Abstract
Melanoma is an aggressive form of skin cancer arising from melanocytes, noted for its high metastatic potential and elevated mortality rate. Conventional treatment approaches are often limited by nonspecific systemic drug distribution, which can result in undesirable adverse effects and premature drug degradation. In this study, we report the development of a microneedle (MN)-mediated delivery platform, incorporating self-assembling polymeric micelles, combining a dissolvable MN patch with polymeric micelles designed to prevent ICG aggregation and co-encapsulate ZER and ICG, and enable combined chemotherapy and photothermal therapy against melanoma. These nanocarriers, referred to as ZER-ICG-polymer micelles, co-encapsulate Zerumbone (ZER)—a lipophilic sesquiterpenoid—and indocyanine green (ICG), a well-known photothermal agent. In vitro cytotoxicity assessments and intracellular distribution studies confirm a marked synergistic therapeutic effect under NIR light exposure, facilitating localized thermal ablation of melanoma cells. In vivo experiments using melanoma-bearing mice demonstrate that a single application of the ZER-ICG-loaded dissolvable microneedle patch, coupled with NIR irradiation, achieves potent photothermal performance and robust tumor growth inhibition, while inducing minimal systemic toxicity and negligible side effects. These findings highlight the promising potential of this transdermal delivery system for the treatment of superficial malignant tumors.
Similar content being viewed by others
Introduction
Melanoma represents the most aggressive and treatment-refractory form of skin cancer1, originating from the malignant transformation of melanocytes and neural crest-derived cells located within the epidermal layer of the skin2. Although it accounts for only approximately 1% of all cutaneous malignancies, melanoma is responsible for the majority of skin cancer-related fatalities, due to its highly invasive nature and pronounced metastatic potential3,4. At present, surgical excision remains the primary and most widely adopted therapeutic approach for managing melanoma5. However, this method has significant limitations, including constraints in the extent of tissue resection, challenges in achieving clear surgical margins, the likelihood of tumor recurrence in situ, and patient-related concerns6. In addition to surgery, a variety of alternative and adjunctive therapeutic strategies have been developed. These include conventional chemotherapy, immune checkpoint blockade therapies, molecularly targeted therapies, sonodynamic (ultrasound-based) therapy7, magnetic response therapy8and photothermal therapy (PTT)9. Among these, PTT has emerged as a promising modality due to its ability to induce localized hyperthermia, leading to irreversible damage to tumor cells10. The application of near-infrared (NIR) laser-irradiated photothermal therapy (PTT) for inducing hyperthermia and reactive oxygen species (ROS) in tumor tissue has been widely integrated with chemotherapy and immunotherapy, leveraging their capabilities for direct tumor ablation and immunogenic cell death (ICD) induction11,12. PTT is increasingly favored for its non-invasive nature, high target specificity, and relatively low systemic toxicity13. Indocyanine green (ICG), a tricarbocyanine-based fluorescent dye approved by the U.S. Food and Drug Administration (FDA), exhibits excellent photothermal conversion efficiency under near-infrared (NIR) light. Due to its strong NIR absorption and ability to generate localized heating, ICG has been extensively investigated in the context of PTT for tumor ablation14. Nevertheless, its physicochemical drawbacks, such as poor aqueous stability and a pronounced tendency to aggregate in water-based solutions, which limits its incorporation into microneedle (MN) matrices commonly fabricated via aqueous processes15. Zerumbone (ZER), a naturally occurring sesquiterpene extracted from the rhizome of Zingiber zerumbet (L.) Smith, exhibits a wide range of bioactive properties, including anti-inflammatory, antioxidant, and anticancer activities16. The chemical structure of ZER is shown in (Fig. 1A). A study conducted by Oh et al. revealed that ZER significantly inhibits melanin production in α-melanocyte-stimulating hormone (α-MSH)-stimulated B16F10 melanoma cells, highlighting its therapeutic potential in pigmentation-related pathologies and skin malignancies17. Multiple studies have further confirmed that ZER demonstrates significant antineoplastic effects under varying concentrations, both in vitro and in vivo. These effects include the selective suppression of tumor cell proliferation, modulation of cancer-related signaling pathways, and a favorable safety profile with minimal cytotoxicity toward normal cells18,19. Despite its promising anti-cancer properties, ZER’s poor solubility in water severely compromises its oral bioavailability and systemic absorption, limiting its practical utility in clinical oncology.
(A) The chemical structure of ZER. (B) The simple schematic diagram of the preparation process of ZER-ICG-NMs. (C) The schematic diagram of the preparation process of ZER-ICG@DNMs. (D) Schematic representation of the transdermal delivery of ZER-ICG@DMNs for the combined chemo-photothermal therapy of melanoma.
In recent years, microneedle (MN)-based transdermal drug delivery systems have gained considerable attention as an innovative and minimally invasive method for enhancing drug penetration into deeper skin layers and tumor tissues, while reducing off-target effects and improving patient adherence20. MNs enable enhanced local drug concentration at the site of action, and by bypassing the gastrointestinal tract and hepatic first-pass metabolism, they offer increased bioavailability21. Among various MN types, dissolving microneedles (DMNs)—fabricated from biocompatible, water-soluble polymers such as hyaluronic acid (HA)—have shown exceptional promise for efficient drug encapsulation and sustained release22. Upon insertion into the skin, DMNs dissolve to release their payload, providing both convenience and improved drug stability23,24. Furthermore, compared to systemic administration via intravenous injection, DMNs can generate a uniformly distributed network of microchannels in the skin, facilitating enhanced drug diffusion into the tumor microenvironment, thereby increasing therapeutic efficacy and simultaneously minimizing systemic toxicity25.
Photosensitizers efficiently absorb light energy, enabling NIR therapy to be carried out at lower power levels, reducing damage to surrounding normal tissues. ZER could achieve efficient killing effect on tumor tissues, significantly enhancing the treatment efficiency. Nanostructures are widely used in drug delivery for cancer treatment due to their potential in bioimaging and phototherapy26. We co-encapsulated two functional agents—ZER, a sesquiterpene derived from ginger (Zingiber zerumbet), and ICG, a clinically approved NIR-responsive photothermal agent—within polymeric nano micelles, generating ZER-ICG-NMs nano micelles with enhanced stability and drug-loading efficiency. These dual-functional nanoparticles were subsequently localized at the needle tips of the DMN patch to fabricate the ZER-ICG @DMNs system, as illustrated schematically in (Fig. 1B). This localized encapsulation strategy enabled not only improved skin penetration but also a more targeted and efficient delivery of the therapeutic agents to melanoma lesions, and allowed for precise photothermal control under NIR exposure. When exposed to 808 nm NIR laser, the released ICG converted the light energy into thermal energy and promoted the production of Reactive oxygen species (ROS), eventually triggering considerable PTT effects to induce tumor cell apoptosis and necrosis. In vivo analyses confirmed that the ZER-ICG-NMs possessed excellent photothermal responsiveness under NIR irradiation, alongside potent cytotoxic effects against melanoma cells. Furthermore, in vivo studies conducted on tumor-bearing mice demonstrated a remarkable therapeutic efficacy of the ZER-ICG @DMNs patch, with significant tumor growth inhibition, robust photothermal responses, and no evident toxicity to surrounding tissues or major organs, as shown in (Fig. 1C). This system promises an administration with high efficiency, paving the way for future development of DMN-integrated nanomedicines targeting other superficial solid tumors.
Materials and methods
Materials and animals
Indocyanine green (ICG), a clinically approved near-infrared photosensitizer, was procured from MedChemExpress (Shanghai, China), whereas the biodegradable copolymer monomethoxy-poly (ethylene glycol)-poly (lactic acid) (mPEG-PLA) was sourced from Tanshtech (Guangzhou, China). All reagents and chemicals utilized throughout this study were of analytical grade and applied directly without further purification.
The cell culture media RPMI-1640, fetal bovine serum (FBS), phosphate-buffered saline (PBS), trypsin–EDTA solution (0.25%), and antibiotics (penicillin–streptomycin) were acquired from Gibco Corp. (California, USA). The cell viability assay reagent, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (CCK-8), was supplied by Abbkine (Wuhan, China). Kits for apoptosis analysis (Annexin V-FITC/PI) were purchased from Elabscience Biotechnology Co., Ltd. (Wuhan, China). Calcein-AM/PI double-staining reagents, nuclear dyes (Hoechst 33342, DAPI), and organelle trackers (LysoTracker Red, MitoTracker Red CMXRos) were provided by Beyotime Biotechnology (Shanghai, China).
Cell culture
Mouse-derived melanoma B16F10 and human melanoma A375 cell lines were provided by Wuhan Servicebio Technology Co., Ltd. (Wuhan, China). Cells were cultivated in RPMI-1640 medium enriched with 10% heat-inactivated FBS and 1% antibiotic mixture (penicillin and streptomycin). Cultures were incubated in a 5% CO₂ atmosphere at 37 °C. All in vitro experiments were conducted using cells in the logarithmic growth phase to ensure consistent biological behavior.
