Abstract
Over-expression of transmembrane serine protease 4 (TMPRSS4), which promotes epithelial-to-mesenchymal transition and cancer cell invasion, is associated with poor prognosis in patients with gastric cancer. This study aimed to develop a new anti-TMPRSS4 small interfering RNA (siRNA) therapeutic strategy by engineering lipid nanoparticles (LNPs). The biodistribution and anti-tumor effect of anti-TMPRSS4-siRNA loaded LNPs were evaluated in vitro and in vivo in mice with gastric cancer tumors (subcutaneous xenograft NUGC-3 cell tumors). LNPs demonstrated enhanced accumulation in the tumor compared with naked siRNA. A significant reduction in gastric cancer tumor growth was observed in mice undergoing combination therapy with LNPs and fluorouracil (5-FU) compared to mice receiving 5-FU alone. Anti-TMPRSS4-siRNA loaded LNPs may be considered a promising therapeutic modality for gastric cancer.
Similar content being viewed by others
Introduction
Although surgical resection is considered the main treatment for operable gastric cancer (GC), most patients at stages II and III require various adjuvant chemotherapies for preventing recurrence1. In 2007, the Adjuvant Chemotherapy Trial of S-1 for GC (ACTS-GC) demonstrated that surgery in addition to postoperative adjuvant chemotherapy with S-1 improved the overall survival (OS) and recurrence-free survival (RFS) rates compared to surgery alone for patients at stages II and III of GC; the 5-year OS rates in patients at stages IIIA and IIIB were 67.1 and 50.2%, respectively, in the S-1 group2,3. In 2019, the Japan Clinical Cancer Research Organization (JACRO) GC-07 demonstrated that an additional therapy of docetaxel to S-1 improved the prognosis of patients with stage III GC; the 3-year RFS rate in patients at stage III was 66% and 50% in the S-1 plus docetaxel S-1 alone groups, respectively4. However, there is scope for further improvements.
The overexpression of transmembrane serine protease 4 (TMPRSS4)5, a type II transmembrane serine protease6, is associated with many cancer types, including pancreatic cancer, colon cancer, biliary cancer, hepatocellular carcinoma, and GC7,8,9,10,11.TMPRSS4 promotes E-cadherin downregulation, which is associated with the epithelial-to-mesenchymal transition (EMT) and cancer cell invasion. TMPRSS4 also activates AKT and ERK signaling to lead to EMT and invasiveness through interaction with integrin-α5 to induce invasiveness12,13,14,. In addition, TMPRSS4 regulates urokinase-type plasminogen activators (uPA), leading to enhanced invasion15.
Previously, we demonstrated that TMPRSS4 overexpression was associated with poor prognosis in patients with stage III GC post-surgery, followed by adjuvant chemotherapy with S-1, and that the silencing of TMPRSS4 by small interfering RNA (siRNA) increased the sensitivity of GC cells to chemotherapy with fluorouracil (5-FU) in vitro8. In a multicenter retrospective study, we obtained results indicating an association between 5-FU chemosensitivity and TMPRSS4 expression in GC16.
RNA interference with siRNA is a powerful technology for silencing specific gene expression, and gene therapy using siRNAs has great potential to treat various diseases, including cancers, by inhibiting specific gene expression17,18. However, the clinical application of the naked form of siRNA is limited owing to enzymatic degradation in the blood and poor uptake by the target cells19. To overcome these problems, various nanocarriers have been developed to efficiently deliver siRNA. Among various nanocarriers, lipid nanoparticles (LNPs) are one of the most advanced delivery systems. LNPs have good compatibility, low toxicity, and the ability to encapsulate siRNA efficiently20.
In the present study, the biodistribution, anti-tumor effect, and safety of the combination therapy of 5-FU and LNP-containing siRNA-TMPRSS4 were evaluated in vivo using a GC nude mouse model and in vitro cellular uptake and subcellular distribution of LNPs.
