Abstract
Soil health is supported by diverse communities of organisms, including springtails and earthworms, facilitating essential processes such as nutrient cycling, organic matter decomposition, and soil structure maintenance. Cultural control methods promoted through Integrated Pest Management (IPM) are often assumed to be environmentally friendly, and their potential effects on soil health have received limited attention. Biofumigation, a cultural tactic, utilizes cruciferous plants like Brassica juncea (Brassicales: Brassicaceae), or their byproducts, to control soil-borne pests, yet their impacts on non-target organisms remain understudied. In this greenhouse study, we evaluated the impact of soil biofumigation with brown mustard seed meal (BMSM) on the springtail Folsomia candida (Entomobryomorpha: Isotomidae) and the earthworm Eisenia fetida (Opisthopora: Lumbricidae). An 85% reduction in springtail populations was recorded within 1 h of BMSM application. However, the springtail population recovered and surpassed the number of springtails in untreated media after 26 days. Earthworms preferred untreated media over BMSM-treated media immediately after incorporation. However, earthworms reared in the biofumigated media had higher body weight and produced more viable cocoons compared to those reared in untreated media. The negative effects of biofumigation on springtails and the deterrence of earthworms appeared to be short-lived and may later contribute to their reproductive fitness.
Similar content being viewed by others
Introduction
Soil health is vital to sustainable agricultural production systems, supporting not only crop productivity but also the complex ecosystem services that maintain agroecological stability1,2. A key component of soil health is the diverse communities of organisms that facilitate essential processes, including organic matter decomposition, nutrient cycling, and soil structure maintenance3,4. Earthworms and springtails are important contributors to these processes and are key members of the soil ecosystem5. Earthworms serve as “ecosystem engineers”, due to their ability to modify existing habitats and establish new ecological niches for various organisms6. This is done through a combination of biological processes, including organic matter fragmentation, tunneling activities, waste deposition, surface feeding, and nutrient translocation7. These activities collectively contribute to improved soil structure, aeration, fertility, and enhanced microbial activity8. Similarly, springtails increase decomposition and mineralization in the soil and are an efficient tool for toxicity assessments in soil habitats9. However, some species are also known to become pestiferous in large numbers, and when resources become limited10,11.
The functional roles of springtails and earthworms in an ecosystem are also influenced by inter- and intra-specific interactions within their subterranean community. For example, some springtails (i.e., F. candida) are reported to prefer the soil previously inhabited by the earthworms (i.e., Aporrectodea caliginosa, Savigny, 1826, and Lumbricus terrestris L.)12. Such interactions are also known to be species-specific; although L. terrestris burrows (i.e., casting tunnels) are attractive to the springtails Isotomiella minor Schaeffer and Isotoma notabilis Schaeffer, others, like Isotoma viridis Bourlet, Protaphorura cf. nemorata Absolon, and Lepidocyrtus lignorum (Fabricius, 1775) avoided the earthworms’ tunnels13. Therefore, in evaluating the impacts of agricultural and pest management practices on subterranean organisms’ behavior and biology, the interspecific interactions should also be considered.
Integrated Pest Management (IPM) promotes ecological-based control strategies and biorational use of pesticides according to a set of decision-making guidelines to manage pests and improve the sustainability of agroecosystems14,15. Various combinations of host plant resistance, cultural, chemical, and biological approaches are often used during the IPM implementation process16,17,18.
Cultural control tactics, such as crop rotation, tillage, cover crops, planting date, harvest date, and sanitation, are widely used practices in conventional and organic production systems19. These tactics can provide crops with a competitive edge against pests, reducing the need for frequent pesticide applications. Cultural management methods are generally accepted as safer alternatives to synthetic pesticides20. However, some cultural practices, such as biofumigation, may also disrupt soil structure and other soil health parameters critical in ensuring the sustainability of production systems21,22.
Soil biofumigation is a practice that involves the soil incorporation of cruciferous plants (plants from the family Brassicaceae) as fresh plant material, also known as green manure, or their byproducts (i.e., seed meal), to control soil-borne pests and promote soil health by supplying nutrients23. Cruciferous cover crops are also known to improve soil organic matter and structure24, while reducing nitrogen leaching25 and soil erosion26.
The biocidal effects of some cruciferous species, such as brown mustard, Brassica juncea (L.) Czern (Brassicales: Brassicaceae) are due to the plant’s glucosinolate contents, which are converted to isothiocyanates (ITCs) upon exposure to moisture following the breakdown of plant tissues27,28. Isothiocyanates are known to reduce insect growth, delay development, and sometime cause mortality when exposed through contact or fumigation29,30,31. Their toxic effects are due to the depletion of glutathione (GSH)32, an antioxidant that insects use to neutralize harmful compounds, as well as the inhibition of detoxification enzymes such as glutathione S-transferases (GST) and esterases31.
These isothiocyanates can be incorporated into the soil through additions of green manure or seed meal33,34. Seed meal is a residual byproduct of oil extraction from cruciferous plants35, therefore, using it as a biofumigant is not only an effective way to recycle this organic waste, but may enhance environmental sustainability36. For example, a higher level of biological activity has been observed with seed meal as compared to the green manure, as the glucosinolates are primarily concentrated in the seed and are retained throughout processing37. Additionally, seed meal can be stored for a longer period with stable glucosinolates due to their low moisture content38. Moreover, a uniform distribution and application rate control are possible with seed meal38. These benefits make seed meal applications an increasingly favored management option among organic producers39,40.
Isothiocyanates vary among plant species, resulting in different degrees of efficacy against various pest and pathogen groups41,42,43,44,45,46. For example, yellow mustard (Sinapis alba L.) primarily contains sinalbin, which hydrolyzes to ionic thiocyanate (SCN¯)37, a compound with herbicidal effects47, but no efficacy against subterranean pests such as wireworms48. In contrast, brown mustard (B. juncea L.) contains sinigrin, which produces allyl isothiocyanate49, a volatile and highly bioactive compound shown to be effective against subterranean arthropods50 and nematodes45,51,52. While the efficacy of B. juncea and B. carinata A. has been demonstrated against several species of subterranean pests and pathogens34,44,53,54,55, no measurable suppression of pests has been reported for B. napus L.56,57,58.