Animals
Female BALB/c nude mice (5 weeks old, ~ 20 g body weight) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd., and housed under sterile and controlled environmental conditions. The animals were maintained in sterile, pathogen-free conditions. All the animal studies were approved by the animal ethics committee of Hebei Medical University (IACUC-Hebmu-2023008). All methods were performed in accordance with the animal experimental guidelines and regulations approved by the animal ethics committee of Hebei Medical University. To euthanize the mice, we first administered CO2 asphyxiation, followed by cervical dislocation to confirm death.To euthanize the mice, we first administered CO2 asphyxiation, followed by cervical dislocation to confirm death. Additionally, we adhered to the ARRIVE guidelines, and all data were reported in compliance with these standards.
Preparation and characterization of ZER-ICG-NMs
The nanoparticle formulation ZER-ICG-NMs was prepared using a vacuum rotary evaporation technique, followed by hydration and filtration. Briefly, we prepared the ICG solution into a stock solution with a concentration of 10 mM, then took 100 μL of this solution and added 4 mg of ZER and 20 mg of mPEG-PLA were dissolved in acetonitrile and sonicated, then ultrasonicated to obtain a homogeneous solution. The organic solvent was then removed using a rotary evaporator at 40 °C under reduced pressure for 20 min to form a thin film. This film was hydrated with 10 mL of ultrapure water and subjected to ultrasonic agitation for an additional 5 min to ensure uniform dispersion. The resulting nanosuspension was filtered through a 0.22 μm membrane filter to yield well-dispersed ZER-ICG-loaded polymeric micelles. The control group (Blank-NMs), single-drug loaded ICG-NMs, and ZER-NMs were synthesized using an identical protocol. The hydrodynamic diameter and size distribution of the nano micelles were determined using dynamic light scattering (DLS) with a Malvern Zetasizer (Malvern Panalytical, UK). The zeta potential was assessed with the same system. Data were reported as the mean ± standard deviation from three independently prepared batches. Transmission electron microscopy (TEM, Hitachi-7800, Japan) was employed to visualize the morphology and structure of the nanoparticles.
The encapsulation efficiency (EE) of ZER and ICG were measured using ultraviolet (UV) spectrophotometry (TU-1950, PERSEE). ZER-ICG-NMs solution was centrifugation at 12,000 rpm for 20 min, collect the supernatant and absorbance was measured at 254 nm. A solution of ZER-ICG-NMs was taken and its absorbance was determined in the same way. For ICG, dialyzed micelles were diluted 20-fold in DMSO to disrupt the micellar structure and release the drug, followed by absorbance measurement at 780 nm. EE% was calculated using: EE (%) = (weight of encapsulated drug / weight of initially added drug) × 100(%).
Drug Loading Capacity (DLC, %) = (loaded drug / total weight of nanoparticles) × 100(%).
In vitro stability of nano micelles
To evaluate long-term colloidal stability, freshly prepared blank micelles and ZER-ICG-NMs were stored at 4 °C for 30 days. DLS analysis was performed at predetermined time points to monitor any changes in particle size or polydispersity.
In vitro photothermal effect of nano micelle
The thermal behavior of ZER-ICG-NMs under NIR irradiation was assessed using an infrared thermal imaging system (Mobir Air2S, Guide Sensmart, China). Water weas used as a negative control to confirm the specificity of ICG-induced photothermal effects. The sample was exposed to an 808 nm near-infrared laser (2 W/cm2). The spot diameter was 8 mm, and the distance between the fiber and the sample was 10 mm. The temperature rise was monitored at 30 s intervals.
To assess photothermal stability, ZER-ICG-NMs at varying concentrations were irradiated for six repeated cycles, each consisting of 3 min irradiation followed by 7 min cooling. The absorbance was monitored at 780 nm during this heating–cooling test, which was designed to verify consistent thermal response and resistance to photobleaching.
ROS generation test
The ability of ZER-ICG-NMs to generate singlet oxygen under NIR exposure was evaluated using a 1,3-diphenylisobenzofuran (DPBF) assay. DPBF, a chemical probe that reacts with singlet oxygen to form non-fluorescent dibenzoylbenzene (DBB), was introduced into both free-ICG and ZER-ICG-NMs aqueous solutions. Samples were irradiated at 808 nm (2 W/cm2) for 5 min. Absorbance reduction of DPBF was measured at 410 nm (excitation/emission) to assess ROS generation efficiency.
Antitumor activity of ZER-ICG-NMs in vitro
In vitro cellular uptake and subcellular localization
To explore the intracellular uptake and subsequent subcellular localization of the ZER-ICG-NMs, B16F10 melanoma cells were initially seeded into specialized confocal dishes at an appropriate density and cultured for 24 h under standard conditions (37 °C, 5% CO₂). After incubation, the culture medium was replaced with fresh medium containing ZER-ICG-NMs, and the cells were further incubated for 1, 2, and 4 h respectively. Following the treatment, unbound micelles were removed by washing the cells thoroughly multiple times with phosphate-buffered saline (PBS). The cells were subsequently fixed using 4% paraformaldehyde for 15 min and stained with the nuclear-specific dye DAPI (6-diamidino-2-phenylindole). Finally, high-resolution cellular images were acquired using a confocal laser scanning microscope (CLSM, FV1200MPE, Olympus, Japan) with appropriate excitation/emission settings (DAPI: 340/488 nm; ICG: 785/813 nm).
Additionally, to evaluate subcellular localization and assess the potential endosomal escape of the nanoparticles, a dual-staining procedure was employed. After incubation with nanoparticle suspension, the cells were stained with LysoTracker Red (C1046, Beyotime, China) and Mito Tracker Red CMXRos (C1999S, Beyotime, China) respectively at 37 °C in the dark, followed by staining with Hoechst33342 (C1027, Beyotime, China) for 10 min, and then imaged via CLSM. (Ex/Em: Hoechst 33342, 346 nm/460 nm; Tracker Red CMXRos 579 nm/599 nm; Lyso-Tracker Red 577 nm/590 nm).
Drug permeation analysis of 3D tumor spheroids
The tumor spheroids model was prepared by the hanging drop method. First, 100 μL of prepared 2% agarose was added to the well of a 96-well plate and irradiated under UV light overnight until the agarose solidified. Next, 10 μL of B16F10 cells, approximately 1 × 104 cells, were seeded onto 96-well plate covers. After 24 h, 100ul of complete medium was added to the Wells of 96-well plates. Afterward, the droplets containing spheroids were transferred into the agarose-coated wells and allowed to mature under standard culture conditions. Fully formed spheroids were then collected and treated with either free ICG or ZER-ICG-NMs for penetration studies. Confocal z-stack imaging was performed at 20 μm intervals to assess the depth and distribution of nanoparticle infiltration into the tumor spheroid cores.
In vitro cytotoxicity assay and photothermal effect
To assess the cytocompatibility of the carrier system, a CCK-8 viability assay was first conducted using Blank-NMs on B16F10 and A375 cells. Briefly, cells were seeded in 96-well plates at a density of 5 × 103 cells per well and cultured overnight. Then, the samples were incubated with different concentrations of Blank-NMs for 24 h. After treatment, 10 μL of the CCK-8 reagent was added to each well and allowed to react for 2 h. Absorbance was then recorded at 450 nm using a microplate reader (SpectraMax Plus384, Molecular Devices, China).
To further evaluate the cytotoxic effects of ZER-ICG-NMs and investigate the synergistic impact of laser-triggered photothermal therapy, B16F10 and A375 cells were treated with ICG-NMs, ZER-NMs, and ZER-ICG-NMs at various concentrations. After incubation for 2 h, the cells were divided into two groups: one group received NIR laser irradiation (808 nm, 2 W/cm2, 5 min), while the other group served as the non-irradiated control. Post-treatment, the medium was replaced with fresh culture medium, and cells were incubated for an additional 22 h before conducting the CCK-8 assay. The cell viability was quantified based on absorbance measurements at 450 nm.
Live/dead cell staining and imaging
To visually examine the effects of photothermal-chemotherapeutic treatments on cell viability, B16F10 cells were cultured in 24-well plates and treated with ICG-NMs, ZER-NMs, and ZER-ICG-NMs. After a 2 h incubation period, cells designated for photothermal treatment were irradiated with an 808 nm NIR laser (2 W/cm2) for 5 min. Subsequently, the cells were stained with Calcein-AM/PI dual dye kit (Beyotime, China), which simultaneously labels live cells (green fluorescence) and dead cells (red fluorescence). Stained cells were then observed under an inverted fluorescence microscope (Dmi8, Leica) for viability assessment.
