spinel (ZnMn2O4/Cu1.5Mn1.5O4) nanocomposite-based supercapacitor devices for efficient energy storage with improved cycle stability. ACS Appl. Energy Mater. 7, 7205–7219 (2024).
Talluri, B., Aparna, M. L., Sreenivasulu, N., Bhattacharya, S. S. & Thomas, T. High entropy spinel metal oxide (CoCrFeMnNi)3O4 nanoparticles as a high-performance supercapacitor electrode material. J. Energy Storage. 42, 103004 (2021).
Ashassi-Sorkhabi, H., Kazempour, A., Mostafaei, J. & Asghari, E. Impact of ultrasound frequency on the corrosion resistance of electroless nickel-phosphorus-nanodiamond plating. Chem. Rev. Lett. 5, 187–192 (2022).
Ashassi-Sorkhabi, H., Mostafaei, J., Kazempour, A. & Asghari, E. Ultrasonic-assisted deposition of Ni-P-Al2O3 coating for practical protection of mild steel: influence of ultrasound frequency on the corrosion behavior of the coating. Chem. Rev. Lett. 5, 127–132 (2022).
Biswas, P., Dev, A., Kumar, P., Singh, R. K. & Kar, M. Adequate energy and power density in double-modified (Ag & Al) manganites for supercapacitor and thermistor applications. J. Power Sources. 653, 237727 (2025).
Jiao, H., Feng, T., Zhang, S. & Wu, M. Electrochemical deposition of ZnCo2O4/NiCo2S4 nanosheet arrays for high-performance supercapacitors. New. J. Chem. 46, 12686–12695 (2022).
Chuo, H. X. et al. Rationally designed hierarchical ZnCo2O4/Ni (OH)2 nanostructures for high-performance pseudocapacitor electrodes. J. Mater. Chem. A. 2, 20462–20469 (2014).
Acknowledgements
The researchers extend their thanks and appreciation to the collaboration and support of the University of Tabriz and the BAP Coordinator of the University of Sakarya (Project No. 2024-25-54-151).
Funding
Funding for this project to provide raw materials and conduct characterization analyses has been provided by the BAP coordinator of Sakarya University.
Author information
Authors and Affiliations
Contributions
Seyyed Mohammad Hossein Jafari-Mousavi: Investigation, Conceptualization, Methodology, Jafar Mostafaei: Methodology, Supervision, Writing – Original DraftMohammad Ahangari: Conceptualization, Methodology, Writing – Original DraftJalal Niazi Saei: Conceptualization, MethodologyAli Çoruh: Conceptualization, Methodology, Writing – Original Draft, Data CurationNagihan Delibaş: Methodology, InvestigationElnaz Asghari: Conceptualization, Writing – Review & Editing, Supervision, Project administrationAligholi Niaei: Conceptualization, Writing – Review & Editing, Supervision, Project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jafari-Mousavi, S.M.H., Mostafaei, J., Ahangari, M. et al. Application of CoFe2 − xMnxO4 spinel structures as an efficient electrode material for supercapacitors. Sci Rep 16, 2743 (2026). https://doi.org/10.1038/s41598-025-32387-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32387-y