Materials and methods
Cell culture and reagents
NUGC3 (obtained from the JCRB Cell Bank) was used as the human GC cell line. The cells were cultured in RPMI-1640 medium (Nacalai Tesque., Kyoto, Japan) supplemented with 10% fetal bovine serum (Life Technologies, Carlsbad, CA) at 37 °C in a humidified atmosphere supplied with 5% CO2.
siRNA transfection in vitro
Cells were transfected with TMPRSS4-siRNA MISSION-siRNA (#SASI_Hs02_00317341, #SASI_Hs02_00317342, and #SASI_Hs02_00317343; Sigma-Aldrich, St. Louis, MO) using Lipofectamine RNAiMAX reagent (Invitrogen; ThermoFisher Scientific, Waltham, MA), according to the manufacturer’s instructions. The siRNA MISSON-siRNA Universal Negative Control (Sigma-Aldrich) was used as a negative control.
Western blotting
The cells were washed with phosphate-buffered saline (PBS) and lysed with RIPA buffer (ThermoFisher Scientific, Waltham, MA)) containing phenylmethanesulfonylfluoride (PMSF: Cell Signaling Technology). Equal amounts of sample proteins were denatured by boiling for 10 min in Sodium dodecyl sulfate (SDS) sample buffer containing 1% beta-mercaptoethanol. Proteins were electrophoresed on an 10% SDS–polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked with 5% skim milk prepared in Tris-buffered saline containing 0.05% Tween 20 for 1 h and then incubated with a primary antibody overnight. The primary antibodies used are as follows: anti-TMPRSS4 rabbit polyclonal antibody (1:2000;11283-1, Proteintech), anti-E-cadherin rabbit monoclonal antibody (1:1000;24E10, Cell Signaling), and anti-beta actin mouse monoclonal antibody (1:10000; 13E5, Cell Signaling). For phosphor-p44/42 MAPK (Erk1/2) rabbit monoclonal antibody (1:2000, D13.14.4E, Cell Signaling Technology), cells were washed with PBS and lysed with RIPA buffer (ThermoFisher Scientific, Waltham, MA) containing protease and phosphatase inhibitor cocktail (ThermoFisher Scientific, Waltham, MA). Proteins were then visualized with anti-mouse and rabbit IgG horseradish peroxidase-conjugated secondary antibody (1:3,000; Cell Signaling Technology) using the ECL Western Blotting Detection System (Bio-Rad Technologies).
Preparation of SiRNA LNP
LNPs were prepared using Fiji Film Toyama Chemical Corporation (Tokyo, Japan). The ionizable lipid components (FL-2266T), 1,2-Distearoyl-sn-glycero-3-phosphocholine, cholesterol, and DMG-PEG-2000, at a molar ratio of 45:10:43.5:1.5, were dissolved in ethanol (total lipid: 12.5 mM/L). TMPRSS-4-siRNA (target sequence: 5’-GGAACUUUCCCACACUACUdTdT-3’) were dissolved in 50 mM sodium acetate (pH 4) (the concentration of siRNA: 0.163 mg/mL). Lipid and siRNA solutions were mixed using a microfluidic system (NanoAssembly Ignite+, Precision Nanosystems, Vancouver, Canada) at a volume ratio of 1:3 to formulate LNP. The LNPs were dialyzed against PBS to remove ethanol, and the formulation was concentrated using a centrifugal ultrafiltration system (100kD Amicon Ultra-15, Merck). The final siRNA concentration and RNA encapsulation efficacy were estimated using Quant-iT RiboGreen RNA Assay Kit (Thermo Fisher Scientific., Tokyo, Japan). The sizes of the synthesized LNPs were measured using a particle size measurement system (ELS-Z2, Ohtsuka Electrics Co., Osaka, Japan). Cyanine 5.5 (Cy.5)-labelled siRNA was prepared by Famac Co., Ltd. (Kanagawa, Japan).
