Abstract
This prospective cohort study aimed to compare changes in vascular density around sclerocorneal and corneal incisions after cataract surgery using anterior segment optical coherence tomography angiography (AS-OCTA). To this end, 20 patients undergoing cataract surgery were divided into sclerocorneal and corneal (n = 10 each) incision groups. AS-OCTA imaging was performed preoperatively and at multiple intervals postoperatively. Vascular densities in the conjunctival and intrascleral layers were analyzed; postoperative changes were quantified using AS-OCTA. In the sclerocorneal incision group, the conjunctival vascular density was significantly reduced on days 1, 7, and 21, while intrascleral vascular density significantly increased on days 1, 3, 5, and 21. The corneal incision group showed an initial increase in conjunctival density on day 1, which rapidly returned to baseline. Intrascleral density was increased on days 1 and 3 but returned to baseline thereafter. Significant between-group differences in intrascleral density were observed on days 3, 5, and 7. In the corneal incision group, a new intrascleral vessel network formed near the incision site between days 14 and 21 postoperatively, which gradually dissipated between days 28 and 90. AS-OCTA reveals that sclerocorneal incisions exhibit immediate and sustained vascular changes, whereas corneal incisions show delayed, transient intrascleral vessel network formation.
Similar content being viewed by others
Introduction
Several surgical techniques have been developed to improve access to the opacified crystalline lens and enhance the efficiency of cataract surgery1,2. Since the adoption of phacoemulsification in the 1980s, sclerocorneal incisions, which create self-sealing wounds that extend from the sclera to cornea, have been the standard approach. However, corneal incisions have become more common as incision sizes have decreased. Corneal incisions offer various advantages over sclerocorneal incisions, including reduced bleeding1 and easier alignment along the steep meridian for astigmatism correction3; however, they are associated with slower wound healing4 and higher risk of wound dehiscence5. Although the healing processes after both incision types have been studied using light microscopy6, corneal topography, anterior segment optical coherence tomography (OCT)7, and slit-lamp microscopy, little is known about the processes involved in conjunctival and scleral healing around the incision sites.
Recent advancements in OCT angiography (OCTA) have enabled non-invasive visualization of blood vessels along with layer-specific retinal and choroidal vasculature analysis. Moreover, anterior segment (AS)-OCTA is increasingly used to monitor corneal neovascularization, assess postoperative pterygium progression, and visualize aqueous outflow pathways through vessels in the cornea, conjunctiva, and sclera8,9,10,11,12,13. In this study, we employed AS-OCTA to conduct temporal, non-invasive imaging of the conjunctiva and sclera surrounding cataract incision sites. Using these data, we compared vascular density and remodeling between corneal and sclerocorneal incisions.
Methods
Ethical approval and informed consent
This study adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Eguchi Eye Hospital (Approval No. N17000115). All patients provided written consent before participation, after being informed of the study’s purpose, procedures, and potential risks.
Patients
This prospective cohort study included 20 eyes in 20 patients who underwent age-related cataract surgery between January and October 2020. The inclusion criteria were as follows: (1) age ≥ 18 years and ability to complete all examinations and (2) presence of cataract with nuclear hardness classified as Emery-Little Grade 2. The exclusion criteria were as follows: (1) prior ocular surgery; (2) history of glaucoma, uveitis, allergic conjunctivitis, other external inflammatory eye diseases, diabetes, or any systemic diseases affecting postoperative wound healing; and (3) use of systemic or topical medications known to affect surgical outcomes.
Surgery
A single surgeon (S.E.) performed all phacoemulsification surgeries with intraocular lens implantation. The study population was divided into two groups based on the incision method. Ten eyes underwent a 2.4-mm single-plane sclerocorneal incision at the 12 o’clock position. The other 10 eyes received a 2.4-mm single-plane corneal incision at the same position. In both groups, sub-Tenon’s anesthesia was performed using 1 mL of 2% xylocaine (Sandoz Pharma K.K., Tokyo, Japan), injected into the sub-Tenon’s space through a small conjunctival incision at the nasal fornix to avoid interference with the surgical field. No intraocular or subconjunctival drugs, such as corticosteroids, antibiotics, or vasoactive agents (including epinephrine), were administered intraoperatively in either group. Phacoemulsification was performed using the Centurion® Vision System (Alcon Inc., Fribourg, Switzerland), and a Clareon® (Alcon Inc.) intraocular lens was implanted in the capsular bag. Phacoemulsification settings were standardized in all cases as follows: ultrasound power was set at 0%, vacuum limit at 450 mmHg, aspiration rate at 30 cc/min, torsional amplitude at 40%, and intraocular pressure was maintained at 20 mmHg during surgery. These parameters were uniformly applied to minimize variability in surgical technique. The surgery was completed after ensuring self-sealing of the wound without the need for sutures. No cauterization was performed in either group.
