Abstract
Escalating insecticide resistance threatens the efficacy of LLINs, undermining malaria control in Africa. We conducted the first experimental hut trials in Uganda using highly resistant free-flying wild Anopheles mosquitoes and F2 hybrids of FANG and Uganda An. funestus to evaluate the performance of bednets. The interceptor G2 (chlorfenapyr) bednet demonstrated superior efficacy compared to Interceptor (pyrethroid-only) net [mortality odds ratio (OR): 18.7 (8.05–48.6) P < 0.0001], achieving an overall mortality rate of 70.6% and 63.2% against An. funestus and An. gambiae respectively. In contrast, PermaNet 3.0 and Olyset Plus (piperonyl butoxide (PBO)) and Royal Guard (pyriproxyfen (PPF)-treated) bednets exhibited significantly lower mortality against An. funestus [Olyset Plus: 36.1%, PermaNet 3.0: 31.0% and Royal Guard (37.6%], though performance against An. gambiae was moderate [PermaNet 3.0: 61.4%, Olyset Plus: 50.0%, Royal Guard: 51.6%]. Interceptor net produced the lowest mortality (~ 25%) against both species. Regarding blood-feeding inhibition (BFI), PBO nets, particularly Olyset Plus, outperformed Interceptor G2 and Royal Guard, while Interceptor produced minimal BFI (< 36%). Further evaluation of Royal Guard’s PPF effect on oviposition revealed no significant reduction in oviposition rates compared to controls with An. funestus (63.9% vs. 63.3%, P > 0.05). Genetic analysis using the hybrid crosses revealed that pyrethroid resistance markers (4.3 Kb-SV and G454A-Cyp9K1) were significantly associated with mosquito survival and blood-feeding success against PermaNet 2.0 (pyrethroid-only) and PermaNet 3.0 but showed no significant association with Interceptor G2 net. These findings support Interceptor G2 as a promising intervention for regions dominated by both highly resistant An. funestus s.l. and An. gambiae s.l. Piperonyl butoxide and PPF nets emerge as a good alternative for areas mostly dominated by resistant An. gambiae s.l. populations. Critically, the demonstrated variable impact of insecticide resistance on bednet efficacy underscores the imperative need for a comprehensive vector distribution mapping, continuous field efficacy assessments, and systematic resistance monitoring. This evidence-based triad should guide strategic LLIN distribution and rotations to sustain malaria control efficacy in resistance-prone settings.
Similar content being viewed by others
Introduction
Sub-Saharan Africa continues to face a high malaria burden despite widespread vector control efforts, particularly through bednets, with progress stalling in recent years1. Insecticide-based interventions, primarily long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), remain the cornerstone of malaria control1. LLINs have been the primary malaria control tool in endemic countries, due to their ease of deployment, cost-effectiveness, and ability to offer both personal and community protection2. Until recently, pyrethroids were the sole insecticide class used in LLINs. However, rising pyrethroid resistance across Africa has significantly reduced the efficacy of pyrethroid-only LLINs, leading to the development of piperonyl butoxide (PBO) LLINs and subsequently other dual-active-ingredient (ai) bednets. The synergist PBO enhances pyrethroid potency by inhibiting cytochrome P450 oxidases (CYPs), key drivers of insecticide resistance in major malaria vectors3,4. In principle, PBO-treated LLINs are expected to exhibit greater insecticidal efficacy than pyrethroid-only LLINs, a finding that has been consistently demonstrated in laboratory studies5,6,7,8. Following a cluster-randomized trial in Tanzania9, the WHO recommended PBO LLINs, prompting their rollout in endemic regions with the target of achieving universal coverage.
