Abstract
To explore the prognostic factors affecting over 80 years of age patients of esophageal cancer with other cancers through the SEER database and provide a scientific basis for the treatment of specific groups of esophageal cancer patients. A total of 2244 patients over 80 years of esophageal cancer with other cancers were selected from the SEER database. Through univariate and multivariate Cox regression analyses, variables were screened to determine independent prognostic risk factors for patients. Patients were divided into Group A and Group B according to sequence number. Propensity score matching (PSM) was performed to adjust for baseline differences between the two groups. Cancer-specific survival (CSS) and overall survival (OS) was calculated using the Kaplan-Meier method and compared using the log-rank test. Subgroup and Multiple Imputation analyses were conducted. This study included 2244 patients over 80 years old of esophageal cancer with other cancers. Cox multivariate analysis revealed that sequence number of primary tumours, surgery and chemotherapy were independent factors for CSS and OS. Age and T stage were an independent prognostic factor for OS but not CSS. Patients were divided into Group A (1st of 2 or more primaries patients) and Group B (2nd of 2 or more primaries, 3rd of 3 or more primaries and ≥ 4th or more primaries patients). There were significant differences in the baseline characteristic of two groups, we performed PSM analyses at a 1:1 ratio to erase significant difference of each variable. After PSM, the median CSS of Group A (31 months, 95% CI 23–47) was significantly better than that of Group B (13 months, 95% CI 10–17) (P < 0.05), the median OS of Group A (19 months, 95% CI 14–25) was significantly better than that of Group B (10 months, 95% CI 7–12) (P < 0.05). Death analysis of 2244 patients revealed that 74.51% (n = 1558) died from disease progression, 8.27% (n = 173) from other malignant tumours, 12.82% (n = 268) from non-tumour diseases, and 4.40% (n = 92) from other causes. Age, T stage, sequence number of primary tumours, surgery and chemotherapy were independent factors affecting the survival of over 80 years of age patients of esophageal cancer with other cancers. Esophageal cancer as the first primary cancer demonstrating markedly better prognosis compared to those with non-first primary esophageal cancers, especially for patients with aged 80–84 years and T1 stage independent of treatments. This study highlight the value of prognosis for over 80 years patients of esophageal cancer with other cancers. Further research is needed to explore the prognostic prediction and treatment.
Similar content being viewed by others
Introduction
With advancements in treatment technology, the survival time of esophageal cancer patients has increased. The prognosis of elderly patients with esophageal cancer is influenced by multiple factors, and patients aged 80 years and above usually have a poor prognosis. Age itself is an important independent adverse prognostic factor. Multiple studies have shown that the survival rate of this group of patients is significantly reduced, and the risk of developing other primary malignant tumours is increased. Research has shown that the type of secondary primary cancer can also affect the prognosis of patients with esophageal cancer who have other malignant tumours. However, the prognosis and treatment plan for these patients are still unclear and require further research. In clinical practice, esophageal cancer patients with multiple primary malignancies often require individualized treatment. This study analysed the data of esophageal cancer patients over 80 years of age with other primary cancers in the SEER database, analysed their independent prognostic factors, and explored the important prognostic factors affecting this patient population. These findings provide evidence for decision-making in clinical practice and provide more reference data for the design of future clinical trials.
Materials and methods
Research subjects
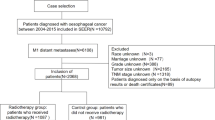
The research subjects were selected from patients diagnosed with esophageal cancer in the SEER database between 1975 and 2021. The inclusion criteria were as follows: (1) diagnosed between 1975 and 2021; (2) had a pathological diagnosis of esophageal cancer; and (3) had other malignant tumours. The exclusion criteria were as follows: (1) under 80 years of age at diagnosis; (2) patients with simple esophageal cancer; (3) patients whose demographic or clinical data were incomplete.