Mitochondrial membrane potential
For mitochondrial membrane potential assay, ICG-NMs, ZER-NMs, and ZER-ICG-NMs with/without NIR treated cells were loaded with the potentiometric dye 500 nM TMRE (C2001S, Beyotime, China) at 37 °C for 20 min. Following staining, cells were imaged under a fluorescence microscope to assess changes based on TMRE fluorescence intensity.
Cell apoptosis by flow cytometric analysis
Similarly, the cell apoptosis assay was investigated by flow cytometry. “The B16-F10 cells were seeded in 6-well plates at a density of 5 × 105 cells/well and pre-cultured for 24 h. After 2 h of incubation with ICG-NMs, ZER-NMs, and ZER-ICG-NMs nanoparticles and the irradiation groups following 808 nm NIR irradiation (2 W/cm2) for 5 min. Then the cells were collected, trypsinized, and stained using the Annexin V-FITC/PI apoptosis detection kit (E-CK-A211, Elabscience, China) according to the manufacturer’s protocol. Apoptotic cells were analyzed using flow cytometry (CytoFLEX LX, Beckman Coulter, USA), and both early and late apoptotic populations were quantified.
qPCR analysis
To investigate the molecular mechanisms underlying apoptosis, the expression levels of apoptosis-related genes (Bax, Caspase-3) were evaluated via qPCR. B16F10 cells were treated with Blank-NMs, ICG-NMs, ZER-NMs, and ZER-ICG NMs for 4 h at 37 ◦C, and cells irradiated with 808 nm and 2 W/cm2 for 5 min were regarded as experimental group. Total RNA was extracted using the Foregene RNA isolation kit and quantified spectrophotometrically (A260/A280). Genomic DNA was eliminated and cDNA was synthesized according to the manufacturer’s protocol. qPCR amplification was conducted using Universal Blue SYBR Green Master Mix, and relative expression levels were calculated using the ΔΔCT method. β-actin served as the internal control gene.
Wound scratch assay and Transwell test
B16f10 cells were seeded with a density of 5 × 105 cells/ mL and dealt with different methods after being full over in six-well plates. Then, a scratch was created with tips, and after washing, the cells were cultured with serum-free medium, ZER-ICG-NMs, and ZER-ICG-NMs with NIR 5 min. 48 h after culture, the relative distance of cells migrating to the wounded area was observed.
For the Transwell migration assay, treated B16F10 cells were suspended in serum-free medium and placed in the upper chambers of 12-well Transwell inserts (8 μm, Corning Incorporated, Corning, NY), with 200 μL in each chamber. The lower wells of the Transwell chambers were supplemented with complete culture medium containing 20% fetal bovine serum (FBS). Following a 48 h incubation period under standard culture conditions, non-migrated cells remaining on the upper surface of the porous membrane were carefully removed using a cotton swab. The cells that had successfully traversed were fixed with 4% paraformaldehyde for 20 min at room temperature. These migrated cells were then stained with a 0.5% crystal violet solution. After thorough washing, stained cells were imaged and counted under a light microscope to quantitatively evaluate the extent of cellular migration.
Preparation and characterization of ZER-ICG @DMNs
We prepared nanoparticle-integrated microneedles (ZER-ICG @DMNs) by multi-step centrifugation27. First, PVP K30 and PVA were prepared into tip solution in a certain proportion, then 1/4 volume of tip solution was added to a certain concentration of nanoparticle suspension to form a tip mixture. The hyaluronic acid (HA) was preexpanded in water overnight to form the substrate solution. Applied an appropriate volume of tip mixture to a poly(dimethylsiloxane) (PDMS) mold and treated under vacuum for 15 min to form the tip of the microneedle. Excess solution and surface bubbles were carefully removed before adding the HA-based backing layer. The mold was placed in a desiccator with silica gel at room temperature for 24 h. Finally, taken out from the PDMS mold gently to get ZER-ICG @DMNs patches. The drug loading capacity was calculated by ultraviolet spectrophotometer after dissolving the whole excisional microneedle tips. Next, we characterized the morphology by scanning electron microscopy (SEM) and CLSM.
In vitro evaluation of ZER-ICG @DMNs
Mechanical testing
To assess the mechanical properties of the MN arrays, a texture analyzer (TA.XT Plus, SMS, UK) in compression mode was used. The test distance is set to 600 µm, the trigger mode is self-dynamic, the speed before and after the test is 1 mm/s, the test speed is 0.1 mm/s, the tip of the MNS is kept upward, the axial compression force is applied to it, and the force is analyzed to obtain the force–displacement curve. Data were recorded and analyzed using Bluehill 2 (version 2.35) and plotted with OriginPro software. The fracture force of ZER-ICG@DMNs was noted at the point of needle breakage. Record the failure stress of ZER-ICG @DMNs when the needle begins to break. Finally, the shape of ZER-ICG @DMNs after compression is observed under SEM. Data are reported as mean SD (n = 5).
Storage stability of ZER-ICG @DMNs
To assess storage stability, ZER-ICG@DMNs were stored at room temperature and 4 °C for 7, 14, and 28 days. After each interval, the microneedles were examined under an optical microscope. The encapsulated ZER was extracted and quantified using a UV spectrophotometer.
Skin insertion capability evaluation
To assess the insertion performance of the DMNs, patches were applied to mouse skin for 5 min. After tip dissolution, the backing layer was removed. The mouse was euthanized, and skin samples were collected for 3D layer scanning via CLSM at 40-μm intervals. The same procedure was also applied to human ex vivo skin. To further evaluate the drug’s penetration performance, ZER-ICG @DMNs patches were applied to the dorsal skin of mice for 5 min and 2 h respectively. Then the skin was excised, fixed with 4% paraformaldehyde, frozen, DAPI-stained, and sectioned (5 µm thick) for CLSM observation.
ZER-ICG @DMNs dissolution in vivo after implantation
ZER-ICG @DMNs patches were applied to the dorsal skin of female Balb/c nude mice. The ZER-ICG @DMNs were removed from the skin at the predetermined intervals of 0.5,1,3, and 5 min. The extent of dissolution was visualized using an optical microscope.
Skin healing experiment
To evaluate skin puncture and healing, ZER-ICG@DMNs were applied to the backs of Balb/c nude mice anesthetized with isoflurane for 5 min. The treated skin was monitored and photographed. After euthanasia, the treated skin was harvested, fixed in paraformaldehyde, dehydrated, embedded in paraffin, sectioned, dewaxed, and stained with hematoxylin and eosin (H&E) for histological observation of healing.
In vivo safety evaluation of blank @DMNs
Based on in vitro results, the in vivo safety of Blank@DMNs was assessed. The Blank @DMNs patch was inserted into the skin of the mice, and the healing time of the pinholes and the inflammation of the back skin of the mice after Blank @DMNs insertion were observed. The mice were treated with Blank @DMNs at fixed times every day for 14 days. During the treatment of Blank @DMNs, the general conditions of the mice were observed every day, including diet, appearance, behavioral activities, mental state, appetite, etc. The mice were weighed once a day. Then mice were anesthetized with isoflurane, and whole blood was collected from the collection vessels containing anticoagulants at 14 days, respectively. The blood was then tested. Mice were then sacrificed, and major organs (heart, liver, spleen, lungs, and kidneys) were excised. Tissues were sectioned and stained with H&E for histopathological examination.
In vivo evaluation of ZER-ICG @DMNs
Female Balb/c nude mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., and housed in sterile conditions with free access to food and water. For tumor model establishment, 100 μL of B16 melanoma cells (5.0 × 10⁶ cells/mL) were subcutaneously injected into the right dorsal area. Tumors were allowed to grow until volumes reached 60–80 mm3. Tumor volume is calculated by the following formula: Tumor volume (mm3) = 1/2 × length × width 2.
In vivo photothermal effect of the ZER-ICG @DMNs
To evaluate the photothermal effect, B16F10 tumor-bearing mice were treated with ZER-ICG@DMNs or ZER@DMNs patches for 5 min. After patch removal, the tumor site was irradiated with an 808 nm NIR laser (2 W/cm2) for 5 min after 30 min. Temperature changes were monitored using a thermal infrared imaging system and recorded at 1-min intervals.
In vivo imaging and biological distribution
The fluorescence distribution of ZER-ICG@DMNs was monitored using an in vivo imaging system (IVIS Lumina XRMS, PerkinElmer, USA) at 0, 0.5, 2, 4, 6, 8, 12, 24, 48, 72, and 96 h. At the final time point, mice were sacrificed, and major organs (heart, liver, spleen, lungs, kidneys) were harvested to observe fluorescence distribution.