In vitro cellular uptake and intracellular distribution
NUGC-3 (6 × 104 cells/well) cells were seeded in 8-well plates. Cells were transfected with LNP encapsulating Cy.5-labelled siRNA (siRNA concentrations of 100 nM) for 3 h. After treatment, the medium containing LNP was discarded, and the cells were incubated with RPMI for 24 and 48 h. The cells were then stained with LysoPrime Green (Dojindo, Tokyo, Japan). Cells were imaged using a BZ-X700 microscope (Keyence, Osaka, Japan).
In vivo tumor xenograft experiments
All the animal experiments were approved by the Institutional Animal Care and Use Committee of Kure Medical Center. A xenograft tumor model was developed using male nu/nu mice at 5–6 weeks of age to study biodistribution. All the mice were purchased from CLEA Inc. (Tokyo, Japan). NUGC-3 cells (2 × 106) suspended in 200 µl PBS were injected subcutaneously into the flanks of male nu/nu mice at 5–6 weeks of age to study the anti-tumor effect of the combination treatment: anti-TMPRSS4 siRNA-LNP and 5-FU. Tumor development was monitored until 2 weeks after the injection of cancer cells when the tumor volume reached approximately 0.1 cm3. Mice were subjected to the following two protocols:
Protocol 1 (low-dose LNP-siTMRSS4): The mice were randomly assigned to four groups, namely PBS, 5-FU, 5-FU plus control-LNP, and 5-FU plus anti-TMPRSS4 siRNA-LNP groups; 0.1 ml of PBS, 0.1 ml of LNP-siTMPRSS4 (15 µg of siTMPRSS4 per injection) solved in PBS, or an equal dose of LNP-siControl (negative control) was intravenously injected 2 times/week for 3 weeks, starting at day 14 after injection of cancer cells. 5-FU (10 mg/kg) was intraperitoneally injected three times/week for 3 weeks, starting at day 14. Tumor volume and body weight were also measured. The tumors, liver, spleen, and lungs were harvested for histological examination on day 35.
Protocol 2 (high dose of LNP-siTMPRSS4): Mice were randomly assigned to two groups, namely 5-FU and 5-FU plus anti-TMPRSS4 siRNA-LNP groups; 0.3 ml of LNP-siTMPRSS4 (120 µg of siTMPRSS4 per injection) solved in PBS was intravenously injected three times for 9 days, starting at day 14 after injection of cancer cells. 5-FU (10 mg/kg) was intraperitoneally injected four times for 9 days, starting on day 14. The dosage of si-TMPRSS4 was determined based on the paper by Adams J. et al.21 Tumor volume and body weight were also measured. The tumors, liver, spleen, and lungs were harvested for histological examination on day 23.
In vivo biodistribution of LNP
The mice were subject to subcutaneous injection of NUGC-3 cells (2 × 106) suspended in 200 µl PBS into the flanks. When the tumor diameter reached to 1 cm, LNP encapsulating Cy.5-labelled siRNA were intravenously injected into mice at a siRNA dose of 80 µg. At 24 h post-injection, tumors and organs, including the heart, lungs, liver, spleen, and kidneys, were collected, and the fluorescence in the organs and tumors was measured using an in vivo imaging system.
Preparation of mice
All procedures were accepted by NHO Kure Medical Center and Chugoku Cancer. All experiments were performed in accordance with relevant guidelines and regulations. All surgical procedures were performed under anesthesia (2% isoflurane). The method of mice euthanasia is cervical dislocation. The study is reported in accordance with ARRIVE guidelines22.
Statistical analysis
Statistical analyses were performed using EZR version 1.52 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)23. All data are expressed as mean ± standard deviation Student’s t-test or analysis of variance (ANOVA) was used for intergroup comparisons. Differences were considered statistically significant if the P value was less than 0.05.