Postoperative medication included 0.5% levofloxacin and 0.01% betamethasone sodium phosphate four times daily and 0.1% diclofenac sodium three times daily for the first week. From 1 week to 3 months postoperatively, the regimen was adjusted to 0.5% levofloxacin and 0.1% fluorometholone four times daily, continuing with 0.1% diclofenac sodium three times daily.
No intraoperative complications were observed in any of the cases included in the study. Additionally, no thermal burns were noted at the incision sites, and good self-sealing was achieved in all cases, ensuring wound closure without sutures.
Examinations
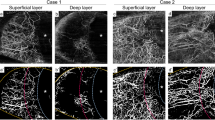
Anterior segment photography and AS-OCTA were performed preoperatively and at multiple intervals postoperatively (days 1, 3, 5, 7, 14, 21, 28, and 90) by a single examiner (Y.O.). AS-OCTA was conducted using a swept-source OCT system (PLEX Elite 9000; Carl Zeiss Meditec, Dublin, CA, USA) with a 10 D adapter lens, capturing 100,000 A-scans per second with a central wavelength of 1040–1060 nm and a bandwidth of 100 nm. The tissue A-scan depth was 3.0 mm, and the axial resolution was approximately 5 μm. Patients were instructed to look downward, and scans were generated by scanning around the wound area using the 6 × 6 mm retinal scan mode, corresponding to approximately 12 × 12 mm in the anterior segment14. En face images were generated using the system’s built-in software with automatic OCTA image segmentation. Flow images were generated for the superficial and deep layers, spanning from the conjunctival epithelium to 200 and 1000 μm (intrascleral)14,15.
In the present study, vascular density analysis was performed using ImageJ software (version 1.52, NIH, USA). First, a region of interest (ROI) of 1024 × 1024 pixels was defined in each image. A bandpass filter was applied (with parameters “Filter large structures down to 12 pixels” and “Filter small structures up to 3 pixels”) to remove the noise and enhance vessel features. Background subtraction was then performed using the “Subtract Background” function with a rolling ball radius of 8 pixels. The processed image was subsequently binarized using Otsu’s thresholding method. Histogram analysis was conducted, and the number of pixels with a signal value of 255 (representing vessels) as well as the total number of pixels within the ROI were obtained. Vascular density (%) was calculated by dividing the number of vessel pixels (signal value 255) by the total number of pixels within the ROI. The same analytic settings were uniformly applied to all images in this study. Postoperative vascular density values were converted to percentages (%) using preoperative values as the baseline for comparison.
Statistical analysis
Data are presented as the mean ± standard deviation. Pre- and postoperative changes in vascular density were evaluated using the Kruskal–Wallis test followed by pairwise Wilcoxon signed-rank tests with Bonferroni correction. Differences in vascular density between the sclerocorneal and corneal incision groups were analyzed using the Wilcoxon rank-sum tests with Bonferroni correction across timepoints. For multiple comparisons across different timepoints, p-values were adjusted using Bonferroni correction to control for family-wise error rate. Unless otherwise specified, the reported p-values in the Results section reflect this adjustment. Statistical analyses were performed using JMP version 15.2.0 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
Results
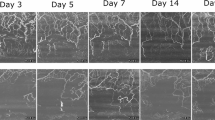
The baseline characteristics of participants are presented in Table 1. In the present study, the corneal incision group exhibited significantly better preoperative corrected visual acuity than the sclerocorneal incision group (p = 0.008). The intraoperative parameters, including phacoemulsification machine settings for all cases (ultrasound power at 0%, vacuum at 450 mmHg, aspiration at 30 cc/min, torsional mode at 40%, and IOP at 20 mmHg), were standardized across groups. No significant difference in total surgical time was observed between the two incision groups. Figure 1 depicts AS-OCTA images showing the changes in conjunctival vessels around the incision site over time. In the sclerocorneal incision group, the vascular networks along the margin of the conjunctival flap were disrupted on postoperative day 1. By day 3, the vessels extended from the conjunctival flap margin to incision site, suggesting active vascular remodeling within the flap. By day 5, the conjunctival vessels had reconnected to the marginal vascular network, and by day 28, the vascular pattern had nearly recovered to its preoperative state.