Data collection
Patient data were extracted via SEER stat 8.4.3 software. Surveillance, Epidemiology, and End Results (SEER) Program (https://seer.cancer.gov/) SEER*Stat Database: Incidence - SEER Research Data, 8 Registries, Nov 2023 Sub (1975–2021). The demographic and clinical information of the patients was retrieved. Demographic information included age, sex, and marital status, whereas clinical information included histology, grade, primary tumour location, T stage, N stage, M stage, tumour size, radiotherapy, chemotherapy, cancer-directed surgery (CDS), and the number of primary tumours. We stratified cancer patients by age group (80–84,85–90 and 90+), summary stage (localized, regional, distant), and AJCC sixth-eighth edition TNM staging manual since time (T1-4 stage, N0/+ stage, M0/+stage ).The SEER database contains public data, so there is no need for informed consent from relevant patients to use the SEER database for research, nor is there a need for ethical review approval. This study was approved by the Hebei General Hospital Ethics Committee (Approval ID: 2025-LW-0150). This study complies with the Declaration of Helsinki. Our request for access to the SEER data was approved by the National Cancer Institute, USA (reference number 19238-Nov2021).
Statistical methods
Data processing and analysis were performed using SPSS soft-ware version 26.0 (lBM SPSS) and R version 4.5.0, along with Storm Statistical Plat fomm (https://www.medsta.cn/software). We employed Multiple Imputation by Chained Equations (MICE) with 5 imputations to address missing data in a methodologically robust manner. This approach preserves statistical power while minimizing bias associated with missing values. For each imputed dataset, we applied chi-square tests and Cox proportional hazards regression models. The estimates and standard errors were subsequently pooled to obtain the final results. The chi-squared test (or Fisher’s exact test, if appropriate) was used to analyze the differences between patients grouped by categorical variables. Survival curves were generated using Kaplan-Meier methods, and compared by log-rank test. Univariate and multivariate Cox regression analyses were used to analyze the independent risk factors for CSS and OS. Only variables with statistical significance (P < 0.05) in univariate analysis were incorporated into multivariate analysis. Hazard ratios (HR) were calculated based on multivariable Cox proportional hazards models to estimate predictors of CSS and OS. All CIs were stated at the 95% confidence level. To adjust for differences between Group A and Group B for patients, we performed PSM analyses at a 1:1 ratio. P < 0.05 indicated a statistically significant difference.
Ethics approval
The study was done in agreement with the declaration of Helsinki and approved by the Hebei General Hospital Ethics Committee (Approval ID: 2025-LW-0179).
Results
Clinical data and patient characteristics
According to the inclusion and exclusion criteria, a total of 2244 patients over 80 years old esophageal cancer with multiple primary malignancies were included. The clinical and pathological features were showed in Table 1.
Prognostic factor analysis for patients over 80 years esophageal cancer with other cancers
Univariate analysis revealed that age, marital status, tumour location, tumour size, stage, T stage, N stage, M stage, CDS, CDS + RT, chemotherapy, CDS + chemotherapy, and sequence number are prognostic factors that affect OS and CSS. Histology is a factor that affects OS but not CSS, as shown in Table 2.
Multivariate analysis revealed that that sequence number of cancer, CDS and CT were independent risk factors for OS and CSS. Age and T stage were an independent risk factor for OS, rather than an independent factor for CSS (see Table 3 for details).
CSS and OS of patients over 80 years esophageal cancer with other cancers
The CSS and OS rates of the entire group of patients at 1, 3, and 5 years were 42.0%, 24.0%, 18.0% and 35.0%, 15.0%, and 8.0%, respectively. The CSS and OS rates of esophageal cancer patients over 80 years of age with 1st of 2 or more primaries were greater than other groups patients (P ≤ 0.05), as shown in Table 4; Fig. 1.
Clinical data and patient characteristics before and after PSM according to the group of sequence number
We have reorganized the entire cohort patients into Group A and Group B, where Group A consists of 1st of 2 or more primaries patients and Group B consists of 2nd of 2 or more primaries, 3rd of 3 or more primaries and ≥ 4th or more primaries patients. There were significant differences in the baseline characteristic of two groups, we performed PSM analyses at a 1:1 ratio to erase significant difference of each variable, respectively (Table 5).
Survival analysis before and after PSM according to the group of sequence number
Before PSM, the median CSS of Group A (31 months, 95%CI:25–47) was significantly better than that of Group B (8 months, 95%CI:7–9), the median OS of Group A (20 months, 95%CI:14–25) was significantly better than that of Group B (6 months, 95%CI:5–6). After PSM, the median CSS of Group A (31 months, 95%CI:23–47) was significantly better than that of Group B (13 months, 95%CI:10–17), the median OS of Group A (19 months, 95%CI:14–25) was significantly better than that of Group B (10 months, 95%CI:7–12) (P < 0.05). (Fig. 2; Tables 6 and 7).