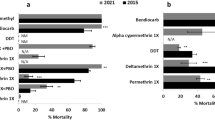
In Uganda, large-scale LLIN distribution began in 2013–14, with pyrethroid-only nets; Olyset net, and PermaNet 2.0, and then every three years as per WHO guidelines10. In 2017, PBO LLINs were progressively introduced in western and eastern districts during the 2017–18 campaign, integrated into a trial across 104 health sub-districts11. Clinical and entomological results from areas that received PBO LLINs compared to pyrethroid-only nets showed lower parasite prevalence and vector density12,13, aligning with a Cochrane review14. Unfortunately, while early semi-field studies (pre-2015) confirmed PBO LLINs’ high efficacy15,16,17, recent trials indicate declining performance, with lower mortality rates against An. gambiae in Benin18,19 and An. funestus in Cameroon5. However, there is a vast variation in the performance of PBO LLINs in different localities which could be attributable to vector behaviours and differences in insecticide resistance profiles.
To counter ongoing resistance threats, new insecticide classes — pyrroles and neonicotinoids — were introduced in 2018 following WHO prequalification20. A pyrrole, chlorfenapyr, is combined with pyrethroids in “new-generation” dual-AI nets like Interceptor® G2. Chlorfenapyr disrupts the energy production in insects, leading to their death21,22. The recent study published by Tchouakui et al revealed that the primary malaria vectors remain susceptible to pyrroles23. Furthermore, semi-field studies highlight Interceptor® G2’s superior efficacy against resistant wild vectors compared to other LLINs24,25,26. In Uganda, chlorfenapyr-treated nets; Interceptor® G2 and PermaNet®Dual were rolled out in November 2023, though its performance remains unevaluated locally. However, trials in Tanzania’s Lake Victoria basin, showed promising results revealing high efficacy26,27. Another dual-AI net, Royal Guard®, combining pyrethroids with pyriproxyfen (PPF) — an insect growth regulator—was distributed in mostly northern and eastern districts of Uganda in 202028. This type of LLIN offers both high mortality and reduces vector oviposition and fecundity29. The entomological impact of PPF-LLINs in Uganda is untested, but epidemiological data suggest performance comparable to PBO nets28. However, trials in Tanzania reported only moderate efficacy against malaria vectors, with performance declining over time27.
In eastern Uganda, two major malaria vectors; An. funestus s.l. (referred to here as An. funestus) and An. gambiae s.l. (referred to here as An. gambiae) dominate8,30 although their relative abundance affected by ongoing control interventions31,32,33,34,35. In Mayuge district where the trial was conducted, we previously showed that An. funestus is the dominant vector and both vectors exhibit extreme pyrethroid resistance, up to ten times the diagnostic dose36,37, threatening LLIN efficacy5,36,37,38. Here, we conducted the first experimental hut trial in Uganda, in a district along the Lake Victoria basin. Following WHO guidelines39, the study simultaneously evaluated the performance of five types of LLINs, including both conventional and next-generation bednets, against these highly resistant vectors.
Methods
Study area
The study was conducted in the eastern region of Uganda in the district of Mayuge (0°23′24.3"N 33°37′36.0"E) (Fig. 1A). The district is along the shores of Lake Victoria in the south and has a tropical savanna climate where the average temperature is 26 °C and rainfall is received throughout the year with an average of 131 mm. The main economic activities include fishing and farming of cash crops such as cotton, coffee, sugar cane and rice. Eastern Uganda has one of the highest malaria transmission rates in the country and Mayuge district belongs to areas categorised as “very high transmission” by the National Malaria Control Program (NMCP) and was among the first districts to receive LLINs in 201240. Although malaria transmission occurs typically throughout the year, it is largely perennial with two annual peaks following two rainy seasons (March–May and August to October).
The experimental hut trial was conducted in Kigandalo sub-county, Kigandalo parish, Kigandalo B village (0°23′24.3"N 33°37′36.0"E) where a total of six (6) huts were constructed (Fig. 1B) according to WHO standard39 . The site is surrounded by permanent water sources, rice fields and sugar cane plantations with permanent flowing streams nearby and a low water table which is suitable for An. funestus breeding as well as An. gambiae. The main malaria vector species are An. funestus and An. gambiae with the former being the predominant vector. Other mosquitoes such as Culex and Mansonia species are also present with the latter being very scarce36,37. Both malaria vector species are very resistant to insecticides especially pyrethroids even at higher concentrations (5 × and 10 × the diagnostic dose) but susceptible to organophosphates, neonicotinoids, and pyrroles36,37,41,42. The experimental hut effect test and trial were conducted against free-flying mosquitoes for a combined 9 weeks between 26th August 2023 to 18th November 2023 during part of the dry season and most of the second rainy season.