Results
Silencing of TMPRSS4 suppresses ERK1/2 signal in vitro
We have previously showed that TMPRSS-4-silencing led to increased chemosensitivity to 5-FU in GC cells. To explore the mechanism underlying the anti-tumor effect of TMPRSS4-silencing, we examined ERK signaling. Western blotting revealed that Lipofectamine-siTMPRSS4 drastically decreased TMPRSS4 protein expression in NUGC-3 cells (Fig. 1 and Supplementary Fig. S1). We observed that TMPRSS4-silencing significantly suppressed the phosphorylation of 44/42-MAP kinase in NUGC-3 cells compared with that in control siRNA-transfected cells (p = 0.03) (Fig. 1).
In vitro silencing of transmembrane serine protease 4 (TMPRSS4) suppresses ERK1/2 signal in NUGC-3 gastric cancer cells. (a) Western blot comparing TMPRSS4, phospho-p44/42MAPK, p44/42MAPK, and β-actin. (b) Phosphorylation of level of MAPK. Band density measured using Image J. *p < 0.05. n = 3. C: control- small interfering RNA (siRNA); T: TMPRSS4 si-RNA.
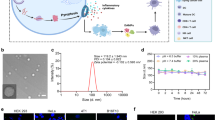
Characterization of LNP-siTMPRSS4, in vitro cellular uptake, and intracellular distribution of LNP-siTMPRSS4
The diameter of the LNP was approximately 100 nm (the particle size ranged from 53.4 nm to 231 nm) (Supplementary Fig. S3), and the encapsulation efficiency was 93%. LNP uptake was observed in the cytoplasm of NUGC-3 cells immediately after 3-h of exposure to LNP (Fig. 2b). Strong co-localization of LNPs on the lysosomes was observed at 3-h post-treatment (the co-localization of red and green markers in the merged image generated yellow fluorescence), while few co-localizations of naked si-RNAs on the lysosomes was observed (Fig. 2a). These results indicated that siRNA-LNPs were efficiently taken up by the GC cells.
Confocal images of NUGC-3 cells. (a) In vitro uptake of lipid nanoparticles containing cy.5-labelled small interfering RNA (siRNA) at 3-h post-treatment and (b) release of siRNA into the cytoplasm at 24-hour post-treatment. si-RNA was labelled cy.5 (red), and Endosome/lysosome was labelled LysoPrime Green (green).
In vivo biodistribution of LNP-siTMPRSS4
The in vivo distribution of LNP encapsulating Cy.5-labelled siRNA was investigated in NUGC-3 tumor-bearing mice and compared with that of naked Cy.5-labelled siRNA. While most naked siRNAs accumulated in the kidneys, most of the LNPs accumulated in the liver (Fig. 3). LNPs demonstrated significantly higher accumulation in the liver, kidney, spleen, and tumor than naked siRNA (Fig. 3). Tumors demonstrated approximately the same density of fluorescence signals as the spleen in mice receiving LNPs (Fig. 3).
In vivo biodistribution of cy.5-labelled small interfering RNA (siRNA) after 24 h in mice subject to intravenous injection. (a) in vivo imaging of cy.5-labelled si-RNA in mice subject to injection of naked si-RNA. (b) in vivo imaging of cy.5-labelled siRNA in mice subject to injection of lipid nanoparticles containing cy.5-labelled siRNA. (c) Quantitative analysis of fluorescence intensity measured from different organs. *p < 0.001, n = 3.