Subgroup analysis of CSS and OS for patients after PSM
We conducted subgroup analysis for Group A and Group B on independent prognostic factors after PSM in the primary and five databases generated after Multiple Imputation to address missing data. We found that patients in Group A with 80–84 years old, and T1 stage had significantly better OS than their counterparts in the Group B (P < 0.05) (Fig. 3). Furthermore, regardless of whether patients undergo CDS or chemotherapy, CSS and OS in Group A were superior to that in Group B patients (P < 0.05) (Figs. 3 and 4).
Subgroup analysis of OS in patients with independent risk factors characteristics after PSM in the Group A and Group B. (OS: Overall survival, CDS/ CDS1/ CDS2/ CDS3/ CDS4/ CDS5:Cancer-directed surgery in the primary/ first/ second/ third/ fourth/ five databases generated after Multiple Imputation, TStage/ TStage1/ TStage2/ TStage3/ TStage4/ TStage5:T stage in the primary/ first/ second/ third/ fourth/ five databases generated after Multiple Imputation)
Subgroup analysis of CSS in patients with independent risk factors characteristics after PSM in the Group A and Group B (CSS: Cancer-specific survival, CDS/ CDS1/ CDS2/ CDS3/ CDS4/ CDS5: Cancer-directed surgery in the primary/ first/ second/ third/ fourth/ five databases generated after Multiple Imputation).
Analysis of the causes of mortality for the entire patients
An analysis of mortality revealed that 74.51% (n = 1558) of patients succumbed to disease progression, whereas 8.27% (n = 173) passed away from other malignant tumours, including those of the respiratory, digestive, urinary, and head and neck systems. Additionally, 12.82% (n = 268) of patients died from non-oncological conditions, such as cardiovascular and cerebrovascular diseases, chronic obstructive pulmonary disease, and infections. Furthermore, 4.40% (n = 92) of patients died from various other causes, including suicide, accidents, and other unknown causes of death. For further details, please refer to Table 8. Among patients with varying numbers of tumours, the primary cause of death was progression of the primary disease, as detailed in Table 9.
Discussion
Research indicates that elderly patients with esophageal cancer exhibit a greater incidence of comorbidities, including other cancers, and these combined factors exacerbate the adverse prognosis for this patient group1,2. A study by Zhang M. revealed that the coexistence of other cancers is one of the factors contributing to decreased survival rates among elderly patients with esophageal cancer. This may impact prognosis by heightening treatment complexity and diminishing patient tolerability, yet aggressive management of comorbid cancers can enhance survival outcomes1. Nevertheless, further investigation into the survival prognosis of esophageal cancer patients aged 80 years and above who have additional cancers is warranted.
This study demonstrated that the prognosis of esophageal cancer patients over 80 years old who also have other cancers is influenced by multiple factors, including patient baseline characteristics, tumour features, and treatment modalities. Age 80 years or above significantly impacts prognosis, and even after adjusting for treatment approaches, advanced age remains correlated with poorer survival outcomes3,4,5. The multivariate analysis in this study revealed that age (≥ 90 years) was an independent risk factor affecting overall survival (OS), but not a factor influencing cancer-specific survival (CSS), suggesting that for patients over 90 years old, tumor-related causes were not the primary reason for death. Frailty and comorbidities in elderly patients, such as cardiovascular diseases or other cancers, are linked to decreased survival rates and a higher incidence of post-treatment complications, which aligns with previous research6,7,8,9. The findings further refine this recommendation: patients aged ≥ 90 years require more frequent comorbidity assessments (e.g., cardiovascular function monitoring), while tumor surveillance intensity can be appropriately adjusted based on CSS data to avoid over-intervention. Future studies should develop prognostic models integrating age, comorbidities, and tumor characteristics to more accurately distinguish between cancer-related and non-cancer-related mortality risks. The treatment choices for over 80 years old patients of esophageal cancer patients with other cancers, including surgery, or chemotherapy, have a significant impact on prognosis. A multi-centre study indicated that the overall survival of resectable esophageal cancer in patients over 80 years old is notably shorter than that of younger individuals. This is attributed to tumour progression and age-related comorbidities10,11. Relevant studies12,13,14,15 also support that age serves as a primary predictor of postoperative complications and mortality. However, compared with nonsurgical patients, elderly esophageal cancer patients who undergo surgery may exhibit superior long-term survival rates16,17. Esophagectomy is feasible in patients aged 80 years and above, with a 5-year survival rate of 30.3% achievable even after rigorous screening16. This study revealed that surgery was an independent favourable prognostic factor for both OS and CSS. Among patients with esophageal cancer accompanied by other primary cancers, postoperative non-cancer deaths, such as cardiovascular and pulmonary complications, constitute a relatively high proportion (approximately 21.3%)18, highlighting the necessity for enhanced postoperative complication management. For esophageal cancer patients aged 80 years or older with coexisting cancers, it is crucial to weigh surgical risks against survival benefits in clinical practice.