Experimental hut design
We opted for the West African design purely for logistical reasons because they were easiest and cheapest to build, which is well-suited to the goal of the study. These experimental huts were constructed following the WHO guidelines (WHO, 2016b). The entire structure is built using cement, sand, and bricks with plastered walls. The roof is built with corrugated iron sheets with a ceiling made of plywood. Four windows; two in front and two on each side are designed with 2 cm angular gap which allows mosquitoes to enter but not escape the huts. A veranda trap is added to the design at the back of the hut and built according to the WHO guidelines (WHO, 2016b). To separate the veranda from the rest of the room, a curtain is used. Before sleeping, each volunteer is required to raise the curtain to give mosquitoes an opportunity to exit the room to the veranda. The curtains are lowered in the morning before mosquito collections begin in the room to prevent exited mosquitoes from coming back into the room hence separating the veranda collections from room/hut collections.
Net treatment arms and comparison
During the trial, five (5) types of LLINs plus a control were assessed which comprised of one pyrethroid-only net, four next-generation bednets and one untreated net as control (Table 1). As per the WHO protocol, square holes measuring 4 cm x 4 cm were cut into bednets, two on each of the long sides and one on each of the short sides.
Hut effect test
Prior to the study, the hut effect was assessed between 26th August and 16th September 2023 to evaluate if the huts and/or the participants were individually attractive. During these 3 weeks, volunteers slept under the untreated nets hanging in the six newly constructed huts and collected mosquitoes every morning.
Experimental hut trial
The experimental hut trial was carried out following a 36-nights protocol as described by WHO guidelines for laboratory and field-testing of long-lasting insecticidal nets43. To correct any specific attractiveness observed during the hut effect assessment, the Latin square design rotation was used to alternate bednets43 such that at the end of the experiment, each bednet would have spent six days in each hut. The huts were cleaned at the end of the week before the next net rotation. Six adult male volunteers were selected to sleep in each hut from 20:00 GMT in the evening to 5:00 GMT in the morning. Each day, the sleepers were rotated such that at the end of the week, a sleeper would have spent one night in each hut. The sleeper rotation was done to correct any bias that could be because of specific attractiveness from the sleepers. Sleepers were blinded to treatment allocation by concealing the net type and covering all visible or readable labels to prevent identification.
Mosquito collections
Every morning, alive and dead mosquitoes were collected using haemolysis tubes5 from: (i) inside the bednets (ii) from the sleeping area (room); on the floor, walls, and ceiling, and (iii) in the veranda exit trap. The mosquitoes collected from each compartment were kept in well labelled separate bags to avoid mixing any samples from the different compartments. The collected samples were then categorized as dead, alive, blood fed or unfed. The ‘alive’ mosquitoes were transferred into paper cups and fed with 10% sugar solution and the mortalities were recorded at 24 h for Interceptor, PermaNet 3.0, Olyset Plus and RG bednets and 72 h for IG2. Furthermore, for RG net and control, alive and blood fed mosquitoes were kept for 4 days and then forced to lay eggs44 to compare the oviposition rates. The collected females were sorted according to morphological keys as previously described45.
Assessing performance of the bednets
Performance of the bednets were assessed relative to the control net (untreated net) as described by WHO43 in terms of:
-
a)
Deterrence rate: the decrease in the proportion of mosquitoes entering the hut as compared to the control. Deterrence (%) = 100 × (Du − Dt)/Du, where Du is the total number of mosquitoes collected in the control hut and Dt is the total number of mosquitoes collected in the treated hut.