LNP-siTMPRSS4 suppressed GC cell growth in combination with 5-FU in vivo
We evaluated the anti-tumor effects of LNP-siTMPRSS4 in combination with 5-FU in a xenograft tumor model implanted with NUGC-3 cells. Protocol 1 was conducted to assess the anti-tumor effect of combination therapy using low-dose LNP in comparison with 5-FU monotherapy (Fig. 4a). Tumor growth in the LNP-TMPRSS4 treatment in combination with 5-FU was smaller compared to that of LNP-siCONT in combination with 5-FU. However, no significant difference was observed in the tumor volume and weight among the four groups: PBS, 5-FU, 5-FU plus control-LNPs, and 5-FU plus anti-TMPRSS4 siRNA-LNPs (Fig. 4b). Thus, Protocol 2 was conducted to assess the anti-tumor effect of combination therapy using a high dose of LNP-siTMPRSS4 (Fig. 5a). Combination therapy using a high dose of LNP-siTMPRSS4 led to a drastic reduction in the tumor weight (control: 962.6 ± 435.5 mg vs. LNP-siTMPRSS4: 487.4 ± 331.7 mg) and volume (control: 2.70 ± 1.36 cm3 vs. LNP-siTMPRSS4: 1.34 ± 0.80 cm3) compared with 5-FU monotherapy (Fig. 5b–d). However, no significant difference was observed when the size was examined based on the maximum tumor diameter (control: 17.09 ± 4.82 cm vs. LNP-siTMPRSS4: 15.29 ± 3.61 cm: p = 0.214). Western blotting revealed that TMPRSS4 expression in the tumor was significantly decreased by the combination of LNP-siTMPRSS4 with 5-FU compared to 5-FU monotherapy (Fig. 6a and Supplementary Fig. S2a), and that the expression of E-cadherin in the tumor was significantly increased by the combination of LNP-siTMPRSS4 with 5-FU compared to 5-FU monotherapy (Fig. 6b and Supplementary Fig. S2b).
High dose of small interfering (si)-transmembrane serine protease 4 (TMPRSS4)- lipid nanoparticle (LNP) in combination with fluorouracil (5-FU) suppressed tumor growth in vivo. (a) Experimental protocol 2 showing tumor inoculation, days of LNP and 5-FU, and endpoint. (b) Comparison of tumor weight obtained at endpoint. *p < 0.05. (c) Tumor growth curve after injection of cancer cells. *p < 0.05. (d) Tumor appearances in mice bearing cancer cells. Upper: control; lower: si-TMPRSS4-LNP, n = 7.
High dose of small interfering (si)-transmembrane serine protease 4 (TMPRSS4)-lipid nanoparticle in combination with fluorouracil suppressed the expression of TMPRSS-4 in tumors and enhanced the expression of E-cadherin in tumor in vivo. (a) Western blotting comparing TMPRSS4 and β-actin. Band density measured by Image J. *p < 0.05. (b) Western blotting comparing E-cadherin and β-actin. Band density measured by Image J. *p < 0.01.
In vivo LNP toxicity
We investigated the in vivo toxicity of the LNP-complex. No significant changes in the body weight (Start: 19.5 ± 1.3 g, End: 22.1 ± 1.4 g) were observed in mice treated with the combination of LNP and 5-FU. Hematoxylin and eosin staining of major organs, including the liver, spleen, and lungs, also demonstrated no marked histological changes (not shown). The serum alanine aminotransferase and creatinine levels in mice treated with LNP were within the normal ranges (Table 1).
Discussion
In stage III GC, additional therapy with docetaxel and S-1 improves the prognosis of patients after surgery4. However, there is scope for further improvement. Previously, we demonstrated that TMPRSS4 overexpression was associated with poor prognosis in patients with stage III GC post-surgery, followed by adjuvant chemotherapy with S-1, and demonstrated that silencing of TMPRSS4 by siRNA induced an increased sensitivity of GC cells to chemotherapy with 5-FU in vitro8,9, indicating TMPRSS4 as an emerging potential therapeutic target in GC. The present study evaluated the anti-tumor effect of targeted therapy against TMPRSS4 using LNPs in combination with 5-FU in mice with GC, since TMPRSS4 knockdown by RNA interference alone may not be sufficient to elicit an adequate therapeutic response.