For elderly patients aged 80 years and above who are deemed inoperable due to esophageal cancer, those receiving curative radiotherapy often face limited long-term overall survival. A retrospective analysis assessed the tolerability and survival rates of 213 patients aged 80 years and older who underwent radical radiotherapy and revealed a lower 5-year survival rate, which was partly due to an increased incidence of noncancer-related deaths, such as cardiovascular events9. Elderly patients (≥ 80 years) receiving radiotherapy have a 3-year overall survival rate of 23.3%, with acceptable treatment tolerability9. However, this study underscores that radiotherapy is not an independent prognostic factor for survival. The coexistence of other cancers is a crucial factor in the prognosis of esophageal cancer patients, yet its impact hinges on the successful management of these comorbid cancers. A study focusing on patients with synchronous dual cancers included 51 patients who received systemic chemotherapy. The findings indicated that when both the target cancer and the comorbid cancer demonstrated high disease control rates–90.9% for the target cancer and 90.7% for the comorbid cancer–patients exhibited significantly better prognoses2. This highlights the disease control status of comorbid cancers as a pivotal factor influencing prognosis. The active management of coexisting cancers, such as through systemic chemotherapy, along with personalized treatments such as radiotherapy or chemotherapy or selective surgery, can partially ameliorate the prognosis. Nevertheless, the overall survival rate for this patient cohort remains low, underscoring the importance of adopting individualized management strategies in clinical practice that consider both the presence of coexisting cancers and age-related risks1,2,19. A study involving 479 patients over 80 years old with esophageal squamous cell carcinoma (ESCC) revealed a low overall survival rate during the average follow-up period, with a notably high proportion of deaths attributed to cardiovascular and cerebrovascular diseases emerged as the second leading cause of death (9.37%)20. In our study, 8.27% (n = 173) patients died from other malignant tumours, and 12.82% (n = 268) of patients died from non-oncological conditions, such as cardiovascular and cerebrovascular diseases and other diseases, further emphasizing the fragility of this age group.
Our study found that the sequence number of multiple primary cancers significantly impacts survival prognosis, with esophageal cancer as the first primary cancer demonstrating markedly better prognosis compared to those with non-first primary esophageal cancers. To eliminate confounding effects of prognostic factors, we categorized patients with esophageal cancer as the first primary malignancy into Group A, and those with non-first primary esophageal cancers into Group B. After performing propensity score matching (PSM) to balance clinical characteristics between groups, the results consistently showed that both cancer-specific survival (CSS) and overall survival (OS) remained significantly superior in Group A compared to Group B. The study by Ye J et al.21 analyzed the survival data of patients with esophageal cancer combined with second primary malignancy (defined as the EC-SPM group, where esophageal cancer was the first among two or more primary cancers). The results showed that the median survival time was 47.00 months (95% CI: 43.87–50.13), and the mean survival time was 74.67 months. However, esophageal cancer as a second primary malignancy is associated with poor overall survival. The study by Deqiang Pan et al.22 demonstrated that EC patients (median age 64 [57–71] years) with prior prostate cancer and bladder cancer had the best survival outcomes (3-year OS rates of 27.7% and 29.2%, respectively), whereas those with prior laryngeal cancer or lung/bronchus cancer showed the worst OS (3-year OS rates of 12.5% and 11.0%, respectively). Leng J et al.23 compared the survival differences between esophageal cancer as a second primary malignancy (esophagus-2) and as a first primary malignancy (esophagus-1). In the study, patients aged over 80 years accounted for 14.7% of the total cohort, with 8.0% being 80–84 years old and 6.7% being 85 years or older. The