In vivo antitumor effect
All animal procedures followed protocols approved by the Ethics Committee of Hebei Medical University. B16F10 tumor-bearing Bulb/c nude mice were randomly divided into seven groups (n = 5): (1) untreated;(2) the ICG @DMNs patches; (3) ICG @DMNs patches + NIR; (4) free-ZER@DMNs patches; (5) the ZER @DMNs patches; (6) ZER-ICG @DMNs patches; (7) ZER-ICG @DMNs patches + NIR. The free-ZER@DMNs patches are made by dissolving the same amount of ZER directly with acetonitrile and then spin steaming and hydration. The remaining steps of preparating microneedles are exactly the same as ZER-ICG@DNMs. Patches were applied to tumor sites for 5 min. For laser-treated groups, tumors were irradiated with an 808 nm laser (2 W/cm2) for 5 min after 2 h of patch application. Treatment began 5 days post-inoculation (designated Day 0). Tumor volumes and body weights were recorded daily. On Day 9, mice were euthanized, and tumors and major organs were collected. Tumor weights were measured, and tissues were stained with H&E for safety evaluation. Tumor sections were further analyzed via immunofluorescence (Melan-A, F4/80, Ki-67) and immunohistochemical staining (Sox-10) to assess therapeutic efficacy. The tumor inhibition rate (IRT) was calculated as:
where Wcon is the mean tumor weight of the control group, Wex is the mean tumor weight of the experimental group.
Statistical analysis
All results are expressed as mean ± standard deviation (SD). One-way ANOVA was used for multiple-group comparisons via SPSS 22.0 software. Differences were considered statistically significant at P < 0.05 (*P < 0.05; **P < 0.01; ***P < 0.001). GraphPad Prism (version 5.01) was used for statistical graph plotting.
Results and discussion
Preparation and characterization of ZER/ICG-NMs
The particle size of Blank-NMs was 19.67 nm, and the zeta potential was 1.26 mV. The average hydrodynamic size of the ZER-ICG-NMs was approximately 243.73 nm, and the zeta potential was -12.3 mV (Fig. 2A,B). Transmission electron microscopy (TEM) was employed to further examine the morphological characteristics of the ZER-ICG-NMs. As depicted in Fig. 2C, the nano micelles displayed a roughly spherical morphology with diameters averaging around 250 nm, which aligns well with the hydrodynamic diameter obtained via DLS measurements. The consistently negative surface charge is indicative of favorable colloidal stability, which is essential for ensuring the dispersion integrity of micelles during subsequent biological experiments (9). To confirm the successful encapsulation of both ZER and ICG within the mPEG-PLA polymeric matrix, ultraviolet–visible (UV–vis) spectrophotometric analysis was conducted. The EE of ZER was determined to be 76.98%, while that of ICG was 22.26%, calculated as the ratio of the mass of encapsulated drug to the initial loading mass for each compound. The DLC of ZER was 13.34%.
Nano micellar solution characterization and photodynamic and photothermal properties. (A) Dynamic light scattering (DLS) size distribution of ZER-ICG-NMs. (B) Zeta potentials of ZER-ICG-NMs. (C) TEM images of ZER-ICG-NMs. (D) Temperature elevation of water, the same ICG concentration of free-ICG, ICG-NMs, ZER-NMs, and ZER-ICG-NMs (mPEG-PLA concentration: 0.5 to 2 mg/mL) aqueous solutions as a function of irradiation time. (E,F) Absorption changes in 1 × 10−4 mol/L DPBF solutions containing ZER-ICG-NMs or free ICG, with the irradiation (2 W/cm2) at 808 nm.
Considering the prospective application of these nanomicelles in microneedle delivery systems, it was crucial to evaluate their kinetic stability under prolonged storage conditions. This stability was quantitatively assessed using DLS. After 30 days of storage, the average hydrodynamic diameter (Dh) of Blank-NMs increased modestly from 19.67 to 42.47 nm, while that of ZER-ICG-NMs exhibited a slight increase from 243.73 to 262.17 nm. Similarly, the polydispersity index (PDI) of Blank-NMs shifted from 0.187 to 0.195, and for ZER-ICG-NMs, the PDI changed from 0.229 to 0.298. These relatively small changes suggest that both nanomicelle formulations exhibit satisfactory kinetic stability over time.
In vitro photothermal effect and measurement of singlet oxygen of nano micelle
The photothermal performance of ZER-ICG-NMs was next evaluated under near-infrared (NIR) irradiation. First, we compare the photothermal conversion ability of different materials under NIR laser (808 nm) irradiation. The 808 nm laser functions in the near-infrared (NIR) spectral region (700–1100 nm), which is characterized by minimal scattering and absorption in biological tissues, earning it the designation of the “optical window”28. These optical characteristics promote greater tissue penetration than shorter wavelengths, for instance, 600 nm red light. Although lasers at 1064 nm exhibit superior penetration depth, the 808 nm wavelength retains suitability for targeting superficial tissues, most notably its widespread clinical availability and cost-effectiveness29. As shown in Fig. 2D, upon exposure to 808 nm NIR laser irradiation, the photothermal effect of ZER-ICG-NMs aqueous suspension is concentration-dependent. Specifically, the temperature of ZER-ICG-NMs, ICG-NMs, and free-ICG maximally increased to 55.2, 55.5, and 50.8 °C, while the ZER-NMs and water, only increased by about 6.3 °C. Under laser irradiation, the formulations of ZER-ICG-NMs and ICG-NMs have a higher temperature response than free ICG. The possible reason is that the ICG encapsulated in ZER-ICG-NMs or ICG-NMs has a higher concentration. The excitation thermal radiation is also encapsulated in the outer shell of the nanoparticles, which leads to higher energy efficiency and lower heat loss in ZER-ICG-NMs or ICG-NMs after laser irradiation. The photostability of the nano micelles was also assessed through repeated heating and cooling cycles. As demonstrated in Figure S1a, consistent temperature elevation was observed across six on/off irradiation cycles, indicating desirable thermal robustness and structural integrity during repeated NIR exposures30. Ideal photothermal therapy nanometer carrier should have an "OFF/ON" switch, used for PTT and near-infrared fluorescence imaging, which the light toxicity of photosensitizer and fluorescence signal can be restrained effectively in the circulation of the blood, and then resumed after it reaches the target site. The different concentrations of ZER-ICG-NMs all showed good stability, the absorbance decreased after laser irradiation, and then the spectrums were unchanged (Figure S1b). This suggests some initial photobleaching of ICG upon the first laser cycle, followed by stability in subsequent cycles. It may imply ICG within NMs is more resistant to further bleaching after an initial exposure compared to free ICG.
The absorbance of DPBF decreases upon reaction with singlet oxygen. As shown in (Fig. 2E,F), both free ICG and ZER-ICG-NMs induced a time-dependent reduction in DPBF absorbance upon NIR irradiation, with noticeable decline after 660 and 900 s, respectively. These observations provide strong evidence that the ZER-ICG-NMs are effective at generating ROS under NIR illumination, further confirming their potential for PTT applications.
In vitro cellular uptake and subcellular localization
To explore the cellular internalization dynamics of ZER-ICG-NMs, B16F10 melanoma cells were incubated with the nano micelles for 1, 2, and 4 h. As indicated in (Fig. 3A), the uptake of ZER-ICG-NMs was time-dependent. 4 h incubation showed the highest fluorescence intensity compared to that at 1 and 2 h (Fig. 3B). Confocal laser scanning microscopy (CLSM) was utilized to assess the subcellular localization of ZER-ICG-NMs. In these experiments, ICG was visualized in green, while mitochondria and lysosomes were labeled in red. The colocalization of ZER-ICG-NMs with these organelles resulted in yellow fluorescence, enabling precise spatial tracking. It was found in Fig. 3C,D, at 1 h that yellow fluorescence appeared, the Pearson’s correlation coefficient (PCC) of ICG and Mito Tracker Red CMXRos was 0.8510 and the Mander’ colocalization coefficient (MCC) was as high as 0.9198, suggesting ZER-ICG-NMs remained in mitochondria (Figure S2a). Whereas, separated green fluorescence was exhibited at 2 h, revealing that ZER-ICG-NMs could escape from mitochondria. After being internalized by B16F10 cells, ZER-ICG-NMs began to enter endosomal/lysosomal compartments at 5 h, with a PCC was 0.8489 and a MCC was 0.9336(Figure S2b), and escaped from lysosomes after 8 h, which indicated that the carried drugs could be effectively released into the cytoplasm of tumor cells to exert their therapeutic functions. Due to the fact that the absorption process takes a considerable amount of time, further optimization of the cell absorption capability is required31.