After LNPs enter the cell, endosomal escape of siRNA is important for post-transcriptional gene silencing in the cytoplasm24. The cellular entry of LNP is followed by their trapping in endosomes, from which only a small fraction can escape. Only 2% of designed siRNAs can escape endosomes in the cytoplasm25. Ionizable lipids play a pivotal role in the fusion of entrapped LNPs with anionic endosomal membrane components, leading to siRNA leakage into the cytoplasm. Ionizable lipids are neutral at physiological pH; they are protonated at low pH and become cationic, which can promote endosomal membrane destabilization and facilitate endosomal escape. We used ionizable lipids (FL-2266T) as the cationic lipids. As shown in Figs. 2 and 3h after transfection, most of the LNPs resided in the endosome, as evidenced by the co-localization of cy-5-siRNA and endosomes (the co-localization of red and green markers in the merged image generated yellow fluorescence). Western blotting revealed that TMPRSS4 expression was drastically downregulated after the addition of LNPs. These results suggested that cy-5-siRNA efficiently escaped from the endosome reservoir and entered the cytoplasm.
In the current study, we observed that combination of LNP-siTMPRSS4 with 5-FU remarkably suppressed tumor growth compared to 5-FU and reduced TMPRSS4 expression in tumors. These results indicated that silencing of TMPRSS4 with siRNA-LNP had an anti-tumor effect in the GC mouse model. In protocol 1, 10-µg siRNA/mice was not sufficient for achieving the anti-tumor effect, while in protocol 2, high dose levels of approximately 120-µg siRNA/mice corresponding to 6-mg siRNA/kg body weight of mice were able to achieve the therapeutic effect. Lee et al. reported that by using LNPs without an active targeting ligand, a dose of 10-mg siRNA/kg of mouse body weight was able to achieve effective gene silencing of the androgen receptor in human prostate cancer-bearing mice21, in contrast with the targeting for hepatocyte, in which a dose of 30-µg siRNA/kg body weight is required to achieve 50% gene silencing26. Optimizing the dosage of siRNA remains a challenge for the future. Polyethylene glycol (PEG)ylated LNPs of 100 nm diameter tend to circulate for a long time and are able to preferentially access the tumor tissue through the leaky tumor vessels: this pathophysiological phenomenon is known as “enhanced permeability and retention (EPR)27. " However, LNPs likely accumulate in the liver due to their well-perfused nature and fenestrations in the hepatic sinusoid and also accumulate in the spleen due to uptake by the spleen phagocytic system. LNPs are unlikely to accumulate in tumors, which is reportedly less than several percent of the total dose of LNP28. Our results demonstrated that the majority of LNPs accumulated in the liver and spleen, which is consistent with the findings reported in the literature29. However, the tumors demonstrated a higher fluorescence signal in the spleen than in previous reports, irrespective of the absence of a targeting ligand. The enhanced accumulation of LNPs in tumors may require active targeting ligands for reducing the siRNA dose. Anthiya et al. adopted truncated Lyp-1 as a target ligand, which has good vascular permeation and tumor homing properties, and achieved enhanced LNP accumulation in the tumor and anti-tumor effects30. Further studies are warranted to explore the effects of active drug targeting.
TMPRSS4 silencing by siRNA induces anti-tumor effects in vivo. TMPRSS4 targeting using siRNA significantly upregulated E-cadherin expression in vivo and suppressed 44/42MAPK phosphorylation in vitro. We have demonstrated that the downregulation of TMPRSS4 is associated with the inactivation of EMT and ERK signaling, although our results did not provide direct evidence that the inhibition of TMPRSS4 induces an anti-tumor effect via the inactivation of EMT and ERK signaling. However, our results are consistent with that reported in the literature12,13. The limitation of this report is that we have not been able to verify the EMT markers (E-cadherin, vimentin, etc.) in vivo. Further studies are warranted to explore the mechanisms underlying the anti-tumor effect of TMPRSS4 downregulation, excluding EMT and ERK.
We evaluated the toxicity of the si-TMPRSS4-LNP in vivo. No side effects of LNPs regarding hepatic or renal function were observed in the group receiving high doses of LNPs. In humans, TMPRSS4 mRNA is weakly detected in the esophagus, stomach, small intestine, colon, and kidneys. However, the physiological roles of TMPRSS4 remain unclear. TMPRSS4 knockout mice are viable, fertile, and have no obvious abnormalities, suggesting the functional redundancy of TMPRSS431. These data indicate that TMPRSS4 silencing using siRNA may have minimal side effects. The use of LNP containing PEGylated lipids is considered safe in clinical settings as RNA drugs and vaccines have been approved by clinics32,33. The safety of siTMPRSS4-LNP is also presumably high, although the safety of these LNP should be further tested.