In the corneal incision group, a distinct increase in conjunctival vascular signal was identified around the incision site and throughout the conjunctival fornix on day 1. By day 3, the vascular signal had returned to preoperative levels, and the overall vascular pattern again closely matched its preoperative appearance.
Figure 2 presents AS-OCTA images demonstrating the changes in intrascleral vessels over time. In the sclerocorneal incision group, vascular density increased markedly from days 1 to 5. While vessels were visible along the incision margin on day 1, they became sparse after day 5. By day 7, a new vascular arcade had formed at the incision margin. The number of vessels around the incision gradually increased; however, the vascular network observed on day 90 still differed from its preoperative pattern.
In the corneal incision group, a significant increase in vascular signal was observed around the incision site and throughout the conjunctival fornix on day 1. By day 3, no evident disruption of the vascular network due to the corneal incision was observed. By day 5, the vascular network organization had become almost identical to its preoperative pattern, with no vessel invasion into the incision site observed. Between days 14 and 21, new vascular networks were observed near the incision site (Fig. 2, white arrows) in all 10 patients in the corneal incision group. These networks were visible a14.1 ± 8.5 days but not 56.9 ± 35.1. In all cases, the vascular network pattern on day 90 was nearly identical to its preoperative pattern.
Quantitative analysis of conjunctival vascular signal density revealed that it was significantly lower on days 1, 7, and 21 than preoperatively (all adjusted p = 0.017), whereas on day 90, it significantly increased compared with that at baseline (mean 103%, adjusted p = 0.017) in the sclerocorneal incision group, showing a reduction of approximately 12% on day 7 (Fig. 3). The reported p-values have been adjusted for multiple comparisons using the Bonferroni method. In the corneal incision group, vascular density increased significantly by approximately 36% on postoperative day 1 compared with its preoperative value (p = 0.0023). The conjunctival vascular density was significantly higher in the corneal incision group than in sclerocorneal group on day 1 (p = 0.0128), with a difference of approximately 38%.
In the sclerocorneal incision group, the intrascleral vascular signal density on days 1, 3, 5, and 21 was significantly higher compared with its preoperative value (adjusted p = 0.01, 0.01,0.01, and 0.017, respectively), with an increase of approximately 89% on day 3. In the corneal incision group, the intrascleral vascular density was significantly higher on days 1 and 3 compared with its preoperative value (p = 0.0001 and 0.0014, respectively), showing an increase of approximately 90% on day 1 (Fig. 4). The intrascleral vascular density was significantly lower in the corneal incision group than in sclerocorneal group on days 3, 5, and 7 (p = 0.0376, 0.0173, and 0.0452, respectively), with a difference of approximately 66% on day 5.
Discussion
In this study, we used AS-OCTA to investigate vascular remodeling in the conjunctiva and sclera after cataract surgery, comparing between sclerocorneal and corneal incisions. Importantly, the corneal incision group had significantly better preoperative corrected visual acuity than the sclerocorneal group. This baseline difference may have influenced postoperative vascular remodeling and visual recovery dynamics. Although our study design limits the ability to fully control for this variable, future research should aim to analyze more homogeneous groups to better understand the impact of corrected visual acuity. In the sclerocorneal group, conjunctival flow density signal initially decreased but gradually recovered, whereas the intrascleral vascular density increased early and remained elevated until postoperative day 21. In contrast, the corneal group exhibited only transient vascular changes immediately after surgery, followed by the delayed appearance of a new intrascleral vascular network around day 14, which regressed by day 90. These findings underscore incision-dependent differences in wound healing-associated vascular responses and highlight the utility of AS-OCTA in capturing these dynamic changes.