In vitro cellular uptake, co-localization, and penetration ability. (A) Cellular uptake of ZER-ICG-NMs after 1,2,4 h incubation (scale bar: 20 μm), and fluorescence intensity of ICG at 1, 2, and 4 h, respectively. (B) Cellular uptake was time-dependent, reaching the peak level of ICG internalization at 4 h. (C,D) Lysosomal (Lyso-tracker, red fluorescence) and mitochondrial (Mito-tracker, red fluorescence) staining of B16F10 tumor cells treated with ZER-ICG-NMs (green fluorescence). Merged red and green signals produced yellow fluorescence, indicating ZER-ICG-NMs remained within the lysosomes/mitochondria, whereas distinct red and green fluorescence implied that ZER-ICG-NMs had escaped from these organelles. (scale bar: 20 μm). (E) Evaluation of the penetration capacity of ZER-ICG-NMs and free ICG in a 3D tumor spheroid model constructed from B16F10 cells. (scale bar: 50 μm). Both ZER-ICG-NMs and free ICG penetrated the spheroids after 3 h, though ZER-ICG-NMs exhibited a significantly stronger fluorescence intensity compared to free ICG.
The ZER-ICG-NMs achieve better penetration into the 3D tumor spheroids model
The ability of the nano micelles to penetrate three-dimensional tumor architectures was evaluated using a spheroid model formed from B16F10 cells. Z-stack CLSM imaging (Fig. 3E) demonstrated that the ZER-ICG-NMs penetrated more efficiently and deeply into the spheroids compared to the free ICG control. As the imaging depth increased to 60 and 80 µm, the fluorescence intensity of free ICG diminished substantially. In contrast, ZER-ICG-NMs fluorescence was uniformly distributed in the tumor spheroids model and could remarkably improve penetration capability.
Nano micelles combined with photothermal effect induced cell apoptosis
Prior to assessing the antitumor effects of the nano micelles against cancer cells, the cytocompatibility of Blank-NMs was evaluated. As shown in Figure S3, after 24 h treatment with various concentrations of Blank-NMs, B16F10 and A375 cells-maintained viability levels above 81.41%, confirming that the blank carrier itself is minimally cytotoxic.
Subsequent investigations examined the cytotoxic efficacy of ZER-NMs, ICG-NMs, ICG-NMs + NIR, ZER-ICG-NMs, and ZER-ICG-NMs + NIR across a range of concentrations (Fig. 4B). Both ZER and ICG demonstrated dose-dependent cytotoxic effects, with greater reductions in cell viability observed at higher concentrations. Among the tested groups, ZER-ICG-NMs subjected to NIR irradiation exhibited the most pronounced cytotoxicity, underscoring the synergistic effect of combining ZER chemotherapy with photothermal therapy. Notably, ZER exhibited appreciable cytotoxic effects only at concentrations above 2.5 µg/mL, whereas below this threshold, no significant impact on cell viability was observed. In parallel, ICG-NMs alone displayed moderate antitumor effects, but the inclusion of 808 nm NIR irradiation significantly enhanced their cytotoxic efficacy, highlighting the importance of light activation in photothermal response.
ZER-ICG-NMs mediated melanoma cells apoptosis, up-regulates Bax and Capsease-3 expression in melanoma cells. (A) Cell viability of B16F10 and A375 cells treated with various NMs with or without NIR exposure. Cells were exposed to different NMs, and cytotoxicity was evaluated using the CCK-8 assay (n = 5). Data are expressed as mean ± standard deviation (SD) from three independent experiments conducted in triplicate; *p < 0.05, **p < 0.01, ***p < 0.001; (B) The living/dead fluorescence images of tumor cells after different treatments (n = 3). (C,D) Images and corresponding quantification results of mitochondrial membrane potential (n = 3). (E,F) Flow cytometry analysis and statistical assessment of apoptosis in B16F10 cells after 2 h of treatment with the indicated groups. The ZER-ICG-NMs combined with NIR treatment triggered the most significant apoptotic response (n = 3). (G,H) Bax and Caspase-3 mRNA expression levels in B16F10 cells were detected by quantitative qPCR. ZER-ICG-NMs with NIR could successfully up-regulate the expression of Bax and Caspase-3 at mRNA levels (n = 3). (I) The wound scratch assay and (K) quantitative analysis of cells with different treatments for 48 h (n = 3). (J) The cell migration ability and (L) quantitative analysis of tumor cells with different treatments (n = 3).
However, when the concentration of ICG was lower than 6.25 μmol/mL, none of the irradiation groups (ICG-NMs + NIR and ZER-ICG-NMs + NIR demonstrated noteworthy cytotoxicity towards melanoma cells. These findings suggested that the high concentration of ICG would induce a potent photothermal effect on tumor cells. Above that concentration, the ZER-ICG-NMs + NIR group exhibited a significantly greater anti-tumor effect than both the ZER-ICG-NMs group and ICG-NMs + NIR group. These findings support the conclusion that the combination of chemotherapy and photothermal therapy within a single nanoplatform provides a more effective therapeutic strategy than monotherapy in melanoma treatment.
To assess the therapeutic efficacy of various nanoparticle formulations, B16F10 melanoma cells were treated with different nano micelles and subsequently irradiated with NIR light under the same conditions as the CCK-8 cytotoxicity assay. The cells were then stained using a Live–Dead viability kit and observed under an inverted fluorescence microscope. The results showed several cells died(red) in the ICG-NMs group, and the number of cell deaths increased in the ICG-NMs + NIR group (Fig. 4B). The proportion of dead cells was similar between the ZER-NMs and ZER-ICG-NMs groups. However, the ZER-ICG-NMs + NIR group exhibited the most pronounced cell death, indicating that the combination of ZER and ICG, when activated by NIR irradiation, exerts a synergistic cytotoxic effect likely mediated by enhanced reactive oxygen species (ROS) generation. These observations are in agreement with the trends reported in the CCK-8 assay.
Under normal conditions, there are a large number of negative charges in the mitochondria, and TMRE as a cation probe accumulates in the mitochondria and can emit bright orange fluorescence. When apoptosis occurred, mitochondrial membrane potential was lost, TMRE was released into the cytoplasm, and the intensity of orange fluorescence in mitochondria decreased significantly. To confirm the effects of different nanoparticles with or without laser irradiation, TMRE staining was used. The results showed that the orange-red fluorescence intensity in the mitochondria was significantly decreased in the ICG-NMs + NIR group compared with the control group and ICG-NMs group (Fig. 4C,D). The fluorescence intensity of the ZER-ICG-NMs group was the weakest, indicating that the most apoptotic melanocytes occurred, indicating that ZER combined with ICG played the strongest role under 808 nm laser irradiation. Previous studies32 have reported that ZER selectively induces apoptosis in cancer cells by impairing mitochondrial function while protecting non-cancerous cells from oxidative stress and damage. The observed decrease in mitochondrial membrane potential is likely attributed to ZER’s ability to elevate intracellular ROS levels and suppress the antioxidant defense mechanisms in malignant cells33.
Apoptosis induction was further quantified using flow cytometric analysis of Annexin V-FITC/PI-stained B16F10 cells (Fig. 4E,F). The results demonstrated that the ZER-ICG-NMs + NIR group induced the highest apoptotic rate (~ 50.54%), which was significantly higher than that of ZER-ICG-NMs (33.35%) and ZER-NMs (28.86%) without NIR exposure. In addition, the ZER-ICG-NMs + NIR group significantly promoted apoptotic cell population, which was higher than that of ZER-ICG-NMs (33.35%) and ZER-NMs (28.86%). The ICG-NMs + NIR group with higher apoptotic cell rate than ICG-NMs group. It could be assumed that the use of ICG and 808 nm laser enhanced higher cell apoptosis rate.
ZER-ICG-NMs combined with photothermal effect-induced cell apoptosis and efficiently increase the expression of Caspase-3 at mRNA and protein levels in vitro
To elucidate the molecular mechanisms underlying the observed apoptotic responses, the expression of key apoptotic markers Bax and caspase-3 were examined at mRNA levels. In the apoptotic signaling pathway of melanoma cells, upregulation of the pro‑apoptotic molecule Bax is often accompanied by activation or increased expression of the downstream effector enzyme caspase‑333,34. Based on prior evidence that ZER induces caspase-3-mediated apoptosis in colorectal cancer cells33, we selected these markers to probe apoptosis in B16F10 cells following various nanoparticle treatments, with or without NIR exposure (Fig. 4G,H). Our data revealed a significant upregulation of Bax and caspase-3 expression (P < 0.05) in cells treated with ZER-ICG-NMs under NIR irradiation. These observations further support the coordinated trend observed in our data, the concurrent increase in Bax expression and caspase‑3 levels during the process of apoptosis.
In vitro cell migration test
As shown in Fig. 4I,K, the ZER-ICG-NMs + NIR group exhibited a substantial reduction in cell migration, with slower wound closure and fewer migrating cells compared to the control. The inhibitory effect on migration was also evident in the ZER-ICG-NMs group without NIR, although to a lesser extent. There was also a notable difference in the cell migration between the control and ZER-ICG-NMs groups (Fig. 4J,L). These results collectively demonstrate that ZER-ICG-NMs significantly suppress melanoma cell migration in vitro, and that this inhibitory effect is further amplified by photothermal activation via NIR irradiation. The combined chemo-photothermal strategy thus presents a promising approach not only for inducing apoptosis but also for restricting metastatic potential in malignant melanoma.