-
b)
Entry rate: the proportion of mosquitoes found to have successfully entered the hut. Entry rate (%) = 100 × (Ht/Hn) where Ht is the total number of mosquitoes found in each hut and Hn is the total number of mosquitoes collected in all the other five treated huts.
-
c)
Exophily (exit rate): the proportion of mosquitoes found to have exited to the veranda trap in each of the huts. Exophily (%) = 100 × (Ev/Et) where Ev is the total number of mosquitoes found in veranda and Et is the total number of mosquitoes found both inside the hut and veranda. A component of an exit rate is Induced exophily which is the proportion of mosquitoes that exited to the veranda trap because of the presence of a treated net. Induced exophily (%) = 100 x (Et-Ec)/(100-Ec) where Et is exophily rate in hut with treated net and Ec is the exophily rate in hut with control net.
-
d)
Blood feeding rate (BFR): the proportion of mosquitoes found to have successfully taken a blood meal. Blood feeding rate = (N mosquitoes fed) × 100/total N mosquitoes. Where “N mosquitoes fed” is the number of mosquitoes that have blood fed, and “total N mosquitoes” is the total number of mosquitoes collected.
-
e)
Blood-feeding inhibition (BFI): the reduction in blood-feeding in comparison with the control hut. BFI is calculated as; (1-Bt/Bc*100) where Bt is the BFR of mosquitoes in hut with the treated net and Bc is the BFR of mosquitoes in hut with the control net. BFI is an indicator of personal protection (PP). Specifically, the PP effect of each bednet is the reduction in the rate of blood feeding induced by the net when compared to the control. Personal protection (%) = 100 × (Bu-Bt)/Bu, where Bu is the total number of blood fed mosquitoes in the control and Bt is the total number of blood-fed mosquitoes in the treated hut.
-
f)
Immediate and delayed mortality: immediate mortality is the proportion of mosquitoes found dead in the morning while delayed mortality is the proportion of alive mosquitoes that die after 24 h to 72 h with access to sugar solution. This study focused on the overall mortality (immediate plus delayed mortality) and is calculated as: Mortality (%) = 100 × (Mt/MT) where Mt is the total number of mosquitoes found dead in the hut even after the holding period and MT is the total number of mosquitoes collected in the hut. Mortality is also an indicator of the killing effect of a bednet which is calculated as; Killing effect (%) = 100 x (Kt – Ku) / Tu, where Kt is the number of mosquitoes killed in the hut with the treated net, Ku is the number of mosquitoes killed in the hut with untreated net and Tu is the total number of mosquitoes collected from the hut with untreated net. For superiority trials we determine whether there is a significant difference (at the 95% significance level) between mortality in the two arms of the trial being considered.
Bednet superiority assessment
The methodology used to assess the superiority of ITNs was proposed by WHO46. A superiority trial could be performed to show if a particular insecticide-treated bednet is superior to another bednet. In this study, we assessed if PPF net (Royal Guard), PBO nets (PermaNet 3.0 and Olyset Plus) and chlorfenapyr net (Interceptor G2) were superior to a pyrethroid-only net (Interceptor). Three parameters; mortality, blood feeding rate and exit rates were considered and the assessment of whether a bednet is superior was made by determining if there is a significant difference (at the 95% significance level) between a treated bednet and untreated net. We further calculated the Odds ratio (OR) produced by each bednet in comparison to Interceptor G2. The analysis was performed in R software as described by Challenger et al.47. Briefly, a regression model is fitted, grouping data of same type of bednets together and the OR with its confidence interval (CI) constructed estimated regression parameters. A bednet is considered superior when there is a statistically significant difference in the measured parameter between the treated and untreated bednets.