The biocidal effects of isothiocyanates may also result in unintended negative effects on non-target subterranean organisms that contribute to soil health59,60. For instance, the effectiveness of entomopathogenic nematodes (e.g., Steinernema carpocapsae (Weiser), S. glaseri (Steiner), and S. riobrave (Cabanillas, Poinar & Raulston, 1994)) (Heterorhabditis bacteriophora (Poinar 1976), H. marelatus (Liu 1996), and H. megidis (Poinar, Jackson & Klein 1987)) in controlling plant parasitic nematodes was reduced when the soil was treated with B. juncea extract and green manure59. Similarly, soil incorporation of B. carinata Braun seed meal, while detrimental to Columbia root-knot nematode (Meloidogyne chitwoodi Golden, O’Bannon, Santo & Finley, 1980), reduced the efficacy of the entomopathogenic nematodes S. feltiae (Filipjev, 1934) and S. riobrave61. Additionally, biofumigation using B. oleracea L. purple sprouting broccoli and wild B. oleracea L. was found to negatively affect the survival and reproduction of springtails (Folsomia candida) as well as the reproduction of earthworms (Eisenia andrei Bouche)60. Despite these studies, the impact of biofumigation with B. juncea seed meal, which has a high glucosinolates concentration62, on non-target and beneficial soil organisms, such as earthworms and springtails, remains poorly understood and requires further investigation.
In the present study, we evaluated the potential impacts of soil biofumigation with brown mustard seed meal (BMSM) on the springtail F. candida (Entomobryomorpha: Isotomidae), and the earthworm E. fetida (Opisthopora: Lumbricidae) in the greenhouse. Specifically, we examined the initial impact of BMSM biofumigation on springtail survival and evaluated the recovery of the population over time. The impact of biofumigation was also examined on earthworm media preference, body weight, and reproduction in the presence or absence of springtails. Understanding the impacts of soil biofumigation on non-target organisms can enable the development of pest management protocols that support the sustainability of the agroecosystem and mitigate environmental risks.
Material and methods
Potting soil and brown mustard seed meal
Sta-Green™ potting mix (Sta-Green Inc., Mooresville, NC), formulated with composted pine bark, sphagnum peat moss, horticultural perlite, and ground dolomitic limestone, was used in this study. The potting mix (pH 5.6) was sterilized at 93 °C for 1.5 h using a Pro-Grow SS-15 soil sterilizer (Pro-Grow Supply, Brookfield, WI) before use.
Brown mustard seed meal (B. juncea) was obtained from BuildAsSoil LLC (Montrose, CO) and applied at the label rate of 5.9 tons/ha, per manufacturer’s recommendation.
Biofumigation effects on springtail population
The springtail F. candida Willem was obtained from West Coast Creatures (Bellingham, WA) to establish a laboratory colony at the Southern Piedmont Entomology Laboratory, Blackstone, Virginia. Pint-sized glass jars containing a 9:5 mixture of plaster of Paris and charcoal at the bottom as the substrate were used to rear the springtails63. The jars were kept out of direct sunlight at room temperature (22 °C), and baker’s yeast was sprinkled weekly as a food source.
The experiments were conducted using square plastic pots (10 cm L × 10 cm W × 8 cm H). Throughout the experiment, the greenhouse temperature was recorded at 23.9 ± 2.8 °C (mean ± se). All pots were lined with folded pieces of fine plastic mesh (0.2 mm) at the bottom to keep springtails from escaping. Pots were filled with approximately 150 g of sterilized potting mix. The moisture content and the temperature of the potting mix were measured using a VG-METER-200™ (Vegetronix, Inc., Riverton, UT) and a pocket dial thermometer (VEE GEE Scientific, Inc. Vernon Hills, IL), respectively. The average moisture and temperature of the potting mix was 35.4 ± 0.81% (mean ± se) and 17.9 ± 0.59 °C, respectively.
One hundred milliliters of tap water was added to the surface of each pot, after which 20 springtails of same size (to reduce age-based variability in response) were introduced. A wet paper towel was placed on top of each pot to prevent the springtails from escaping; springtails tend to jump to relocate, and this approach was effective in containing the individuals, as confirmed in preliminary trials. The springtails were left undisturbed for 24 h as described by OECD guidelines 23264, to establish and habituate in the potting mix prior to biofumigation (i.e., brown mustard seed meal soil incorporation).
The brown mustard seed meal (BMSM)-treated treatment had 6 g of BMSM (equivalent to an application rate of 5.9 tons/ha) added to each pot and mixed gently using a lab spatula into the top 6–8 cm of the potted soil . The untreated control pots were also mixed gently to simulate similar conditions to treated pots. Each pot was watered on a weekly basis with 100 ml of tap water.
The experiment included a total of 140 pots divided into two groups: 70 of the pots treated with BMSM, and 70 untreated control pots. To determine the initial impact of BMSM application on springtail populations, 10 pots in each of the two groups were inspected after 1 h. The remaining observations were performed at 7 (N = 10), 12 (N = 10), 19 (N = 10), 26 (N = 10), 33 (N = 10), and 40 (N = 10) days after soil incorporation; these over time observations aimed to assess the recovery time of the springtail populations after BMSM biofumigation. Recovery time in our study is defined as the first time point at which the total springtail population in the BMSM treatment reaches statistically similar levels to the control treatments. This experiment was repeated twice (two time-blocks). The 7th day observation time was included only in the second time block.
At each observation time, the medium from each pot was gently spread in a 41 × 27 × 9 cm (length × width × depth) plastic tray partially filled with approximately 1.5 L of water. Springtails are hydrophobic and float on the surface of the water, facilitating the counting process60,65,66. For the first four observation times (1 h, 7 d, 12 d, and 19 d), all individual springtails were counted (absolute numbers) due to the relatively lower numbers. For the remaining 3 observation times (26 d, 33 d, and 40 d), as the numbers increased greatly, a grid-based estimation method was adopted. Each tray was photographed60 using a Canon EOS Rebel T7 camera equipped with an EF-S 18–55 mm lens mounted on a tripod, 112 cm above the tray (Supplementary Materials, Fig. S1). Each image was then pasted into a standardized (33.7 × 19.05 cm2) PowerPoint slide, and a 6 × 10 grid (2.5 cm2 /grid cell) was layered over the image. The number of springtails in five random grid cells were counted, averaged, and then multiplied by 60 to estimate the total number of springtails in each pot. The error rate of the grid-based estimation method was determined for ten pots from day 26 observation to validate the accuracy of our approach, using the formula below (Eq. 1). The error rate averaged 8.3% with a standard deviation of 5.7%.