Fabrication and characterization of ZER-ICG@DMNs patches
Despite the promising therapeutic potential of nanoparticles, clinical studies have highlighted their limited delivery efficiency in human tumors—a factor critical to the overall efficacy of anticancer treatments. To address this limitation and enhance local delivery and absorption of nano micelles, we developed a transdermal drug delivery system using dissolvable microneedles (DMNs) for direct administration into tumor tissues. Hyaluronic acid (HA), known for its superior biocompatibility and biodegradability, was selected as the primary matrix material to construct the DMNs. This biopolymer not only facilitates biocompatible interaction with skin but also contributes to the mechanical integrity required for dermal penetration. ZER-ICG-NMs were incorporated into the microneedle tips after concentration via ultrafiltration, resulting in the formation of ZER-ICG@DMNs patches optimized for epidermal delivery.
Scanning electron microscopy (SEM) revealed a highly ordered array of microneedles with a consistent geometric configuration. Each pyramid-shaped microneedle measured approximately 507 µm in height, with a base diameter of 259 µm and an inter-needle spacing of 459 µm. The DMN array was configured in a 15 × 15 grid, exhibiting uniform architecture suitable for skin application (Fig. 5A). Furthermore, energy-dispersive X-ray spectroscopy (EDS) mapping confirmed the even distribution of constituent elements such as C, O, Na, Cl, S, and Ca across the DMN matrix (Fig. 5B,C), supporting the successful fabrication of homogeneous microneedle systems.
Characteristics of ZER-ICG @DMNs patches. (A) SEM images of ZER-ICG@DMNs array. (B,C) The EDS elements mapping and content of ZER-ICG@DMNs. These elements were labeled with corresponding colors. (D) Force–displacement profiles of the MNs (n = 5). (E) SEM-imaged of ZER-ICG@DMNs, ZER@DMNs, ICG@DMNs, and Blank @DMNs tip morphologies after compression; (F) Drug content of ZER-ICG@DMNs patches at above terms (n = 3). (G) Morphological structure of ZER-ICG@DMNs patches in various states (RT, 4℃ for 7, 14, 28 days).
In vitro evaluation of ZER-ICG @DMNs
Mechanical testing and storage stability of MNs
There was little difference between the mechanical strength of the ZER-ICG @DMNs, ZER@DMNs, ICG@DMNs, and Blank @DMNs (Fig. 5D). When the displacement was 600 μm, none of the four types of DMNs were broken. Notably, all four types of DMNs withstood a displacement of up to 600 µm without fracturing, indicating that their mechanical strength exceeded the threshold required for successful transdermal insertion35. As shown in Fig. 5E, the tip of the DMNs was bent after compression.
The loaded drug doses of the ZER-ICG@DMNs were 0.805 ± 0.017 μg/mg. To determine the storage stability of ZER embedded in the DMNs, the patches were stored under different temperature conditions and monitored over a 28-day period. Morphological examination indicated that the microneedles retained their structural integrity and pyramidal shape throughout the storage period (Fig. 5G). The drug content remained consistent at 0.798 ± 0.007 μg/mg and 0.903 ± 0.014 μg/mg, respectively, under both conditions (Fig. 5F), which preliminarily indicated that the DMNs had good stability.
Skin insertion ability and drug delivery of ZER-ICG @DMNs
Efficient drug delivery using DMNs requires successful penetration into the skin to bypass the stratum corneum. The penetration depth and microchannel formation were analyzed using histological imaging (Fig. 6A). The diameter of the microchannels decreased progressively with depth, and the loss of green fluorescence at approximately 280 ± 40 µm indicated the maximum penetration depth of the microneedles. In addition, there was no evident difference in puncture depth between mouse skin and human skin (Fig. 6B). The rapid drug delivery of ZER-ICG @DMNs was further assessed in vivo. The penetration ability of ZER-ICG @DMNs was confirmed by the CLAM. After administration for 5 min, the green fluorescence of ICG and microchannels created by MNs were observed in the mice skin. Next, the frozen section of ZER-ICG @DMNs administered skin indicated a rapid drug delivery within 2 h (Fig. 6C). It was suggested that the ZER-ICG @DMNs were capable of penetrating the stratum corneum, creating microchannels, and delivering incorporated compounds efficiently to the skin of mice.
CLSM-imaged microchannels formed by ZER-ICG @DMNs in nude mice skin (A) and people skin (B); (C) CLSM images of nude mice skin sections after application of ZER-ICG @DMNs patch for different durations; (D) Dissolution of DMNs after puncture of nude mice skin. Skin recovery images (E) and H&E staining of inserted skin sections (F) before and after DMNs treatment. (G) H&E staining of the main organs 14 days after Blank@DMNs treatment.
ZER-ICG @DMNs dissolution in vivo after implantation
Dissolving microneedles are composed of biodegradable polymers that disintegrate upon insertion, facilitating controlled drug release. To monitor the dissolution kinetics of ZER-ICG@DMNs in vivo, we observed the residual height of the microneedles on mouse skin at multiple time intervals using optical microscopy. As shown in Fig. 6D the residual height of DMNs after the application of DMN arrays to the nude mice skin for 0, 0.5, 1, 3, and 5 min. The experiments showed that the ZER-ICG @DMNs completely dissolved within 5 min, which helped the loaded drugs to be released and accumulate in the skin.
Skin healing experiment
The biocompatibility and safety of microneedle administration were further evaluated by observing the healing process of the skin after patch removal. Visual inspection immediately after application revealed small puncture marks, which gradually faded and became nearly undetectable after 2 h (Fig. 6E,F). Hematoxylin and eosin (HE) staining of skin tissue sections corroborated this observation, revealing that the micropores induced by the microneedles diminished over time and were nearly closed within 2 h post-application. No signs of inflammation, redness, swelling, or exudate were observed, indicating that the microneedle-based administration system was well-tolerated and safe for transdermal drug delivery.
In vivo biosafety evaluation of blank @DMNs
To assess the in vivo biosafety profile of Blank @DMNs patches, a 14-day observation period was conducted, during which no mortality or visible signs of physiological abnormalities were detected among the test animals. Histopathological examination of major organs, including the heart, liver, spleen, lungs, and kidneys, was performed using hematoxylin and eosin (H&E) staining. The results revealed no noticeable tissue injury, inflammatory infiltration, or alterations in organ morphology (Fig. 6G), thus confirming that the Blank @DMNs patches exhibited excellent biocompatibility and systemic safety in vivo. Furthermore, to evaluate potential systemic toxicity related to hepatic and renal functions, standard blood biochemical parameters were analyzed post-treatment. These included serum levels of ALT (alanine aminotransferase), AST (aspartate aminotransferase), BUN (blood urea nitrogen), and CR (creatinine). In addition, a routine complete blood count (CBC) was performed. The blood test, liver, and kidney of mice did not show any abnormality (Fig. 7A,B), further suggesting satisfactory biosafety of Blank @DMNs patches in vivo.
In vivo biosafety evaluation of blank @DMNs. (A) White blood cells, red blood cells, platelets, neutrophils and other routine blood tests were generally normal (n = 5); (B) AST, ALT, BIL and other liver and kidney function tests were normal. In vivo evaluation of ZER-ICG @DMNs (n = 5). (C) NIR-induced photothermal response in B16F10 tumor-bearing mice over 5 min (808 nm laser, 2 W/cm2). (D) Temperature variation curves recorded at the tumor sites. (E) Fluorescence imaging of mice at various time intervals following treatment with ZER-ICG @DMNs (n = 5). (F) Ex vivo fluorescence distribution in major organs at 96 h post ZER-ICG @DMNs administration.
In vivo evaluation of ZER-ICG @DMNs
In vivo infrared thermal imaging
To investigate the photothermal response of the ZER-ICG-loaded microneedles in a melanoma-bearing mouse model, infrared thermal imaging was employed following near-infrared (NIR) laser irradiation. Mice receiving the ZER-ICG @DMNs patch displayed a rapid and significant rise in temperature at the application site, with temperatures escalating from approximately 30 °C to beyond 55.9 °C upon 5 min of NIR exposure (2 W/cm2). In contrast, the temperature increment in animals treated with ZER @DMNs lacking ICG was negligible (Fig. 7C,D). This pronounced temperature elevation underscores the superior photothermal conversion efficiency conferred by ICG encapsulation within the DMNs. The results validate the potential of ZER-ICG @DMNs as a powerful platform for in vivo photothermal therapy (PTT) in melanoma treatment.
In vivo fluorescence imaging and biological distribution.