This study had certain limitations. First, we did not explore the anti-tumor effects of siTMPRSS4-LNP without 5-FU. Whether siTMPRSS4-LNP enhances chemosensitivity to 5-FU in vivo remains unclear. Further research is warranted to explore these anti-tumor actions, including the enhancement of the responsiveness of GC cells to 5-FU.
Data availability
The datasets used and/or analyzed in this study are available upon reasonable request. If someone wants to request the data from this study, he/she may request them from the corresponding author.
References
GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 303, 1729–1737. https://doi.org/10.1001/jama.2010.534 (2010).
Sakamoto, S. et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl. J. Med. 357, 1810–1820. https://doi.org/10.1001/jama.2010.534 (2007).
Sasako, M. et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 29, 4387–4393. https://doi.org/10.1200/JCO.2011.36.5908 (2011).
Yoshida, K. et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J. Clin. Oncol. 37, 1296–1304. https://doi.org/10.1200/JCO.18.01138 (2019).
Kim, S. TMPRSS4, a type II transmembrane Serine protease, as a potential therapeutic target in cancer. Exp. Mol. Med. 55, 716–724. https://doi.org/10.1038/s12276-023-00975-5 (2023).
Tanabe, L. M. & List, K. The role of type II transmembrane Serine protease-mediated signaling in cancer. FEBS 284, 1421–1436. https://doi.org/10.1111/febs.13971 (2017).
Exposito, F. et al. Targeting of TMPRSS4 sensitizes lung cancer cells to chemotherapy by impairing the proliferation machinery. Cancer Lett. 453, 21–33. https://doi.org/10.1016/j.canlet.2019.03.013 (2019).
Tazawa, H. et al. Utility of TMPRSS4 as a prognostic biomarker and potential therapeutic target in patients with gastric cancer. J. Gastrointest. Surg. 26, 305–313. https://doi.org/10.1007/s11605-021-05101-2 (2022).
Tazuma, S. et al. Effects of transmembrane Serine protease 4 on the survival in patients with pancreatic ductal adenocarcinoma undergoing surgery followed by adjuvant chemotherapy. Surg. Today. 54, 1208–1219. https://doi.org/10.1007/s00595-024-02824-y (2024).
Shibata, Y. et al. Transmembrane Serine protease 4 expression in the prognosis of radical resection for biliary tract cancer. World J. Gastrointest. Surg. 16, 2555–2564. https://doi.org/10.4240/wjgs.v16.i8.2555 (2024).
Dong, Z. R. et al. TMPRSS4 drives angiogenesis in hepatocellular carcinoma by promoting HB-EGF expression and proteolytic cleavage. Hepatology 72, 923–939. https://doi.org/10.1002/hep.31076 (2020).
Kim, S. et al. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin α and its signaling pathways. Carcinogenesis 31, 597–606. https://doi.org/10.1093/carcin/bgq024 (2010).
Larzabal, L. et al. Tmprss4 regulates levels of integrin α5 in NSCLC through miR-205 activity to promote metastasis. Br. J. Cancer. 110, 764–774. https://doi.org/10.1038/bjc.2013.761 (2014).
Min, H. J., Lee, M. K., Lee, J. W. & Kim, S. TMPRSS4 induces cancer cell invasion through pro-uPA processing. Biochem. Biophys. Res. Commun. 446, 1–7. https://doi.org/10.1016/j.bbrc.2014.01.013 (2014).
de Aberasturi, A. L., Calvo, A. & TMPRSS TMPRSS4: an emerging potential therapeutic target in cancer. Br. J. Cancer. 112, 4–8. https://doi.org/10.1038/bjc.2014.403 (2015).