results demonstrated that in the first 5 years after diagnosis, patients with esophagus-2 had similar risk of overall mortality with those with esophagus-1 but increased risk thereafter. As for non-cancer related mortality, esophagus-2 patients had higher risk all along. To further analyze survival differences, subgroup analyses in this study demonstrated that among patients aged 80–84 years and T1 stage, Group A showed superior overall survival (OS) compared to Group B. Moreover, Group A exhibited better survival outcomes than Group B regardless of whether they received surgery or chemotherapy. However, no significant survival differences were observed between Group A and Group B in patients aged ≥ 85 years, those with T2 + 3 + 4 stages. These findings suggest that the intrinsic biological aggressiveness of advanced-stage esophageal cancer may override other prognostic factors, effectively diminishing the survival advantages typically associated with the tumor’s sequence number or other favorable patient characteristics. This observation offers valuable clinical insights for physicians managing cases of locally advanced or metastatic disease.The subgroup analysis in Figs. 3 was conducted using both the original database and five imputed databases to account for missing values. The results indicate that the impact of T4 on subgroups could not be fully determined, warranting further validation in subsequent studies. Additionally, advanced esophageal cancer-related multiple primary malignancies significantly increase diagnostic and therapeutic challenges, leading to poorer overall prognosis. The first primary contributing factors include decreased physiological reserve, reduced treatment tolerance, potentially higher tumour aggressiveness, and increased complexity of treatment strategies24,25. However, secondary or subsequent primary esophageal cancers show reduced overall survival (OS) and cancer-specific survival (CSS), likely due to compromised physical condition following prior anticancer therapies26. Conversely, when esophageal cancer treatment is administered for other cancers as secondary or later primaries (Group A), patients exhibit lower EC cancer mortality rate compared to those when these other cancers were first primary malignancies (Group B). Our final tumor mortality analysis (Table 9) revealed: When esophageal cancer is the first primary tumor (Group A): Esophageal cancer mortality: 62%, Other cancer mortality: 17%. When esophageal cancer is the secondary or subsequent primary tumor (Group B): Esophageal cancer mortality: >70%, Other cancer mortality: <10%. Further prospective studies are warranted to validate these observations.
Our research indicates that among esophageal cancer patients aged over 80 with other malignancies, Non-tumour deaths account for a considerable proportion 12.82% (Table 8). This may be attributed to the higher prevalence of comorbidities in elderly patients, as well as potential mortality induced by anti-cancer treatments. Studies demonstrate that underlying cardiovascular disease (CVD) is a critical concern in esophageal cancer patients undergoing radical chemoradiotherapy or surgery. These treatments may induce or exacerbate cardiovascular events, including myocardial infarction, heart failure, and arrhythmias27. Chemotherapeutic agents, particularly platinum-based regimens (e.g., cisplatin), may increase thromboembolic risk28. The high trauma of esophagectomy could impose additional burden on patients with pre-existing CVD , while high complication rates (e.g., anastomotic leakage, infections) may indirectly aggravate cardiovascular/cerebrovascular risks, especially in elderly patients or those with comorbid CVD29. The literature lacks controlled studies directly comparing the impact of different treatment modalities on non-cancer mortality, with most evidence derived from single-center retrospective analyses or small prospective trials. In summary, current evidence supports an association between esophageal cancer treatments and non-cancer mortality (particularly cardiovascular/cerebrovascular events), but more prospective studies are needed to clarify causal mechanisms. Clinical decision-making should be based on comprehensive assessment of patient comorbidities and treatment-related risks.