To verify the specific active targeting efficiency and the duration of ZER-ICG @DMNs, the fluorescence signals of mice were detected at different time points after administration of ZER-ICG @DMNs. Notably, within 5 min of patch removal, strong fluorescence signals were localized predominantly at the tumor site, signifying rapid drug release and specific tissue accumulation (Fig. 7E). Moreover, the tumor fluorescence could still be observed even after 96 h post-DMNs treatment, indicating a prolonged circulation time. The fluorescence intensity decreased gradually with the extension of time. The mice were sacrificed at 96 h to isolate and visualize the major organs under the same conditions (Fig. 7F). The absence of significant fluorescence signals in these organs confirmed minimal systemic distribution and off-target accumulation, thereby reinforcing the suitability of local DMN-mediated delivery as a precise and controlled strategy for localized melanoma therapy.
Anti-melanoma effect of ZER-ICG @DMNs patches in vivo
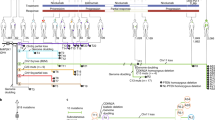
Based on the efficient B16F10 tumor-killing property of ZER-ICG NMs in vitro, we further investigate the anti-melanoma effect of ZER-ICG @DMNs patches in vivo. Balb/c mice bearing B16F10 tumors were randomly divided into 7 groups as mentioned in Section “In vivo antitumor effect”. Each group was treated via topical application of microneedle patches directly onto the tumor site with gentle pressure maintained for 5 min to ensure transdermal penetration. In NIR-treated groups, an 808 nm laser was applied at a power density of 2 W/cm2 for 5 min, commencing 2 h post-administration to allow optimal drug absorption (Fig. 8A).
Therapeutic performance of ZER-ICG @DMNs combined with NIR irradiation in B16F10 tumor-bearing mice. (A) Schematic representation of the in vivo treatment procedure. (B) Representative images of excised tumors from each treatment group. (C) Final body weights of mice at the conclusion of the experiment (n = 5). (D) Tumor growth curves and (E) final tumor mass across different groups (n = 5). (F) Immunofluorescence staining for Melan-A (green), F4/80 (red), and Ki-67 (pink) in tumor tissues (n = 5). (G) SOX-10 immunohistochemical staining of tumor sections (n = 5). All images include a scale bar of 50 μm. (H) H&E stained images of major organ slices were collected from mice of each group at day 9 post-treatment. No appreciable sign of organ damage appeared in all major organs of mice (scale bar 100um) (n = 5).
Upon termination of the study, tumors and vital organs were excised, weighed, and documented (Fig. 8B). At the end of the experiment, there was basically no difference in the final body weight among the various mouse strains (Fig. 8C). Analysis of tumor volumes revealed that while ICG @DMNs alone did not yield significant tumor suppression compared to the untreated control, partial tumor inhibition was observed in mice treated with free-ZER @DMNs, ZER @DMNs, and ZER-ICG @DMNs. However, the free-ZER group demonstrated inferior efficacy, possibly due to reduced stability and limited tumor-specific retention of the free drug, which may diffuse nonspecifically into adjacent tissues. More pronounced anti-tumor effects were evident in mice receiving ICG @DMNs + NIR and ZER @DMNs treatments, which demonstrated tumor inhibition rates (IRT) of 26.75 and 60.35%, respectively, suggesting that both photothermal and chemotherapeutic modalities contribute to tumor suppression. Notably, the combination therapy group treated with ZER-ICG @DMNs + NIR exhibited the most substantial therapeutic benefit, achieving a remarkable IRT of 99.35%, with complete tumor ablation observed in three mice. These findings are consistent with prior studies highlighting the synergistic effects of combinational photothermal-chemotherapy using nanoplatforms36. Tumor mass trends corroborated volume reduction findings (Fig. 8D, E).
The tumor tissue with different treatments was collected and stained. The melan-A and F4/80 were downregulated in the ZER @DMNs group, ZER-ICG @DMNs group, ZER-ICG @DMNs + NIR group and free-ZER @DMNs group (Fig. 8F), which corresponds to the tumor volume and weight results. We know that tumor-associated macrophages (TAMs), especially M2 macrophages, promote tumor progression37. F4/80 as macrophage marker is shown to be downregulated in effective treatment groups. This implies reduced tumor-associated macrophages, and maybe considered beneficial in this context, which redacts pro-tumor M2 TAMs furthermore. Apoptotic cell populations were most prominent in the ZER-ICG @DMNs + NIR group, while proliferation, assessed through Ki67 staining, was significantly reduced in this cohort. Ki67, a nuclear marker associated with cellular proliferation and known to be elevated in malignant melanomas, was found at its lowest expression in the ZER-ICG @DMNs + NIR group, suggesting effective tumor growth suppression. This finding is clinically relevant, as high Ki67 indices are often associated with aggressive melanoma and poor prognosis. The proliferation index of most melanomas increases, usually exceeding 5–10%38. The positive signal of Ki67 staining in the ZER-ICG @DMNs + NIR group was the least in the seven groups (Fig. 8F), which demonstrated the superior inhibiting effect on tumor expansion. Collectively, these results demonstrated that the drug-photothermal therapy posed by the ZER-ICG @DMNs patches had satisfactory efficacy in inhibiting tumor growth without obvious toxicity. In humans, SOX-10 expression has been demonstrated in melanoma. In addition, SOX-10 had the highest diagnostic sensitivity (96.7%) in melanoma39, further confirmed therapeutic efficacy; tumors in the ZER-ICG @DMNs + NIR group exhibited the weakest SOX-10 positivity (Fig. 8G).
In addition to its anti-tumor efficacy, we also evaluated the safety of DMN administration. The H&E staining images of major organ tissues (Fig. 8H) revealed no signs of inflammation, necrosis, or tissue damage, verifying that microneedle-mediated drug delivery caused no off-target toxicity. Taken together, these results strongly suggest that the ZER-ICG @DMNs patches offer a highly effective and safe approach for the synergistic chemo-photothermal treatment of melanoma, with potential translational applications in treating other superficial malignancies.
Conclusion
In summary, this study successfully developed a near-infrared (NIR)-responsive transdermal drug delivery platform by integrating photothermally active and chemotherapeutic nanoparticles into a dissolvable microneedle system. The designed nanocarriers were synthesized by incorporating Indocyanine Green (ICG), a clinically approved photothermal agent, into a ZER-based framework, which was further functionalized with hyaluronic acid to enhance biocompatibility and tumor-targeting capability. The resulting ZER-ICG nanomicelles (ZER-ICG-NMs) exhibited a homogeneous size distribution, improved colloidal stability, and robust responsiveness to NIR irradiation both in vitro and in vivo. Functionally, these hybrid nanoparticles demonstrated enhanced antitumor activity by promoting melanoma cell apoptosis and inhibiting metastatic migration, attributed to the synergistic interplay of chemotherapeutic action from Zerumbone (ZER) and the localized heat generation by ICG under laser exposure. Notably, encapsulating ICG within the nanocarrier matrix significantly improved its photothermal conversion efficiency and stability compared to free ICG, which is typically prone to photobleaching and degradation. Moreover, ZER-ICG-NMs showed superior skin permeability and cellular uptake, leading to improved therapeutic bioavailability over the administration of free drug formulations. When administered via dissolving microneedle (DMN) patches, the system enabled efficient intradermal delivery of nanoparticles directly to the tumor microenvironment in a murine B16F10 melanoma model. Upon insertion into the skin, the DMNs rapidly dissolved, ensuring localized release of ZER-ICG-NMs at the tumor site. Under NIR laser irradiation, the treated tumors exhibited significant temperature elevation, resulting in effective tumor ablation with minimal damage to surrounding tissues. This therapeutic approach achieved potent in vivo anti-melanoma efficacy without inducing systemic toxicity, as confirmed by histological analysis and blood biochemical assessments. Collectively, these findings validate the potential of ZER-ICG @DMNs as a minimally invasive and highly effective therapeutic strategy for the treatment of melanoma and potentially other superficial tumors. The combination of photothermal and chemotherapeutic modalities, delivered through a biocompatible and dissolvable microneedle system, offers a promising avenue for future clinical translation. Nonetheless, further investigations are warranted to fully elucidate the underlying mechanisms of action, optimize drug release kinetics, and comprehensively evaluate the pharmacokinetics and long-term safety of ZER in vivo. These future studies will be essential to support the clinical application of this dual-modality nanoplatform for improved cancer therapy and recurrence prevention.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
An, L. et al. A sulfur dioxide polymer prodrug showing combined effect with doxorubicin in combating subcutaneous and metastatic melanoma. Bioact. Mater. 6(5), 1365–1374. https://doi.org/10.1016/j.bioactmat.2020.10.027 (2021).
Lai, M., La Rocca, V., Amato, R., Freer, G. & Pistello, M. Sphingolipid/ceramide pathways and autophagy in the onset and progression of melanoma: Novel therapeutic targets and opportunities. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20143436 (2019).
Saginala, K., Barsouk, A., Aluru, J. S., Rawla, P. & Barsouk, A. Epidemiology of melanoma. Med. Sci. (Basel). https://doi.org/10.3390/medsci9040063 (2021).