Biofumigation effects on earthworms
Earthworm preference
The earthworms used in this study were obtained from HomeGrownWorms (Grand Junction, CO) and were kept in cocopeat in the greenhouse setting throughout the experiment (23.8 ± 2.8 °C). Dried cow manure (10 g), collected from pasture-based livestock maintenance facility Southern Piedmont AREC, was used as food substrate, added and gently mixed into each pot on a weekly basis. Earthworm preference was assessed through dual- choice experiments conducted in two-way polyvinyl chloride (PVC) olfactometers (300 PVC, 3.8 cm) (Supplementary Materials, Fig. S2), a modified version of the olfactometer used by Zirbes et al.67. In our study each olfactometer consisted of a central 4 cm pipe (5 cm, diameter) fitted with two 10 cm pipes on each side of the central piece (Supplementary Materials, Fig. S2). Two elbow pieces (31–1220 2A) (Supplementary Materials, Fig. S2) were fitted onto each end of the 10 cm pipes, facing up. A 1.5 cm diameter hole was drilled into a central pipe, through which the earthworm was introduced. The experimental setup included the following comparisons: i) biofumigated soil with brown mustard seed meal vs. untreated soil, ii) biofumigated soil with brown mustard seed meal containing springtails vs. untreated soil and iii) soil with only springtails vs. untreated soil. For treatments that included springtails, 0.2 mm cloth mesh pouches filled with 1 g of sterilized potting mix, and 50 springtails were used (Supplementary Materials, Fig. S3). The same size pouches, filled with only sterilized potting mix, were placed on the untreated side of the olfactometer. These pouches were placed in the potting mix medium on the opposite ends of the olfactometer immediately after BMSM applications.
The olfactometer was filled with sterilized potting mix (Sta-Green Inc., Mooresville, NC), leaving some airspace for crawling. To generate BMSM treatment on one side of the olfactometer, 2 g of BMSM (equivalent to 5.9 ton/ha) was added to the surface of the media at one end of the olfactometer and mixed gently. After biofumigation, the media exposed on each side of the olfactometer with the elbow were moistened with 2 ml of water and covered with plastic for 5 min. The moisture content and the temperature of the potting mix were measured using a VG-METER-200™ (Vegetronix, Inc., Riverton, UT) and a pocket dial thermometer (VEE GEE Scientific, Inc. Vernon Hills, IL), respectively. The average moisture of the media was 45.4 ± 2.4% before adding water, and the average soil temperature in olfactometers was 20.5 ± 3.1 °C.
The earthworms were deprived of their food substrate (cow manure) for one week prior to the test. In each test, a single earthworm with well-developed clitellum was introduced into the central portion of the olfactometer immediately after the incorporation of BMSM and left undisturbed for 30 min. After 30 min, the olfactometer was disassembled, and the location of the earthworm was recorded. Earthworms found in the middle portion of the olfactometer would have been considered non-responsive; however, all the earthworms were responsive in this bioassay. Each pairwise bioassay was replicated 20 times.
BMSM effects on earthworm fitness traits
Impact of biofumigation on body weight: Earthworm weight change following BMSM applications was evaluated in 4 × 21 cm (diameter (D) × height (H)) Ray Leach cone-tainers™ filled with 144 g of sterilized potting mix. Three treatments were imposed: i) recommended rate of brown mustard seed meal (8 g/cone-tainer, 5.9 tons/ha), ii) high rate of brown mustard seed meal (10 g/cone-tainer, 7.4 tons/ha), and iii) untreated control. Earthworms (juveniles) were weighed before and after the study21, using a Scout™ pro Electronic Balance, Ohaus-SP2001 (OHAUS Corporation, Parsippany, NJ). To weigh, earthworms were removed from cocopeat (before the experiment) or potting mix (after the experiment), gently washed, dried on paper towels, and placed on the scale in a 20 ml weighing dish/boat. The earthworms used in the experiments weighed between 105 and 515 mg.
Each treatment was replicated ten times, and the experiment was repeated twice (two time-blocks). Brown mustard seed meal was applied at the specified rates and mixed gently using a laboratory spatula. Dried cow manure (10 g) was added to each cone-tainer as a food substrate at the start of the experiment as previously described. The moisture content and the temperature of the potting mix were measured using a VG-METER-200™ (Vegetronix, Inc., Riverton, UT) and a pocket dial thermometer (VEE GEE Scientific, Inc. Vernon Hills, IL), respectively. The average media moisture in the olfactometer was 54.6 ± 3.2% and the average soil temperature was 22.7 ± 2.6 °C. Two earthworms were placed on the surface of the media in each cone-tainer and 5 ml of water was added to the surface. The cone-tainers were then sealed with a fine plastic screen (0.3 mm mesh) at both the top and bottom to prevent earthworm escape. The cone-tainers were then wrapped with plastics for 24 h to maximize earthworm exposure to the biofumigant following BMSM application in BMSM treatments.
Earthworms were kept for 28 days, and the cone-tainers were watered on a weekly basis (5 ml/cone-tainer). The percentage change relative to the initial weight68 was calculated as follows (Eq. 2):
where Weight (t2) is the average weight of two earthworms at the end of the experiment, and Weight (t1) is the initial average weight of the two earthworms.
Impact of biofumigation on reproduction: Food grade plastic containers (946 cc; 12 × 14-cm (D × H) were modified by puncturing approximately 100 randomly placed holes around each container, using an insect dissecting needle. An 8 cm (D) hole was cut out from each of the lids and covered with 0.3 mm cloth mesh to allow airflow into the containers. The study consisted of three treatments: i) recommended rate of brown mustard seed meal (10 g/container, 5.9 tons/ha), ii) high rate of brown mustard seed meal (13 g/container, 7.4 tons/ha), and iii) control (no brown mustard seed meal).
Each container was filled with 180 g of sterilized potting mix (as previously described), and brown mustard seed meal was applied to the surface at the specified rates and mixed gently into the top 6–8 cm of the media using a laboratory spatula. Dry cow manure (10 g) was added as a food substrate at the start of the experiment. Two adult earthworms were introduced into each container, and 5 ml of water was added to the media surface. The moisture content and the temperature of the potting mix were measured using a VG-METER-200™ (Vegetronix, Inc., Riverton, UT) and a pocket dial thermometer (VEE GEE Scientific, Inc. Vernon Hills, IL), respectively. The average moisture was 48.6 ± 3.3%, and the average soil temperature was 17.2 ± 2.4 °C. The containers were sealed with plastic wrap for 24 h after BMSM application.