In this study, phacoemulsification machine settings—including ultrasound power at 0%, vacuum at 450 mmHg, aspiration rate at 30 cc/min, torsional mode at 40%, and intraocular pressure maintained at 20 mmHg—were standardized across the sclerocorneal and corneal incision groups. Moreover, no significant difference was observed in total surgical time between groups. This strict standardization ensures that the differences in vascular remodeling detected in this study are attributable primarily to the incision location rather than procedural variability. However, it should be noted that despite uniform machine settings, the inherent tissue impact and wound healing dynamics likely differ between the two incision methods, which warrants further mechanistic investigation.
Although conjunctival flow density signal was decreased in the sclerocorneal incision group, local vascular remodeling was activated between the corneal limbus and incision site starting on postoperative day 3. The conjunctival vessels that had been disrupted at the incision site gradually reconnected with the limbal vascular network. By postoperative day 28, the overall vascular signal pattern in the conjunctiva was almost equivalent to that observed before surgery. Despite the reduction in overall vascular density, vessels outside the remodeling area remained unchanged, suggesting that the avascular region created by the sclerocorneal incision may have influenced the analysis.
In contrast to the conjunctival changes, the intrascleral vascular density in the sclerocorneal incision group was increased significantly immediately after surgery. From postoperative day 7 onward, new vascular arcades formed at the incision margins. The overall intrascleral vascular organization remained altered compared with its preoperative pattern, even at 90 days postoperatively. The increased intrascleral vascular signal from immediately after surgery until postoperative day 5 coincides with the period of active wound healing. In a model for cataract surgery, Hikichi et al.16 reported that connective tissue penetrated the full thickness of the surgical wound by postoperative day 3, became denser and aligned with the stromal architecture by day 5, and showed further progression of wound healing by day 7. Similarly, in a model for skin wound healing, Tonnesen et al.17 demonstrated that angiogenic capillary buds invaded the fibrin/fibronectin-rich wound clot and became organized into a microvascular network throughout the granulation tissue within a few days. Hirasaka et al.6 showed that after sclerocorneal incisions, fibroblast activation and neutrophil infiltration were observed around the wound in rabbit eyes from day 1, with fibroblast numbers continuing to rise until day 7. Therefore, the significant increase in intrascleral vascular signal observed with AS-OCTA from immediately after surgery to day 5 aligns temporally with the active wound healing process from immediately after surgery to day 7, as reported in these previous studies. These findings suggest that the increased intrascleral vascular signal, as assessed using AS-OCTA, may reflect the process of active wound remodeling.
In the corneal incision group, the conjunctival and intrascleral flow density signal was transiently increased immediately after surgery (postoperative day 1) but quickly declined to near baseline levels by postoperative days 3–5. The early, transient increase in vascular density supports the role of limbal vessels in corneal wound healing, aligning with previous reports of early leukocyte recruitment via limbal vessels and cytokine-mediated vascular responses18,19,20. However, the rapid normalization may reflect a limited role of scleral vessels in corneal incisions compared with sclerocorneal incisions, suggesting a potentially greater role of other factors, such as corneal stromal cells and aqueous humor-derived macrophages, in the early wound healing after corneal incisions21,22.
Notably, in all the cases included in the corneal incision group, we observed a significant increase in vascular density in the limbal and adjacent scleral vessels near the incision site between postoperative days 14 and 21. This increase occurred although these areas were not directly manipulated during surgery. According to Hirasaka23, fibroblast proliferation remains active in corneal incision wounds at 2 weeks post-surgery. Additionally, Ernest et al.24 noted that fibroblast response and subsequent wound healing in corneal incisions were delayed by more than 1 week compared with those in sclerocorneal incisions. This finding is particularly interesting, because it demonstrates that wound healing after a corneal incision induces vascular remodeling not only at the incision site but also in the surrounding limbal and scleral regions that are not physically manipulated. The transient increase in vascular density in these non-manipulated areas suggests that the wound healing process after a corneal incision involves a broader vascular response than previously recognized. Hence, the appearance of this new vascular network around postoperative day 14 likely reflects the delayed fibroblast response and indicates ongoing processes involved in corneal wound healing.