Wei, M. et al. Oxygen-generating transdermal nanoplatform codelivering BRD4 proteolysis-targeting chimera/verteporfin/CaO2 synergistically remodels immunosuppressive melanoma microenvironment to potentiate combination immunotherapy. ACS Nano 19(28), 25830–25850. https://doi.org/10.1021/acsnano.5c04580 (2025).
Villani, A. et al. The treatment of advanced melanoma: Therapeutic update. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23126388 (2022).
Poklepovic, A. S. & Luke, J. J. Considering adjuvant therapy for stage II melanoma. Cancer 126(6), 1166–1174. https://doi.org/10.1002/cncr.32585 (2020).
Tu, L. et al. Ultrasound-controlled drug release and drug activation for cancer therapy. Exploration (Beijing) 1(3), 20210023. https://doi.org/10.1002/exp.20210023 (2021).
Wang, Y. et al. Engineering ferrite nanoparticles with enhanced magnetic response for advanced biomedical applications. Mater. Today Adv. 8, 100119. https://doi.org/10.1016/j.mtadv.2020.100119 (2020).
Zhao, Y. et al. Intelligent and spatiotemporal drug release based on multifunctional nanoparticle-integrated dissolving microneedle system for synergetic chemo-photothermal therapy to eradicate melanoma. Acta Biomater. 135, 164–178. https://doi.org/10.1016/j.actbio.2021.09.009 (2021).
Sousa-Junior, A. A. et al. Immunogenic cell death photothermally mediated by erythrocyte membrane-coated magnetofluorescent nanocarriers improves survival in sarcoma model. Pharmaceutics https://doi.org/10.3390/pharmaceutics15030943 (2023).
Huang, Y. et al. Engineered macrophages as near-infrared light activated drug vectors for chemo-photodynamic therapy of primary and bone metastatic breast cancer. Nat. Commun. https://doi.org/10.1038/s41467-021-24564-0 (2021).
Yin, Y. et al. Microneedle patch-involved local therapy synergized with immune checkpoint inhibitor for pre- and post-operative cancer treatment. J. Control Release 379, 678–695. https://doi.org/10.1016/j.jconrel.2025.01.051 (2025).
Wei, S., Quan, G., Lu, C., Pan, X. & Wu, C. Dissolving microneedles integrated with pH-responsive micelles containing AIEgen with ultra-photostability for enhancing melanoma photothermal therapy. Biomater. Sci. 8(20), 5739–5750. https://doi.org/10.1039/d0bm00914h (2020).
Shirata, C. et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci. Rep. 7(1), 13958. https://doi.org/10.1038/s41598-017-14401-0 (2017).
Dong, X. et al. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials 209, 111–125. https://doi.org/10.1016/j.biomaterials.2019.04.024 (2019).
Hari Prasad, D. et al. Bioactive Compounds from Zingiber montanum and their pharmacological activities with focus on Zerumbone. Appl. Sci. 11(21), 10205. https://doi.org/10.3390/app112110205 (2021).
Oh, T. I. et al. Zerumbone, a tropical ginger sesquiterpene of Zingiber officinale roscoe, Attenuates α-MSH-induced melanogenesis in B16F10 cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19103149 (2018).
Girisa, S. et al. Potential of Zerumbone as an anti-cancer agent. Molecules https://doi.org/10.3390/molecules24040734 (2019).
Kalantari, K. et al. A Review of the biomedical applications of Zerumbone and the techniques for its extraction from ginger rhizomes. Molecules https://doi.org/10.3390/molecules22101645 (2017).
Li, X., Zhao, Z., Zhang, M., Ling, G. & Zhang, P. Research progress of microneedles in the treatment of melanoma. J. Control Release 348, 631–647. https://doi.org/10.1016/j.jconrel.2022.06.021 (2022).
Han, Y. et al. Microneedle-based approaches for skin disease treatment. Nano-Micro Lett. https://doi.org/10.1007/s40820-025-01662-y (2025).
Dabholkar, N. et al. Biodegradable microneedles fabricated with carbohydrates and proteins: Revolutionary approach for transdermal drug delivery. Int. J. Biol. Macromol. 170, 602–621. https://doi.org/10.1016/j.ijbiomac.2020.12.177 (2021).
Liu, C., Zhao, Z., Lv, H., Yu, J. & Zhang, P. Microneedles-mediated drug delivery system for the diagnosis and treatment of melanoma. Coll. Surf. B Biointerfaces. 219, 112818. https://doi.org/10.1016/j.colsurfb.2022.112818 (2022).
Zhang, Y. et al. Microneedle system for tissue engineering and regenerative medicine. Exploration (Beijing) 3(1), 20210170. https://doi.org/10.1002/EXP.20210170 (2023).
Moreira, A. F. et al. Microneedle-based delivery devices for cancer therapy: A review. Pharmacol. Res. 148, 104438. https://doi.org/10.1016/j.phrs.2019.104438 (2019).
Kang, Y. et al. Nanocarrier-based transdermal drug delivery systems for dermatological therapy. Pharmaceutics https://doi.org/10.3390/pharmaceutics16111384 (2024).
Hou, A. et al. Rational design of rapidly separating dissolving microneedles for precise drug delivery by balancing the mechanical performance and disintegration rate. Adv. Healthc. Mater. 8(21), e1900898. https://doi.org/10.1002/adhm.201900898 (2019).
Duan, W. et al. Bimetallic plasmonic nanozyme-based microneedle for synergistic ferroptosis therapy of melanoma. Adv. Sci. https://doi.org/10.1002/advs.202504203 (2025).
Duan, W. et al. Engineering dual-driven pro-angiogenic nanozyme based on porous silicon for synergistic acceleration of burn infected wound healing. Mater Today Bio. 31, 101522. https://doi.org/10.1016/j.mtbio.2025.101522 (2025).
Qin, W. et al. Dissolving microneedles with spatiotemporally controlled pulsatile release nanosystem for synergistic chemo-photothermal therapy of melanoma. Theranostics 10(18), 8179–8196. https://doi.org/10.7150/thno.44194 (2020).
Tang, L. et al. Micro/nano system-mediated local treatment in conjunction with immune checkpoint inhibitor against advanced-stage malignant melanoma. Chem. Eng. J. https://doi.org/10.1016/j.cej.2024.154499 (2024).
Rosa, A. et al. Dietary zerumbone from shampoo ginger: new insights into its antioxidant and anticancer activity. Food Funct. 10(3), 1629–1642. https://doi.org/10.1039/c8fo02395f (2019).
Sithara, T. et al. Zerumbone, a cyclic sesquiterpene from Zingiber zerumbet induces apoptosis, cell cycle arrest, and antimigratory effects in SW480 colorectal cancer cells. J. Agric. Food Chem. 66(3), 602–612. https://doi.org/10.1021/acs.jafc.7b04472 (2018).
Al Saqr, A. et al. Elucidating the anti-melanoma effect and mechanisms of Hispolon. Life Sci. 256, 117702. https://doi.org/10.1016/j.lfs.2020.117702 (2020).
Fakhraei Lahiji, S. et al. Tissue interlocking dissolving microneedles for accurate and efficient transdermal delivery of biomolecules. Sci. Rep. 9(1), 7886. https://doi.org/10.1038/s41598-019-44418-6 (2019).
Liu, J., Li, L., Zhang, R. & Xu, Z. P. The adjacent effect between Gd(III) and Cu(II) in layered double hydroxide nanoparticles synergistically enhances T(1)-weighted magnetic resonance imaging contrast. Nanoscale Horiz. 8(2), 279–290. https://doi.org/10.1039/d2nh00478j (2023).
Xia, Y. et al. Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. https://doi.org/10.1002/adma.202002054 (2020).
Vyas, N. S. et al. Observational study examining the diagnostic practice of Ki67 staining for melanocytic lesions. Am. J. Dermatopathol. 41(7), 488–491. https://doi.org/10.1097/dad.0000000000001379 (2019).
King, E. et al. Evaluation of SOX-10 immunohistochemical expression in canine melanoma and non-melanocytic tumors by tissue microarray. Vet. Pathol. 61(6), 896–903. https://doi.org/10.1177/03009858241273318 (2024).
Funding
We acknowledge the financial support from the following funding: S&T Program of Hebei (No. 226Z776G). The authors thank Hebei Medical University Core Facilities and Centers for technical assistance.
Author information
Authors and Affiliations
Contributions
QZ wrote the main manuscript text, KZ, BW and JL prepared all figures, HW and JZ review the draft, DW, YY and LZ carried out statistical analysis on the data, BX supervise, FZ and GZ revised the original draft. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Q., Zhang, K., Wang, B. et al. A dissolvable microneedle patch co-delivering Zerumbone and ICG for combined chemo-photothermal therapy of melanoma. Sci Rep 16, 3943 (2026). https://doi.org/10.1038/s41598-025-33891-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-33891-x