Earthworms were maintained in containers for 60 days, receiving 5 ml of water weekly. After 60 days, the contents of the containers were hand-sorted, as described by Fouche’ et al.21 and OECD guideline for testing chemicals: earthworm reproduction test69, to count the unhatched cocoons and newly hatched juveniles. The unhatched cocoons were kept for another 30 days to assess the hatching success rate. The hatched juveniles and adult earthworms were discarded from the containers. Each treatment was replicated ten times, and the experiment was repeated twice. The cocoon hatch rate was calculated as follows (Eq. 3):
Statistical analysis
Statistical analyses were performed using IBM-SPSS ver.29 (IBM, Armonk, NY). Generalized linear mixed models (GLMM) with time and treatment as the fixed factors and repeat (time-block) as a random factor were used to compare springtail populations between BMSM-treated and untreated treatments. Springtail counts were square root log-transformed to meet GLM assumptions. The least significant difference (LSD) was used for pairwise comparisons.
Two-tailed sign test was used to analyze earthworm preference. Analysis of variance (ANOVA) with treatment as the fixed factor was used to compare earthworms weight change, followed by Tukey honestly significant difference (HSD) tests for pairwise comparisons.
The nonparametric Kruskal–Wallis test, followed by Wilcoxon signed rank tests for pairwise comparisons, was used to compare the number of cocoons between treatments. The cocoon hatch rate of earthworms was compared between treatments using a one-way ANOVA followed by Tukey HSD for pairwise comparison.
Results
Biofumigation effects on springtail population
Overall, springtail populations were significantly affected by BMSM application (F1,245 = 114.3, P < 0.001; Fig. 1). Changes in the springtail population were also significantly influenced by time (F6,237 = 817.9, P < 0.001). Moreover, there was significant interaction between treatment and time (F6,245 = 115.6, P < 0.001; Fig. 1).
An 85% reduction in springtail population was observed within an hour of biofumigation in treated pots compared to the control (P < 0.001). A similar pattern was observed at 7- and 12-days post-treatment, where control pots had 4.5- and 2.7-fold higher populations than the BMSM-treated pots, respectively. However, the difference in springtail numbers between the BMSM-treated and control pots disappeared by day 19 (P = 0.36) (Fig. 1). The trend then reversed, with the springtail population in biofumigated pots significantly exceeding those in control pots by day 26 (1.73-fold higher, P < 0.001), 33 (1.99-fold higher, P < 0.001), and 40 (3.97-fold higher, P < 0.001) (Fig. 1). A supplementary figure using untransformed data (actual average numbers of springtails) is also included (Supplementary Materials Fig. S4), with a bar graph (days 1- 40) and an inset focusing on the early phase (days 1–19) to better visualize initial treatment effects.
Earthworm preference
Earthworms displayed a significant preference toward the untreated soil over BMSM-treated soil (sign test: N = 20; P < 0.001) (Fig. 2a). This preference remained significant when earthworms were given a choice between soil treated with BMSM containing springtails and the non-treated soil (N = 20; P = 0.01) (Fig. 2b). However, no preference was detected between soil containing only springtails and control soil (N = 20; P = 0.1) (Fig. 2c.).
BMSM effects on earthworm fitness traits
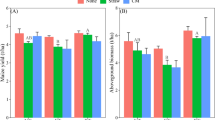
Impact of biofumigation on earthworm body weight: There was a significant (F2, 57 = 57.33, P < 0.001) effect of treatment on earthworm body weight, as the earthworms exhibited an increase in body weight in soils treated with the high (P < 0.001) and recommended (P < 0.001) rates of BMSM compared to the untreated control, where weight loss was reported. There was no significant difference in weight gain between earthworms exposed to the recommended rate and the high rate of BMSM (P = 0.86) (Fig. 3).
Percentage change relative to initial earthworm weight after biofumigation with the high and the recommended rates of brown mustard seed meal (BMSM), and in comparison, with the untreated control. Significant differences (P < 0.05) are indicated by different letters. Error bars represent standard errors (± 1SE).
Impact of biofumigation on earthworm reproduction: A significant treatment effect was observed on the number of cocoons deposited in the media (ꭓ22 = 30.12, P < 0.001) and the cocoons hatch rate (F2,57 = 5.66, P = 0.0057).
The average number of cocoons per earthworm (± SE) was significantly higher in the high (15.7 [± 0.54], P < 0.001) and recommended (14.7 [± 0.54], P < 0.001) rates of BMSM compared to the untreated control (9.2 [± 0.46]). However, no significant difference was observed between the two BMSM application rates (P = 0.61) (Fig. 4).
Impact of biofumigation with the high and the recommended rates of brown mustard seed meal (BMSM), and in comparison, with the untreated control, on the total number of cocoons produced by earthworms. Significant differences (P < 0.05) are indicated by different letters. Error bars represent standard errors (± 1SE).
The average hatch rate [± SE] was significantly higher in the high rate of BMSM (0.63 [± 0.01], P = 0.005) compared to the untreated control (0.57 [± 0.02]). In contrast, the hatch rate at the recommended BMSM rate (0.60 [± 0.02], P = 0.07) did not differ significantly from the control or from the high BMSM rate (P = 0.54) (Fig. 5).
Discussion
The springtail F. candida and the earthworm E. fetida are both significant contributors to soil health8,70,71,72, and our results demonstrated that soil biofumigation with brown mustard seed meal (BMSM) can affect different aspects of their biology and ecology.
Within an hour of biofumigation, springtail populations experienced a sharp decline, with mortality rates reaching 85%. This observation supported a previous study showing the toxicity of B. oleracea glucosinolates as a green manure on F. candida survival60. Despite this initial negative impact, the springtail population in BMSM-treated media recovered and ultimately surpassed that of the untreated control group. This suggests that, as expected, biofumigation effects were temporary and in the form of acute toxicity, due to the breakdown of isothiocyanates (ITCs)73,74,75,76. The exponential increase in springtail populations after BMSM application may be explained by the increase in the availability of nitrogen and organic matter following BMSM incorporation27. An increase in soil nitrogen content following fertilization is known to contribute to high springtail densities77,78. Alternatively, or in addition, hormoligosis following exposure to sublethal doses of isothiocyanates in springtails that survive the initial impact, may also explain the significant increase in reproduction in comparison to the untreated controls79,80,81. Future studies are needed to identify the underlying mechanism of this over-time reproduction success in springtail populations following BMSM applications.