There are several limitations to this study. First, the retrospective nature of the SEER database may result in incomplete or missing data. Despite employing multiple imputation techniques to address missing data, the inherent limitations of incomplete datasets precluded more precise analyses in this investigation. Second, the SEER database does not release specific and detailed data on surgical methods and radiation and chemotherapy. Third, this study focuses on analyzing the unique prognostic characteristics of esophageal cancer combined with other primary cancers (MPC), rather than the independent prognostic factors of esophageal cancer alone. The exclusion of patients with esophageal cancer alone is to ensure the homogeneity of the study cohort and avoid interference from confounding factors in extracting MPC-related conclusions. By focusing specifically on MPC cases, this study design allows for more accurate characterization of the distinct biological behaviors, treatment challenges, and survival patterns associated with esophageal cancer when it occurs as part of multiple primary malignancies. Even if it cannot be directly compared with simple esophageal cancer, the research results can still provide important references for personalized treatment of MPC patients. Fourth, this study is constrained by the absence of comprehensive data regarding tumor types, disease stages, and treatment modalities for other malignancies, potentially introducing selection bias. To mitigate this limitation, subsequent analyses will incorporate datasets containing detailed information on multiple primary tumors. Furthermore, future research should prioritize multicenter, large-scale cohort studies along with prospective double-blind randomized clinical trials to robustly validate these findings.
In summary, age, T stage, surgery, chemotherapy and the sequence number of esophageal cancers are closely related to the prognosis of over 80 years of age patients of esophageal cancer with other cancers. Esophageal cancer as the first primary cancer demonstrating markedly better prognosis compared to those with non-first primary esophageal cancers, especially for patients with aged 80–84 years and T1 stage independent of treatments. It has reference significance for the diagnosis and treatment of over 80 years of age patients of esophageal cancer with other cancers and helps physicians make clinical treatment decisions.If esophageal cancer is the first primary tumor, it usually requires prioritized treatment, particularly in early-stage cases where surgery or definitive chemoradiotherapy are the main options. For subsequent second primary cancers, treatment strategies need to be adjusted based on the therapeutic outcomes of esophageal cancer and patient tolerance. When esophageal cancer occurs as a second primary malignancy, the cumulative toxicity risks from prior treatments (such as radiotherapy) must be carefully evaluated. The sequence of tumor occurrence directly influences treatment priorities, modality selection, and prognosis assessment. In clinical practice, multidisciplinary team consultations should be implemented to optimize decision-making.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to proprietary restrictions, but are available from the corresponding author on reasonable request.
References
Zhang, M. et al. Psoas muscle mass index and peak expiratory flow as measures of sarcopenia: relation to outcomes of elderly patients with resectable esophageal cancer. Front. Oncol. 13, 1303877 (2023).
Sohda, M. et al. Efficacy of chemotherapy for comorbid cancer in patients with simultaneous double cancers: a multi-center study. Surg. Today. 53 (1), 98–108 (2023).
Li, C. et al. Two distinct age-prognosis patterns in patients with esophageal cancer undergoing surgical and radiotherapy treatments: a combined analysis of 3JECROG and SEER databases. Ther. Adv. Med. Oncol. 16, 17588359241261009 (2024).
Koterazawa, Y. et al. Risk factors for poor long-term outcomes in elderly patients with esophageal squamous cell carcinoma after minimally invasive esophagectomy. Surg. Today. 55 (5), 659–667 (2025).
Qin, L. et al. Development and validation of a prognostic nomogram for predicting cancer-specific survival in lymph node-negative elderly esophageal cancer patients: A SEER-based study. Med. (Baltim). 102 (30), e34441 (2023).
Dai, Y. et al. A comparative study of elective nodal irradiation and involved field irradiation in elderly patients with advanced esophageal cancer. Front. Oncol. 13, 1323908 (2023).
Wang, M. et al. Effect of extracapsular lymph node involvement on the prognosis of patients with esophageal squamous cell carcinoma. Technol. Health Care. 31 (5), 1771–1786 (2023).
Wei, M. X. et al. Clinicopathological characteristics and postoperative prognosis of patients with nuclear pedigree of esophageal squamous cell carcinoma. Front. Oncol. 13, 1190457 (2023).
Zhai, T. et al. Radiotherapy for patients with esophageal cancer aged 80 years or older: A 16-year experience. J. Cancer Res. Ther. 20 (2), 678–683 (2024).
Ahmed, N. et al. Survival and perioperative outcomes of octo- and nonagenarians with resectable esophageal carcinoma. Dis. Esophagus. 36 (12), doad043 (2023).
Cooper, L. et al. Esophageal cancer in octogenarians: should esophagectomy be done? J. Geriatr. Oncol. 15 (2), 101710 (2024).
Shimonosono, M. et al. Clinical outcome after esophagectomy or definitive chemoradiotherapy in elderly patients (≥ 80 Years) with esophageal Cancer. Anticancer Res. 42 (8), 3953–3961 (2022).