Assessment of impact of 4.3 Kb-SV and AG454A-Cyp9K1 resistance markers on bednet performance against An. funestus mosquitoes
To assess the impact of resistance markers on the efficacy of bednets, we conducted a second experimental hut trial using F₂ hybrids derived from field-collected An. funestus mosquitoes from Mayuge crossed with the FANG laboratory-susceptible strain, which is maintained at the Centre for Research in Infectious Diseases (CRID) in Cameroon. The use of hybrid mosquitoes was intended to obtain the segregated genotypes of two key resistance markers, 4.3 Kb-SV and G454A-Cyp9K1, which are already fixed in Ugandan populations (only RR genotype present). This approach allowed for the segregation of genotypes, enabling robust genotype–phenotype association analyses as successfully done in the past48,49,50. These two markers were selected as proxies for pyrethroid resistance, as they have been previously validated to be strongly associated with resistance phenotypes51,52.
Field mosquitoes collected in November 2024 were reared to F1 progeny, then crossed with the FANG strain to generate F2 hybrids. The experimental hut trial was conducted over three nights using a release-recapture protocol that featured two key modifications: (1) windows were sealed internally to prevent mosquito escape, and (2) 75–100 mosquitoes were released per hut. Six treatments were evaluated (i) Untreated net, (ii) PermaNet 3.0, (iii) Olyset Plus, (iv) PermaNet Dual, (v) Interceptor G2 and (vi) PermaNet 2.0.
In this study, we assessed impact of resistance on collected mosquitoes from PermaNet 3.0 (representing PBO nets), Interceptor G2 (representing chlorfenapyr nets), and PermaNet 2.0 (representing pyrethroid-only net). The alleles of resistance markers were genotyped in a subset of these treatments to determine the impact of pyrethroid resistance on the performance of the bednets. The following phenotypes were included: the dead, alive, blood-fed, and unfed mosquitoes in the veranda, inside the net and in the room. However, only two LLIN performance parameters were considered; mortality and blood feeding status, since enough samples could be obtained. The association between the resistance and the performance of each net was assessed using ‘vcd’53and ‘epitools’54 packages in R software, to calculate the Fisher’s exact probability test using a contingency table.
Data analysis
The number of mosquitoes collected in each hut with different treatments were assessed using negative binomial regression. The proportions of entomological outcomes assessing performance of bednets such as blood feeding, exophily, mortality, for a given experimental hut treatment were assessed using binomial generalized linear mixed models (GLMMs) with a logit link function, fitted using the ‘lme4’ package55 for R software (version 4.2.2). An independent model was fitted for each entomological outcome and mosquito species. To complement the fixed effect of each treatment, random effects were included in each model to account for these sources of variation; between the six huts, between the six sleepers, between the six weeks of the trial. Hut trial data was analysed using experimental hut trial data analysis pipeline developed by Challenger et al47. Finally, for the Hut effect test, the one-way analysis of variance (ANOVA) was used to determine if there was a significant difference in the means of mosquitoes collected from the six huts. For the statistical analyses in this study, level of significance (alpha) was set at a cut-off value of 0.05 and correction was done for multiple testing. The relationship between bednet performance and resistance marker genotypes was evaluated by conducting a phenotype-genotype association analysis, examining mortality and blood feeding rates using Unconditional Maximum Likelihood Estimation (MLE) and Wald normal approximation method in R software using packages ‘epitools’54 and ‘oddsratio’56.
Results
Hut effect test and overall mosquito abundance
During the Hut Effect Test, a total of 740 mosquitoes were collected from the six huts. Out of the 740 mosquitoes, 253 (34.2%) were An. funestus females, 85 (11.5%) were An. gambiae females, 81 (11.0%) were Anopheles males of any species but ignored, 311 (42.0%) were Culex species and 10 (3.3%) were Mansonia species (Fig. 2A). Culex species were considered in the analysis for being a significant biting nuisance in this area that grows rice which can also transmit lymphatic filariasis in Uganda. There was no significant difference in the number of mosquitoes collected in the huts during the effect test (ANOVA, DF = 17, F-statistic = 0.7112, p = 0.6267) but there was a significant difference in number of mosquitoes collected per week (p = 0.00917). Focusing on the malaria vectors, mosquito average collections per hut were highest with An. funestus compared to An. gambiae (Fig. 2B and 2C). The blood-feeding rate differed significantly between the malaria vector species (z = 5.9005, p < 0.00001), with the highest rate observed in An. funestus, where 79.4% of mosquitoes successfully fed, compared to 45.9% in An. gambiae (Fig. 2D). During the hut trial, a total of 3068 mosquitoes were collected. Out of the total collections, 1326 (43.2%) were An. funestus, 314 (10.2%) were An. gambiae, 731 (23.8%) were Anopheles males, 690 (22.5%) were Culex species and 7 (0.2%) were Mansonia species (Tables 2, 3 and 4). During the trial, there was an overall increase in the mosquito collections as the trial progressed with more mosquitoes collected from Day 21 to Day 36 compared to the earlier days for both An. funestus (Fig. 2B) and An. gambiae (Fig. 2C).