The initial mortality of springtails highlights a potential risk associated with the use of biofumigation in agricultural systems, as they play a crucial role in residue decomposition and nutrient cycling5 and their reduction could temporarily affect these processes. However, the recovery of the springtail population suggests that biofumigation can still be a viable IPM tool. Moreover, the temporary delay in decomposition and nutrient cycling may not be entirely negative. It is possible that such a delay could better synchronize nutrient release with the crop’s demand during the later developmental stage, potentially enhancing nutrient use efficiency82. Nevertheless, field studies are needed to confirm the greenhouse results.
Despite their role in decomposition and nutrient cycling, springtails can become pestiferous when their population densities are high and soil organic matter or food sources are limited in the soil10,11. For instance, F. candida has been documented to cause damage in lettuce83 and sugar beet seedlings84, but the inflicted damage was reduced when an alternative food source was provided83. In our study, while the initial mortality demonstrated the BMSM biocidal effect against springtails, the same biocidal isothiocyanates can also cause crop damage due to their known phytotoxic properties38. The reported phytotoxicity and the observed increase in springtail populations over time following BMSM applications highlight the importance of further research on the timing of applications in different soil types (i.e., organic matter contents) to maximize crop development and minimize phytotoxicity.
The earthworm E. fetida showed a preference for untreated soil media over BMSM-treated media regardless of the presence of springtails. Earthworms are able to detect and avoid harmful substances in the soil as shown in previous studies85,86,87. Exploiting this evasive behavior, mustard extracts have also been used to extract earthworm from the soil as they try to escape the released isothiocyanates (ITCs)88,89,90. The observed earthworm avoidance of the BMSM-treated soil may be concerning as this behavioral response could influence their distribution and activity in biofumigated fields. Field studies are needed to trace earthworm movement through the soil profile over time after biofumigation.
Despite the observed earthworm avoidance of the BMSM-treated soil, no earthworm mortality was observed in BMSM-treated or untreated soil. Moreover, the observed positive impacts of BMSM on the earthworms’ body weight and reproduction in the present study contrasts earlier reports noting negative effects of biofumigation on earthworm reproduction60. There are several variables that may explain the observed inconsistency in findings including the species of mustard and the type of media. In our study, E. fetida were exposed to the B. juncea seed meal mixed into composted pine bark potting mix, whereas Zuluaga and colleagues60 used B. oleracea plant material (leaf) as a biofumigant incorporated into a peat-based potting mixture. Our results indicate that earthworms can survive and later benefit from the BMSM-treated soil, likely due to increased nitrogen availability in the soil91. Additionally, the observed positive impacts on earthworms’ body weight and reproduction rate, despite initial avoidance of BMSM- treated soil suggest that once isothiocyanates released following BMSM breakdown, earthworms could redirect their preference toward treated soil . However, future studies are warranted to validate this contention.
Moreover, our results showed no significant difference in weight gain and oviposition of the earthworms between the recommended and higher rates of BMSM. This indicates that if higher rates of B. juncea are required to achieve effective pest suppression, they can be applied without adversely affecting earthworm growth and reproduction. Future field studies should further explore the relationship between application rates, soil conditions, and efficacy to optimize biofumigation protocols for different agricultural systems.
Overall, these findings suggest that biofumigation could be incorporated into IPM strategies, but caution must be exercised to ensure that the benefits of pest control do not come at the expense of soil health and ecosystem function. Strategies that minimize the negative effects on the non-target organisms, such as the timing of biofumigation application to coincide with periods of low biological activity92,93 or using lower concentrations of biofumigant, should be explored.
One limitation of our study is that it does not represent a real-world scenario of an agroecosystem. Our study used by-products of brown mustard (BMSM), focusing on high glucosinolate content under controlled environmental conditions. However, under field conditions, factors like soil type, soil moisture, temperature, organic matter, and microbial communities could influence the release and the dispersal of the biofumigants and subsequently the impact on non-target organisms94,95,96,97,98. Additionally, our study only focused on two organisms, however, other crucial soil fauna like microbial communities may also be affected by biofumigation99,100,101,102 and warrants further research. In relation to this, it has been documented that a continuous inclusion of Brassica napus L., which contains considerably less concentrations of glucosinolates compared to B. juncea, into the crop rotation schedule in wheat (Triticum aestivum) production systems can negatively impact soil microbial activity103. The long-term impacts of repeated soil biofumigation remain unclear, and future studies should address these gaps to better understand the broader ecological effects of biofumigation.
Soil biofumigation with brassica green manures and their by-products are promoted as contributors to soil health and alternative to systemic pesticides. Our results demonstrate that while BMSM biofumigation causes initial mortality in springtails, both springtail and earthworm populations can recover and ultimately benefit from this practice. These findings fill a critical knowledge gap by demonstrating that non-target effects of biofumigation are more complex than previously understood and provide evidence that B. juncea seed meal effects are reversible and may enhance soil organisms fitness over time.
Data availability
The data sets generated during the study are available on request from the corresponding author.
References
Lehmann, J., Bossio, D. A., Kögel-Knabner, I. & Rillig, M. C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 1(10), 544–553 (2020).
Kibblewhite, M. G., Ritz, K. & Swift, M. J. Soil health in agricultural systems. Philos. Trans. Royal Soc B Biol. Sci. 363(1492), 685–701 (2008).
Reddy, H. R., Khan, M., Pasarkar, A. Soil invertebrates as sentinels of soil health: A zoological approach to soil quality assessment. J. Adv. Zool. 45(1). (2024).
Menta, C. & Remelli, S. Soil health and arthropods: From complex system to worthwhile investigation. Insects. 11(1), 54 (2020).
Brussaard, L. Ecosystem services provided by the soil biota. Soil Ecol. Ecosyst. services. (2012)(1995).
Römbke, J., Jänsch, S. & Didden, W. The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 62(2), 249–265 (2005).
Brown, G. G. How do earthworms affect microfloral and faunal community diversity?. Plant Soil 170(1), 209–231 (1995).
Stork, N. E. & Eggleton, P. Invertebrates as determinants and indicators of soil quality. Am. J. Altern. Agric. 7(1–2), 38–47 (1992).
Mogîldea, D., Gomoiu, I., Ştefănuţ, S., Onete, M., Comanescu, M., Honciuc, V., et al. Species monitoring in the central Parks of Bucharest. (2008).
Boetel, M. A., Dregseth, R. J., Schroeder, A. J. Control of subterranean springtails in sugarbeet using granular, liquid, and seed treatment insecticides. Entomology Research Sugarbeet Research and Education Board of Minnesota and North Dakota 28 January 2016. 2008.).