The newly formed vascular network was transient, gradually regressing between postoperative days 28 and 90, and closely resembled the preoperative pattern by day 90. Supporting our findings, previous research24 in feline models demonstrated that, while sclerocorneal wounds achieve structural integrity within approximately 7 days, complete recovery of purely corneal incisions may require up to 60 days. Thus, the delayed normalization of the vascular pattern observed in the corneal incision group in our study likely reflects the inherently slower wound healing process of corneal incisions than that of sclerocorneal incisions. Despite these observations, only a few studies23,24 have investigated the temporal changes in limbal vascularity following corneal incisions, underscoring the need for further research to clarify the relationship between these vascular alterations and the mechanisms underlying corneal wound healing.
Both scleral and corneal incisions are utilized in cataract surgery, and the choice of surgical technique has been suggested to affect the risk of postoperative endophthalmitis, a serious postoperative complication25,26,27,28,29. Our study observed that the increased vascularization associated with sclerocorneal incisions may promote wound healing but also potentially enhance inflammatory responses. Notably, the sustained elevation of vascular density until postoperative day 21 in the sclerocorneal group suggests a prolonged phase of vascular activity that could influence infection risk and necessitates careful inflammation management. In contrast, the corneal incision group displayed more transient vascular changes, with early normalization of vascular density, indicating a potentially shorter inflammatory period. This difference may contribute to optimizing postoperative steroid use, potentially reducing overtreatment and associated risks. Quantitative and stratified analyses using AS-OCTA analyses offer new insights into cataract-incision healing and may help refine surgical planning and postoperative management. Based on our AS-OCTA findings, selecting a sclerocorneal incision, which promotes greater vascularization, may be advantageous for patients at high risk of impaired wound healing, such as those with diabetes mellitus, atopic dermatitis, or those receiving steroid treatment30. Furthermore, postoperative monitoring with AS-OCTA could enable non-invasive assessment of wound healing by tracking vascular changes, allowing tailored postoperative management and limiting the overuse of steroids.
Our study has some limitations. First, while posterior segment OCTA devices are equipped with sophisticated eye-tracking systems that compensate for eye movement by continuously monitoring retinal vessel position, such systems are designed for retinal use and may not function optimally for anterior segment scans, potentially resulting in reduced image quality owing to the noise resulting from eye movement. In this study, scanning positions were manually adjusted by referencing major conjunctival vessels identified in slit-lamp photographs of each eye. Nevertheless, the development of eye-tracking systems specific to AS-OCTA is needed. Second, although the cornea was excluded from the analysis range, the sclerocorneal incision site appeared as an avascular region within the sclera (Fig. 1), whereas the corneal incision site was barely visible as an avascular region (Fig. 2). Therefore, the vascular density in the sclerocorneal incision group may have been underestimated compared with that in the corneal incision group, particularly in the early postoperative period. Third, this study included a small sample size of 10 participants per group, which limited its statistical power and generalizability. A post hoc power analysis was performed for primary group comparisons. The statistical power for the postoperative day 1 superficial layer comparison was 0.29, reflecting low power owing to the limited sample size. For the deep layer comparisons at postoperative days 3, 5, and 7, the powers were 0.56, 0.66, and 0.66, respectively, indicating moderate statistical power. These findings highlight the need for cautious interpretation of the results and future studies with larger samples to confirm findings. However, this study is positioned as an exploratory study, and the findings obtained are intended to serve as foundational data for future large-scale studies. Fourth, surgical duration was not actively controlled for or included as a primary variable in this study, although there was no significant difference in operative time between groups (Table 1). Surgical duration may influence wound healing dynamics and therefore vascular density measurements. This aspect should be addressed in future research. Finally, the conjunctival and scleral vascular densities may have been affected by factors unrelated to wound healing, including medications and surgical disinfectants, which can transiently increase vascular density. Although efforts were made to minimize these effects, eliminating their influence within the adopted study design was challenging. While various factors essential for wound healing are known to be released from limbal vessels19,22,24, to the best of our knowledge, no reports have directly linked increases in conjunctival and scleral vascular density to wound healing. Further research efforts, including pathological studies, are needed to elucidate these relationships.