Performance of bednets against An. funestus mosquitoes
In the 36-night experimental hut trial, Interceptor G2 emerged as a preeminent mosquito control tool, exhibiting efficacy across multiple metrics (Fig. 3). It achieved the highest overall mortality at 70.6% and a killing effect of 23.0% (Table 2), with 43% of killed mosquitoes being unfed and 27.0% blood fed (Fig. 3A), outperforming the other next-generation bednets; Royal Guard (37.6%), PermaNet 3.0 (31.0%) and Olyset (36.1%), and pyrethroid-only net, Interceptor (25.6%). The highest proportion of successfully blood fed and alive mosquitoes were observed in Interceptor (43.0%) and lowest interceptor G2 (12.0%) (Fig. 3A). Interceptor G2 also excelled in deterrence (48.4%) although this was within similar range as PermaNet 3.0 (45.5%) and Olyset Plus (51.6%) but slightly higher than Interceptor (38.5%) and Royal Guard (29.4%) (Table 2). However, the PBO net, Olyset Plus, proved to be most adept at thwarting blood-feeding, with only 23.5% of mosquitoes being able to blood feed hence inhibiting blood feeding by 67.9%. If we consider deterrence rates, which was highest in Olyset Plus, the blood feeding rate was even lowered further to 11.0% compared to the other nets; Royal Guard (32.0%), PermaNet 3.0 (21.0%) Interceptor (29.0%) and Interceptor G2 (20.0%). Overall, blood feeding rates were significantly lowered by action of deterrence in most of the bednets (Fig. 3B). Subsequently, Olyset plus delivered the highest personal protection (84.46%) compared to PermaNet 3.0 (71.71%) – with a similar protection rate as Interceptor G2 (72.51%), while personal protection was relatively lower in Interceptor (60.16%) and Royal Guard (55.78%) (Table 2). The daily mortality estimates with tight confidence intervals show that interceptor G2 produced less daily variability compared to the other bednets (Supplementary Fig. 1), underscoring its reliability as a control tool for resistant An. funestus populations.
Data from the experimental hut trial, showing nightly variability in mosquito mortality, blood-feeding, and numbers caught for An. funestus. (A) Breakdown of mosquito numbers in the control arm (untreated net), Interceptor, Interceptor G2, Royal Guard, PermaNet 3.0 and Olyset Plus LLINs over the course of the trial. The height of each bar indicates the total number of mosquitoes entering the hut each night. Bar colour denotes the mosquito mortality at 24 and 72 h and blood-feeding status at 24 h following collection (see the legend in panel A (Olyset Plus) or panel B for the description of the mosquito status). (B) Summary measures over the whole trial for the outcome of a single feeding event by a blood-feeding mosquito: being deterred from entering the hut (grey shading, calculated by the difference in the number of mosquitoes caught in the control arm relative to treated nets), mosquitoes being alive and unfed, unfed and dead, fed and dead or successfully blood-fed and alive (green, note the percentage is in the second plot is different from pie charts in panel due to the actions of deterrence).