Tazawa, H. et al. TMPRSS4 as a prognostic biomarker after gastric cancer surgery. Sci. Rep. 15, 8385. https://doi.org/10.1038/s41598-025-93422-6 (2025).
Dykxhoorn, D. M. & Lieberman, J. Knocking down disease with SiRNAs. Cell 126, 21–25 (2006).
Kulkarni, J. A. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643. https://doi.org/10.1038/s41565-021-00898-0 (2021).
Kanasty, R., Dorkin, J. R., Vegas, A. & Anderson, D. Delivery materials for SiRNA therapeutics. Nat. Mater. 12, 967–977. https://doi.org/10.1038/nmat3765 (2013).
Semple, S. C. et al. Rational design of cationic lipids for SiRNA delivery. Nat. Biotechnol. 28, 172–176. https://doi.org/10.1038/nbt.1602 (2010).
Lee, J. B. et al. Lipid nanoparticle siRNA systems for silencing the androgen receptor in human prostate cancer in vivo. Int. J. Cancer 131, e781-790 (2012).
Carol, K. et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013).
Niculescu, A. G., Bîrcă, A. C. & Grumezescu, A. M. New applications of lipid and polymer-based nanoparticles for nucleic acids delivery. Pharmaceutics 13, 2053. https://doi.org/10.3390/pharmaceutics13122053 (2021).
Gilleron, J. et al. Image-based analysis of lipid nanoparticle-mediated SiRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638–646. https://doi.org/10.1038/nbt.2612 (2013).
Coelho, T. et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl. J. Med. 379, 11–21 (2018).
Islam, W. et al. EPR-effect enhancers strongly potentiate tumor-targeted delivery of nanomedicines to advanced cancers: further extension to enhancement of the therapeutic effect. J. Pers. Med. 11, 487. https://doi.org/10.3390/jpm11060487 (2021).
Kalita, T., Dezfouli, S. A., Pandey, L. M. & Uludag, H. SiRNA functionalized lipid nanoparticles (LNPs) in management of diseases. Pharmaceutics 14, 2520. https://doi.org/10.3390/pharmaceutics14112520 (2022).
Zhang, R. et al. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater. Sci. 9, 1449–1463. https://doi.org/10.1039/d0bm01609h (2021).
Anthiya, S. et al. Targeted SiRNA lipid nanoparticles for the treatment of KRAS-mutant tumors. J. Control Release. 357, 67–83. https://doi.org/10.1016/j.jconrel.2023.03.016 (2023).
Keppner, A. et al. Epithelial sodium channel-mediated sodium transport is not dependent on the membrane-bound Serine protease CAP2/TMPRSS4. PLOS One. 10, e0135224. https://doi.org/10.1371/journal.pone.0135224 (2015).
Adams, D. et al. Patisiran, an RNAi Therapeutic, for hereditary transthyretin amyloidosis. N Engl. J. Med. 379 (1), 11–21. https://doi.org/10.1056/NEJMoa1716153 (2018).
Wang, J. et al. Recent advances in lipid nanoparticles and their safety concerns for mRNA delivery. Vaccines 12 (10), 1148. https://doi.org/10.3390/vaccines12101148 (2024).
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Author information
Authors and Affiliations
Contributions
H. Tazawa and H. Tashiro wrote the manuscript. Y. Ishida maintained the cell cultures and mouse management. T. Suzuki and Y. Shimizu conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was reviewed and approved by the NHO Central Ethics Committee [Approval Number: 2019-93].
Informed consent
Written informed consent was obtained from all of the participants involved in this study. All procedures were accepted by NHO Kure Medical Center and Chugoku Cancer.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tazawa, H., Ishida, Y., Suzuki, T. et al. Delivery of lipid nanoparticles containing small interfering RNA targeting transmembrane serine protease 4 in a human gastric cancer model using nude mice. Sci Rep 15, 45053 (2025). https://doi.org/10.1038/s41598-025-32407-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32407-x