Kim, J. Y. et al. Evaluating the perioperative risks in esophageal resection and reconstruction for esophageal carcinoma among elderly patients: A retrospective propensity score matching analysis. Eur. J. Surg. Oncol. 51 (3), 109542 (2025).
Mengardo, V. et al. The effect of aging on short- and long-term results after esophagectomy: an international multicenter retrospective analysis. Dis. Esophagus. 37 (2), doad057 (2024).
Laurent, A. et al. Esophageal cancer: outcome and potential benefit of esophagectomy in elderly patients. Thorac. Cancer. 13 (19), 2699–2710 (2022).
Li, K. et al. Surgical vs nonsurgical treatment for esophageal squamous cell carcinoma in patients older than 70 years: a propensity score matching analysis. J. Gastrointest. Surg. 28 (5), 611–620 (2024).
Moletta, L. et al. Short- and long-term outcomes in elderly patients with resectable esophageal cancer: upfront esophagectomy compared to surgery after neoadjuvant treatments. J. Clin. Med. 13(14), 4271.
Aoyama, T. et al. The clinical impact of other primary cancer in patients who received curative treatment for esophageal cancer. Anticancer Res. 42 (11), 5635–5641 (2022).
Hu, L. L. et al. Prognosis of radiotherapy for esophageal cancer in elderly patients exceeding seventy-five years old. World J. Gastrointest. Oncol. 16 (12), 4636–4649 (2024).
Ryu, D. G. et al. Clinical outcomes of esophageal squamous cell carcinoma in patients aged over 80 years. Korean J. Intern. Med. 40 (2), 230–242 (2025).
Ye, J. et al. Better prognosis and survival in esophageal cancer survivors after comorbid second primary malignancies: A SEER Database-Based study. Front. Surg. 9, 893–429 (2022).
Pan, D. et al. Survival outcomes in esophageal cancer patients with a prior cancer. Med. (Baltim). 100 (7), e24798 (2021).
Leng, J. et al. Recommendations for broadening eligibility criteria in esophagus cancer clinical trials: the mortality disparity of esophagus cancer as a first or second primary malignancy. J. Thorac. Dis. 16 (6), 3882–3896 (2024).
Cui, Y. et al. Research Progress of Multiple Primary Malignancies Associated With Esophageal Cancer. Cancer Control 30, 10732748231176640 (2023).
Tsai, P. C. et al. Overall survival for esophageal squamous cell carcinoma with multiple primary cancers after curative Esophagectomy-A retrospective Single-Institution study. Cancers (Basel). 14 (21), 5263 (2022).
Ohmori, M. et al. Excessive risk of second-cancer incidence and cancer mortality in patients with esophageal cancer. J. Gastroenterol. 56 (5), 434–441 (2021).
Søndergaard, M. M. A. et al. Cardiovascular burden and adverse events in patients with esophageal cancer treated with chemoradiation for curative intent. JACC CardioOncol. 3 (5), 711–721 (2021).
Rousseau, J. & Wen, P. Y. Cerebrovascular and peripheral vascular complications in cancer patients. Curr. Neurol. Neurosci. Rep. 25 (1), 46 (2025).
Khaw, S. P. et al. Predictors of readmission after esophagectomy for esophageal cancer: a systematic review and meta-analysis. Int. J. Surg. November 14, (2025).DOI: 10.1097/JS9.0000000000003860.
Acknowledgements
We would like to sincerely thank the original SEER database and related consortia for sharing and managing the summary statistics. The authors wish to thank all the hands and minds involved in this study.
Funding
This work was supported by the Medical Science Research Project Plan of the Hebei Provincial Health Commission (20220920, 20241812, 20210768).
Author information
Authors and Affiliations
Contributions
Study concepts and study design: Xiaolu Yan and Qiaofang Li. Data acquisition, quality control of data and algorithms: Yitong Li and Lei Tian. Data analysis and interpretation and manuscript preparation: Kuo Wang and Qiaofang Li. Statistical analysis: Mengchang Gao. Manuscript editing and review: all the authors. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Q., Gao, M., Wang, K. et al. Study on the survival prognosis of over 80 years old patients of esophageal cancer with other cancers-based on SEER data analysis. Sci Rep 16, 3950 (2026). https://doi.org/10.1038/s41598-025-34081-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-34081-5