Conclusion
This study quantitatively evaluated vascular remodeling around sclerocorneal and corneal cataract incisions using AS-OCTA. Our key findings indicate that sclerocorneal incisions induce an immediate and robust vascular response characterized by sustained changes in conjunctival and intrascleral vascular density. In contrast, corneal incisions produce a limited initial response but are followed by the delayed formation of a transient new intrascleral vascular network between postoperative days 14 and 21, a phenomenon not previously captured by conventional imaging modalities. Herein, AS-OCTA enables precise visualization of these subtle yet clinically relevant vascular changes, emphasizing its utility as a non-invasive diagnostic tool for detailed assessment and management of surgical wound healing.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al Mahmood, A. M., Al-Swailem, S. A. & Behrens, A. Clear corneal incision in cataract surgery. Middle East. Afr. J. Ophthalmol. 21, 25–31. https://doi.org/10.4103/0974-9233.124084 (2014).
Piao, J. & Joo, C. K. Site of clear corneal incision in cataract surgery and its effects on surgically induced astigmatism. Sci. Rep. 10, 3955. https://doi.org/10.1038/s41598-020-60985-5 (2020).
Bar-Sela, S. M. & Spierer, A. Astigmatism outcomes of scleral tunnel and clear corneal incisions for congenital cataract surgery. Eye (Lond). 20, 1044–1048. https://doi.org/10.1038/sj.eye.6702082 (2006).
Buzard, K. A. & Febbraro, J. L. Transconjunctival corneoscleral tunnel blue line cataract incision. J. Cataract Refract. Surg. 26, 242–249. https://doi.org/10.1016/s0886-3350(99)00343-0 (2000).
Hurvitz, L. M. Late clear corneal wound failure after trivial trauma. J. Cataract Refract. Surg. 25, 283–284. https://doi.org/10.1016/s0886-3350(99)80140-0 (1999).
Hirasaka, T., Katakami, C. & Yamamoto, M. Corneoscleral wound healing after self-sealing cataract surgery–2. Cellular proliferation. Nippon Ganka Gakkai Zasshi. 99, 755–762 (1995).
Gharaee, H. et al. Comparing morphologic features and complications of main clear corneal incision between junior and senior residents observed using anterior segment optical coherence tomography. Med. Hypothesis Discov Innov. Ophthalmol. 12, 18–27. https://doi.org/10.51329/mehdiophthal1466 (2023).
Bergmann, M. T., Koch, D. D. & Zeiter, J. H. The effect of scleral cautery on corneal astigmatism in cadaver eyes. Ophthal Surg. 19, 259–262. https://doi.org/10.3928/1542-8877-19880401-10 (1988).
Bahl, V. J., Malik, K. P. S. & Guliani, B. P. Evaluation of cautery in manual small-incision cataract surgery. Indian J. Ophthalmol. 70, 3883–3887. https://doi.org/10.4103/ijo.IJO_1540_22 (2022).
Ruiz-Lozano, R. E. et al. The clinical and pathogenic spectrum of surgically-induced scleral necrosis: a review. Surv. Ophthalmol. 66, 594–611. https://doi.org/10.1016/j.survophthal.2020.12.008 (2021).
Makita, S. et al. Optical coherence angiography. Opt. Express. 14, 7821–7840. https://doi.org/10.1364/oe.14.007821 (2006).
Yasuno, Y. et al. In vivo high-contrast imaging of deep posterior eye by 1-microm swept source optical coherence tomography and scattering optical coherence angiography. Opt. Express. 15, 6121–6139. https://doi.org/10.1364/oe.15.006121 (2007).
Eguchi, S. et al. Impact of scleral cautery on limbal vasculature after cataract surgery assessed using optical coherence tomography angiography. Sci. Rep. 14, 22530. https://doi.org/10.1038/s41598-024-73677-1 (2024).
Akagi, T. et al. Conjunctival and intrascleral vasculatures assessed using anterior segment optical coherence tomography angiography in normal eyes. Am. J. Ophthalmol. 196, 1–9. https://doi.org/10.1016/j.ajo.2018.08.009 (2018).