Chelongar, Y., Poorjavad, N., Khajehali, J., Ardestani, M. M. Assessing damages of collembolan species to some vegetables in greenhouses and their chemical control. Paper presented at: Proceedings of the Zoological Society2022.
Wickenbrock, L. & Heisler, C. Influence of earthworm activity on the abundance of collembola in soil. Soil Biol. Biochem. 29(3), 517–521 (1997).
Tiunov A, Kuznetsova N. Environmental activity of anecic earthworms (Lumbricus terrestris L.) and spatial organization of soil communities. biology bulletin-russian academy of sciences c/c of izvestiia-rossiiskoi akademii nauk seriia biologicheskaia. 27(5), 510–8. (2000)
Ehler, L. E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 62(9), 787–789 (2006).
Romeh, A. A. Integrated pest management for sustainable agriculture. In Sustainability of Agricultural Environment in Egypt: Part II: Soil-Water-Plant Nexus. 215–34. (Springer, 2018)
Zhou, W., Arcot, Y., Medina, R. F., Bernal, J., Cisneros-Zevallos, L., Akbulut, M. E. Integrated Pest Management: An Update on the Sustainability Approach to Crop Protection. ACS Omega. (2024).
Way, M. & Van Emden, H. Integrated pest management in practice—Pathways towards successful application. Crop Prot. 19(2), 81–103 (2000).
Prokopy, R. J. Two decades of bottom-up, ecologically based pest management in a small commercial apple orchard in Massachusetts. Agr. Ecosyst. Environ. 94(3), 299–309 (2003).
Bajwa, W. I., Kogan, M. Cultural practices: Springboard to IPM. In: Integrated pest management: Potential, constraints and challenges. 21–38 (CABI Publishing, 2004).
Hossain, L., Rahman, R., Khan, M. S. Alternatives of pesticides. Pesticide residue in foods: sources, management, and control. 147–65, (2017).
Fouché, T., Maboeta, M. & Claassens, S. Effect of biofumigants on soil microbial communities and ecotoxicology of earthworms (Eisenia andrei). Water Air Soil Pollut. 227(8), 256 (2016).
Castellano-Hinojosa, A., Boyd, N. S. & Strauss, S. L. Impact of fumigants on non-target soil microorganisms: A review. J. Hazard. Mater. 427, 128149 (2022).
Kumar, R., Mahajan, G., Srivastava, S., Sinha, A. Green manuring: A boon for sustainable agriculture and pest management-a review. Agricultural Reviews. 35(3) (2014).
Walker, B. A. R. et al. Ten years of green manuring and biofumigation alters soil characteristics and microbiota. Appl. Soil. Ecol. 187, 104836 (2023).
Brown, P. D. & Morra, M. J. Brassicaceae tissues as inhibitors of nitrification in soil. J. Agric. Food Chem. 57(17), 7706–7711, (2009).
Paudel BR. Green manures and Brassica soil amendments for soil health and soil microbial community in Douglas-fir nurseries and potato. (Washington State University, 2016).
Kirkegaard, J. & Matthiessen, J. Developing and refining the biofumigation concept. Agroindustria. 3(3), 233–239 (2004).
Abul-Fadl, M., El-Badry, N. & Ammar, M. Nutritional and chemical evaluation for two different varieties of mustard seeds. World Appl. Sci. J. 15(9), 1225–1233 (2011).
Agrawal, A. A. & Kurashige, N. S. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J. Chem. Ecol. 29(6), 1403–1415 (2003).
Beekwilder, J. et al. The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. PLoS ONE 3(4), e2068 (2008).
Du, Y., Grodowitz, M. J. & Chen, J. Insecticidal and enzyme inhibitory activities of isothiocyanates against red imported fire ants, solenopsis invicta. Biomolecules 10(5), 716 (2020).
Jeschke, V., Gershenzon, J. & Vassão, D. G. A mode of action of glucosinolate-derived isothiocyanates: Detoxification depletes glutathione and cysteine levels with ramifications on protein metabolism in Spodoptera littoralis. Insect Biochem. Mol. Biol. 71, 37–48 (2016).
Lazzeri, L., Leoni, O. & Manici, L. M. Biocidal plant dried pellets for biofumigation. Ind. Crops Prod. 20(1), 59–65 (2004).
Matthiessen, J. N. & Kirkegaard, J. A. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 25(3), 235–265 (2006).
Vichare, S. A. & Morya, S. Exploring waste utilization potential: nutritional, functional and medicinal properties of oilseed cakes. Front. Food Sci. Technol. 4(2024), 1441029 (2024).
Santana-Méridas, O., González-Coloma, A. & Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 11, 447–466 (2012).
Borek, V. & Morra, M. J. Ionic thiocyanate (SCN-) production from 4-hydroxybenzyl glucosinolate contained in Sinapis alba seed meal. J. Agric. Food Chem. 53(22), 8650–8654 (2005).
Serrano-Pérez, P., De Santiago, A. & Rodríguez-Molina, M. D. C. Biofumigation with pellets of defatted seed meal of brassica carinata: Factors affecting performance against phytophthora nicotianae in pepper crops. Front. Sustain. Food Syst. 5, 664531 (2021).
Mazzoncini, M. et al. Effect of defatted oilseed meals applied as organic fertilizers on vegetable crop production and environmental impact. Ind. Crops Prod. 75, 54–64 (2015).
Zaccardelli, M., Villecco, D., Celano, G. & Scotti, R. Soil amendment with seed meals: Short term effects on soil respiration and biochemical properties. Appl. Soil. Ecol. 72, 225–231 (2013).
Desaeger J, Csinos A, Seebold K. Evaluation of chemical and biological isothiocyanate generators on soilborne pest and disease control in squash. (2005).
Blank, R., Robertson, L., McMeikan, W. & Piggot, G. Summer crops of direct-drilled kale (Brassica oleracea) and fodder radish (Raphanus sativus) to control australian soldier fly (Inopus rubriceps). N. Z. J. Exp. Agric. 10(1), 83–86 (1982).
Akiew, S., Trevorrow, P. Biofumigation of bacterial wilt of tobacco. Paper presented at: Proc. 1st Australas. Soilborn Dis. Symp. (1999).
Stephens, P., Davoren, C. & Wicks, T. Effect of methyl bromide, metham sodium and the biofumigants Indian mustard and canola on the incidence of soilborne fungal pathogens and growth of grapevine nursery stock. Australas. Plant Pathol. 28, 187–196 (1999).