Performance of bednets against An. gambiae mosquitoes
Similarly, as reported against An. funestus mosquitoes, Interceptor G2 also exhibited high efficacy across multiple metrics against An. gambiae mosquitoes (Fig. 4). It equally achieved the highest overall mortality at 63.2% but relatively lower killing effect of 16.0% (Table 3), with 42.0% of killed mosquitoes being unfed and 21.0% blood fed (Fig. 4A). Unlike with An. funestus, Interceptor G2 did not outperform other new generational bednets since mortality was within similar range for Royal Guard; 51.6% (39.17–64.05), PermaNet; 61.4% (46.98–75.75) and Olyset; 50.0% (34.50–65.50), but did outperform pyrethroid-only net, Interceptor; 25.0% (12.21–37.79). Similar to findings observed with An. funestus, the highest proportion of successfully blood fed and alive mosquitoes were observed in Interceptor (43.0%) but the lowest proportion was with Olyset Plus (0.0%) instead of Interceptor G2 (Fig. 4A). Further contrasts were observed in deterrence rates where except for Royal Guard (27.9%), Interceptor, Interceptor G2, PermaNet 3.0 and Olyset Plus all produced similar rates (within 47–56%). (Table 3). Olyset Plus still significantly proved to be most adept at thwarting blood-feeding, with only 7.5% of mosquitoes being able to blood feed giving a blood feeding inhibition rate of 88.06%. Considering deterrence rates, which was highest in Interceptor G2, the blood feeding rate was lowest in Olyset Plus (3.0%), followed by Royal Guard (15.0%), PermaNet 3.0 (15.0%), Interceptor G2 (20.0%) and Interceptor (23.0%) (Fig. 4B). Overall, blood feeding rates were significantly lowered by action of deterrence in An. gambiae compared to An. funestus for all the bednets, except Interceptor G2. Again, Olyset Plus delivered the highest personal protection with An. gambiae (94.44%) compared to PermaNet 3.0 (75.93%) – with a similar protection rate as Royal Guard (75.93%), while personal protection was relatively lower in Interceptor G2 (68.52%) and Interceptor (62.96%) (Table 3). The daily mortality estimates with tight confidence intervals show that the most of nets produced less daily variability (Supplementary Fig. 2), underscoring its reliability in as a control tool for resistant An. gambiae populations.
Data from the experimental hut trial, showing nightly variability in mosquito mortality, blood-feeding, and numbers caught for An. gambiae. (A) Breakdown of mosquito numbers in the control arm (untreated net), Interceptor, Interceptor G2, Royal Guard, PermaNet 3.0 and Olyset Plus LLINs over the course of the trial. The height of each bar indicates the total number of mosquitoes entering the hut each night. Bar colour denotes the mosquito mortality at 24 and 72 h and blood-feeding status at 24 h following collection (see the legend in panel A (Olyset Plus) or panel B for the description of the mosquito status). (B) Summary measures over the whole trial for the outcome of a single feeding event by a blood-feeding mosquito: being deterred from entering the hut (grey shading, calculated by the difference in the number of mosquitoes caught in the control arm relative to treated nets), mosquitoes being alive and unfed, unfed and dead, fed and dead or successfully blood-fed and alive (green, note the percentage is in the second plot is different from pie charts in panel due to the actions of deterrence).
Performance of bednets against Culex mosquito species
Against Culex mosquitoes, deterrence rate was higher with Interceptor at 50.3%, surpassing next-generation bednets, which clustered around a similar range; Interceptor G2 (39.9%), Royal Guard (37.0%), PermaNet 3.0 (35.8%), and Olyset Plus (38.2%). Exophily rates followed a comparable pattern, but only Interceptor (61.6%), PermaNet 3.0 (58.6%) and Olyset Plus (57.9%) showed statistically significant exit rates compared to the control.
All bednets except Royal Guard yielded significant mortality rates relative to the control. Interceptor had the highest mortality at 40.7% (30.31–51.08), followed closely by Interceptor G2 (37.5%; 28.20 – 46.80), PermaNet 3.0 (38.7%; 26.68–47.80) and Olyset Plus (36.4%; 27.33–45.57). Overall, mortality rates were marginally lower than observed with An. gambiae and An. funestus malaria vectors.