Akagi, T. et al. Anterior segment optical coherence tomography angiography imaging of conjunctiva and intrasclera in treated primary open-angle glaucoma. Am. J. Ophthalmol. 208, 313–322. https://doi.org/10.1016/j.ajo.2019.05.008 (2019).
Hikichi, T. et al. Wound healing of scleral self-sealing incision: a comparison of ultrasound biomicroscopy and histology findings. Graefes Arch. Clin. Exp. Ophthalmol. 236, 775–778. https://doi.org/10.1007/s004170050157 (1998).
Tonnesen, M. G., Feng, X. & Clark, R. A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 5, 40–46. https://doi.org/10.1046/j.1087-0024.2000.00014.x (2000).
Byeseda, S. E. et al. ICAM-1 is necessary for epithelial recruitment of γδ T cells and efficient corneal wound healing. Am. J. Pathol. 175, 571–579. https://doi.org/10.2353/ajpath.2009.090112 (2009).
Li, Z. et al. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am. J. Pathol. 178, 1106–1116. https://doi.org/10.1016/j.ajpath.2010.12.001 (2011).
Wilson, S. E. Corneal wound healing. Exp. Eye Res. 19, 108089. https://doi.org/10.1016/j.exer.2020.108089 (2020).
Jaffe, N. S. Wound healing. in Cataract surgery and its complications, 4th edMosby,. (1984).
Azar, T. D. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American ophthalmological society thesis). Trans. Am. Ophthalmol. Soc. 104, 264–302 (2006).
Hirasaka, T. Corneoscleral wound healing after self-sealing cataract surgery–4. Scleral incision vs. corneal incision. Nippon Ganka Gakkai Zasshi. 99, 770–777 (1995).
Ernest, P. et al. Is there a difference in incision healing based on location? J. Cataract Refract. Surg. 24, 482–486. https://doi.org/10.1016/s0886-3350(98)80288-5 (1998).
Taban, M. et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch. Ophthalmol. 123, 613–620. https://doi.org/10.1001/archopht.123.5.613 (2005).
Oshika, T. et al. Incidence of endophthalmitis after cataract surgery in Japan. Acta Ophthalmol. Scand. 85, 848–851. https://doi.org/10.1111/j.1600-0420.2007.00932.x (2007).
Khanna, R. C. et al. Risk factors for endophthalmitis following cataract surgery-our experience at a tertiary eye care centre in India. Int. J. Ophthalmol. 8, 1184–1189. https://doi.org/10.3980/j.issn.2222-3959.2015.06.19 (2015).
Fine, I. H., Hoffman, R. S. & Packer, M. Profile of clear corneal cataract incisions demonstrated by ocular coherence tomography. J. Cataract Refract. Surg. 33, 94–97. https://doi.org/10.1016/j.jcrs.2006.09.016 (2007).
Shi, S. L. et al. Incidence of endophthalmitis after phacoemulsification cataract surgery: a meta-analysis. Int. J. Ophthalmol. 15, 327–335. https://doi.org/10.18240/ijo.2022.02.20 (2022).
Grzybowski, A., Kanclerz, P., Huerva, V., Ascaso, F. J. & Tuuminen, R. Diabetes and phacoemulsification cataract surgery: difficulties, risks and potential complications. J. Clin. Med. 8, 716. https://doi.org/10.3390/jcm8050716 (2019).
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors have no additional acknowledgments to declare.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Conceptualization: Tatsuaki AmariMethodology: Tatsuaki Amari, Shuichiro EguchiFormal analysis and investigation: Tatsuaki Amari, Yusuke OniyanagiWriting—original draft preparation: Tatsuaki AmariWriting—review and editing: Suguru Nakagawa, Shuichiro EguchiSupervision: Shuichiro EguchiGuarantor: Tatsuaki Amari is the guarantor of this work and accepts full responsibility for the finished article, had access to the data, and controlled the decision to publish.Non-author contributors: None.All individuals named here have given permission to be included.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Amari, T., Eguchi, S., Oniyanagi, Y. et al. Anterior segment optical coherence tomography angiography assessment of limbal vasculature changes after sclerocorneal vs. corneal cataract incisions. Sci Rep 16, 2793 (2026). https://doi.org/10.1038/s41598-025-32604-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32604-8