Walker, G. & Morey, B. Effect of brassica and weed manures on abundance of Tylenchulus semipenetrans and fungi in citrus orchard soil. Aust. J. Exp. Agric. 39(1), 65–72 (1999).
Mojtahedi, H., Santo, G., Wilson, J., Hang, A. Managing Meloidogyne chitwoodi on potato with rapeseed as green manure. (1993).
Morra, M. J., Popova, I. E. & Boydston, R. A. Bioherbicidal activity of Sinapis alba seed meal extracts. Ind. Crops Prod. 115, 174–181 (2018).
McCaffrey, J., Williams, L. III., Borek, V., Brown, P. & Morra, M. Toxicity of ionic thiocyanate-amended soil to the wireworm Limonius californicus (Coleoptera: Elateridae). J. Econ. Entomol. 88(4), 793–797 (1995).
Saini, A. R. K. Aspects of brassica juncea meal toxicity: allyl isothiocyanate release and bioassay. (2009).
Williams, L., Morra, M. J., Brown, P. D. & McCaffrey, J. P. Toxicity of allyl isothiocyanate-amended soil to Limonius californicus (Mann.)(Coleoptera: Elateridae) wireworms. J. Chem. Ecol. 19, 1033–1046 (1993).
Rahman, L. & Somers, T. Suppression of root knot nematode (Meloidogynejavanica) after incorporation of Indian mustard cv. Nemfix as green manure and seed meal in vineyards. Australas. Plant Pathol. 34, 77–83 (2005).
Daugovish, O., Downer, J., Becker, O., Browne, G. & Dunniway, J. Mustard-derived biofumigation for vegetable crops and strawberries. Agroindustria. 3, 335–338 (2004).
Furlan, L. et al. The efficacy of biofumigant meals and plants to control wireworm populations. Ind. Crops Prod. 31(2), 245–254 (2010).
Gouws, R. & Wehner, F. Biofumigation as alternative control measure for common scab on seed potatoes in South Africa. Agroindustria. 3, 309–312 (2004).
Van Os, G. et al. Biofumigation against soil borne diseases in flower bulb culture. Agroindustria. 3(3), 295–301 (2004).
Davis, J. et al. Effects of green manures on Verticillium wilt of potato. Phytopathology 86(5), 444–453 (1996).
Stirling, G. & Stirling, A. The potential of Brassica green manure crops for controlling root-knot nematode (Meloidogyne javanica) on horticultural crops in a subtropical environment. Aust. J. Exp. Agric. 43(6), 623–630 (2003).
Johnson, A., Golden, A., Auld, D. & Sumner, D. Effects of rapeseed and vetch as green manure crops and fallow on nematodes and soil-borne pathogens. J. Nematol. 24(1), 117 (1992).
Ramirez, R. A. II., Henderson, D. R., Riga, E., Lacey, L. A. & Snyder, W. E. Harmful effects of mustard bio-fumigants on entomopathogenic nematodes. Biol. Control 48(2), 147–154 (2009).
Zuluaga, D. L. et al. Biofumigation using a wild Brassica oleracea accession with high glucosinolate content affects beneficial soil invertebrates. Plant Soil 394, 155–163 (2015).
Henderson, D. R., Riga, E., Ramirez, R. A., Wilson, J. & Snyder, W. E. Mustard biofumigation disrupts biological control by Steinernema spp. nematodes in the soil. Biol. Control. 48(3), 316–322 (2009).
Okunade, O. A., Ghawi, S. K., Methven, L., Niranjan, K. Thermal and pressure stability of myrosinase enzymes from black mustard (Brassica nigra LWDJ Koch. var. nigra), brown mustard (Brassica juncea L. Czern. var. juncea) and yellow mustard (Sinapsis alba L. subsp. maire) seeds. Food chemistry. 187, 485–90. (2015)
Moore, J. C., Tripp, B. B., Simpson, R.T., Coleman, D. C. Springtails in the classroom: Collembola as model organisms for inquiry-based laboratories. Am. Biol. Teacher. 512–9. (2000).
OECD. Test No. 232: Collembolan Reproduction Test in Soil. 2016;https://doi.org/10.1787/9789264264601-en).
Gundersen, H., Leinaas, H. P. & Thaulow, C. Surface structure and wetting characteristics of Collembola cuticles. PLoS ONE 9(2), e86783 (2014).
Fischer, J., Szabó, B., Manikhin, L. & Filser, J. A hidden artefact: How surfactants can distort the results of springtail reproduction tests. Environ. Toxicol. Chem. 44(5), 1410–1421 (2025).
Zirbes, L., Deneubourg, J. L., Brostaux, Y. & Haubruge, E. A new case of consensual decision: Collective movement in earthworms. Ethology 116(6), 546–553 (2010).
Dittbrenner, N., Schmitt, H., Capowiez, Y. & Triebskorn, R. Sensitivity of Eisenia fetida in comparison to Aporrectodea caliginosa and Lumbricus terrestris after imidacloprid exposure. Body mass change and histopathology. J. Soils Sediments 11(6), 1000–1010 (2011).
(OECD) OfEc-oad. Earthworm reproduction test (Eisenia fetida/Eisenia andrei). paris;2004. Test No. 222, OECD Guidelines for the Testing of Chemicals, Section 2.
Alves PRL, Bandeira FO, Hennig TB. Ecological Role of Earthworms as Bioindicators of Soil Health. Life Sciences Research and Development. 51. (2022)
Neemisha. Role of soil organisms in maintaining soil health, ecosystem functioning, and sustaining agricultural production. Soil health. 313–35. (2020).
Yadav, R. S., Kerketta, D., Kumar, D. Biodiversity of soil arthropods: Symbol of soil health. Research trends in life sciences. 25–44. (2018)
Hanschen, F. S., Yim, B., Winkelmann, T., Smalla, K. & Schreiner, M. Degradation of biofumigant isothiocyanates and allyl glucosinolate in soil and their effects on the microbial community composition. PLoS ONE 10(7), e0132931 (2015).
Dungan, R. S., Gan, J. & Yates, S. R. Accelerated degradation of methyl isothiocyanate in soil. Water Air Soil Pollut. 142, 299–310 (2003).
Borek, V., Morra, M. J., Brown, P. D. & McCaffrey, J. P. Transformation of the glucosinolate-derived allelochemicals allyl isothiocyanate and allylnitrile in soil. J. Agric. Food Chem. 43(7), 1935–1940 (1995).
Gimsing, A. L., Strobel, B. W. & Hansen, H. C. Degradation and sorption of 2-propenyl and benzyl isothiocyanate in soil. Environ. Toxicol. Chem. Int. J. 28(6), 1178–1184 (2009).
Betancur-Corredor, B., Lang, B. & Russell, D. J. Organic nitrogen fertilization benefits selected soil fauna in global agroecosystems. Biol. Fertil. Soils 59(1), 1–16 (2023).
Wang, Z. et al. Effects of nitrogen addition on soil springtail (Collembolan) community in a poplar plantation. J Nanjing Forest. Univ. 48(4), 243 (2024).
Guedes, R., Smagghe, G., Stark, J. & Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 61(1), 43–62 (2016).
Jager, T., Crommentuijn, T., van Gestel, C. A. M. & Kooijman, S. A. L. M. Simultaneous modeling of multiple end points in life-cycle toxicity tests. Environ. Sci. Technol. 38(10), 2894–2900 (2004).
Santos, M. et al. Imidacloprid-mediated effects on survival and fertility of the Neotropical brown stink bug Euschistus heros. J. Pest. Sci. 89, 231–240 (2016).
Agyeman, K., Berchie, J., Gaisie, E. & Adomako, J. Synchronization of nutrient release from Gliricidia sepium with nutrient uptake by maize through biomass decomposition. Int. J. Sci Adv. Technol. 3(3), 49–56 (2013).
Tamura, K. & Moché, J. Reducing crop damage caused by folsomia candida by providing an alternate food source. J. Emerg. Investig. https://doi.org/10.59720/17-116) (2018).
Boetel, M., Dregseth, R., Khan, M. Springtails in Sugarbeet: Identification, Biology, and Management. (2001).
Da Luz, T. N., Ribeiro, R. & Sousa, J. P. Avoidance tests with collembola and earthworms as early screening tools for site-specific assessment of polluted soils. Environ. Toxicol. Chem Int. J. 23(9), 2188–2193 (2004).
Lukkari, T. & Haimi, J. Avoidance of Cu-and Zn-contaminated soil by three ecologically different earthworm species. Ecotoxicol. Environ. Saf. 62(1), 35–41 (2005).
Yeardley, R. B. Jr., Gast, L. C. & Lazorchak, J. M. The potential of an earthworm avoidance test for evaluation of hazardous waste sites. Environ. Toxicol. Chem. Int. J. 15(9), 1532–1537 (1996).
Pelosi, C. et al. A new method to measure allyl isothiocyanate (AITC) concentrations in mustard—Comparison of AITC and commercial mustard solutions as earthworm extractants. Appl. Soil. Ecol. 80, 1–5 (2014).
Lawrence, A. P. & Bowers, M. A. A test of the ‘hot’ mustard extraction method of sampling earthworms. Soil Biol. Biochem. 34(4), 549–552 (2002).
Singh, J., Singh, S. & Vig, A. P. Extraction of earthworm from soil by different sampling methods: A review. Environ. Dev. Sustain. 18, 1521–1539 (2016).
Marchetti, R., Lazzeri, L., D’Avino, L. & Ponzoni, G. Nitrogen and carbon mineralization in soils amended with biofumigant or non-biofumigant plant materials. Ind. Crops Prod. 75, 65–72 (2015).
Potvin, L. R. & Lilleskov, E. A. Introduced earthworm species exhibited unique patterns of seasonal activity and vertical distribution, and Lumbricus terrestris burrows remained usable for at least 7 years in hardwood and pine stands. Biol. Fertil. Soils 53(2), 187–198 (2017).
Badejo M, Van Straalen N. Seasonal abundance of springtails in two contrasting environments. Biotropica. 222–8. (1993)
Morra, M. & Kirkegaard, J. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 34, 1683–1690 (2002).
Jensen, C. R., Mogensen, V. O., Mortensen, G., Fieldsend, J. K., Milford, G. F. J, Andersen, M. N., et al. Seed glucosinolate, oil and protein contents of field-grown rape (Brassica napus L.) affected by soil drying and evaporative demand. Field Crops Res. 47(2), 93–105. (1996).
Van Dam, N. M., Tytgat, T. O. & Kirkegaard, J. A. Root and shoot glucosinolates: A comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem. Rev. 8, 171–186 (2009).
Velasco, P., Cartea, M. E., González, C., Vilar, M. & Ordás, A. Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J. Agric. Food Chem. 55(3), 955–962 (2007).
Gu, Z.-x, Guo, Q.-h & Gu, Y.-j. Factors influencing glucoraphanin and sulforaphane formation in brassica plants: A review. J. Integr. Agric. 11(11), 1804–1816 (2012).
Siebers, M. et al. Disruption of microbial community composition and identification of plant growth promoting microorganisms after exposure of soil to rapeseed-derived glucosinolates. PLoS ONE 13(7), e0200160 (2018).
Wang, L. & Mazzola, M. Field evaluation of reduced rate brassicaceae seed meal amendment and rootstock genotype on the microbiome and control of apple replant disease. Phytopathology 109(8), 1378–1391 (2019).
Ma, Y. et al. Impact of brassicaceous seed meals on the composition of the soil fungal community and the incidence of Fusarium wilt on chili pepper. Appl. Soil. Ecol. 90, 41–48 (2015).
Mazzola, M., Hewavitharana, S. S. & Strauss, S. L. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen reinfestation. Phytopathology 105(4), 460–469 (2015).
Hansen, J. C., Schillinger, W. F., Sullivan, T. S. & Paulitz, T. C. Soil microbial biomass and fungi reduced with canola introduced into long-term monoculture wheat rotations. Front. Microbiol. 10, 1488 (2019).
Acknowledgements
This study was supported by the College of Agriculture and Life Science, and the Southern Piedmont Agricultural Research and Extension Center.
Funding
Funding for this study was provided by the College of Agriculture and Life Sciences, and the Southern Piedmont Agricultural Research and Extension Center, Virginia Tech.
Author information
Authors and Affiliations
Contributions
Conceptualization, design, analysis, and interpretation of the data: U. P, and A. R Data acquisition, visualization, and writing—original draft preparation: U.P Conceptualization, writing—review, and editing: A.J Writing—review and editing, funding acquisition, and project administration: A.R
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Panta, U., Jernigan, A. & Rashed, A. Effects of soil biofumigation on non-target springtails (Collembola) and earthworms (Opisthopora). Sci Rep 16, 3551 (2026). https://doi.org/10.1038/s41598-025-33634-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-33634-y