For personal protection, PBO nets excelled again, with PermaNet 3.0 (87.69%) and Olyset Plus (86.15%) showing the highest rates, though the difference was not significant. Blood-feeding inhibition mirrored this trend. Despite its high mortality, Interceptor recorded the lowest personal protection (80.0%) and blood feeding inhibition (59.77%). Generally, personal protection rates across all bednets were comparable and averaged higher than those in malaria vectors (Table 4).
Superiority test for mortality, blood feeding and exit rates in Anopheles vectors
Mosquito mortality rates for An. funestus (Fig. 5A, Supplementary Fig. 3A) and An. gambiae (Fig. 5B, Supplementary Fig. 3B) revealed that Interceptor G2 is significantly superior to the standard pyrethroid-only net, achieving the highest mortality rates in both populations of highly resistant vectors but with higher superiority in An. funestus [OR = 18.7 (8.05–46.8), P < 0.001] than An. gambiae [OR = 6.57 (2.13–24.24), P = 0.002]. Similarly, except for PermaNet 3.0, Royal Guard [OR = 2.79 (1.29–6.2), P = 0.01] and Olyset Plus [OR = 2.72 (1.22–6.22), P = 0.01] also showed superiority against pyrethroid-only net in resistant An. funestus populations, but this was weaker than observed with Interceptor G2. In resistant An. gambiae populations, all new-generation bednets demonstrated comparable superiority to Interceptor G2, with PermaNet 3.0 showing the closest performance [OR = 5.69 (1.91–19.76), P = 0.003], followed by Olyset Plus [OR = 3.45 (1.16–11.46), P = 0.03], and Royal Guard [OR = 3.64 (1.34–11.03), P = 0.014].
Odds ratio calculation during superiority assessment. The plots show estimates of mosquito mortality after 24 h or 72 (A, B), blood feeding rates (C, D) and exit rates (E, F) in the experimental hut trial (A, C and E are for An. funestus while B, D, F, are for An. gambiae). The odds ratio graph showing points (with 95% confidence interval estimates on the horizontal lines) of treated LLINs and untreated control net (grey). Overall estimate of the LLIN mortality, blood feeding rates and exit rates is shown in blue if it is above OR = 1.0 or red if it is below OR = 1.0, compared to pyrethroid-only Interceptor net, along with their confidence intervals. Grey dotted horizontal line is OR = 1.0 (cut-off level).
Blood feeding rates for An. funestus and An. gambiae (Fig. 5C & 5D, Supplementary Fig. 3C & 3D) were marginally different. In An. funestus, the assessment revealed only PBO nets were significantly superior to the standard pyrethroid-only net, achieving the lowest blood feeding rates for Olyset Plus [OR = 0.35 (0.22–0.55), P < 0.001] while PermaNet 3.0 was borderline [OR = 0.65 (0.43–0.99), P = 0.0475]. In An. gambiae, only Olyset Plus was again significantly superior at levels higher than observed in An. funestus [OR = 0.1 (0.02–0.35), P < 0.001], as well as Royal Guard [OR = 0.33 (0.14–0.8), P = 0.0142]. Despite the superior mortality rate, Interceptor G2 was not superior over standard nets.
In assessing superiority looking at exit rates (Fig. 5E & 5F, Supplementary Fig. 3E & 3F), all the next-generation nets, except for Interceptor G2, were superior to the standard pyrethroid-only net, achieving significant high exit rates in An. funestus population [Royal Guard; OR = 1.95 (1.2–3.19), P = 0.0068, Olyset Plus; OR = 2.51 (1.51–4.27), P = 0.0005 and PermaNet 3.0; OR = 1.87 (1.12–3.14), P = 0.0154]. However, no bednet was superior to standard nets in populations of An. gambiae.
Generally, in highly resistant populations of An. funestus and An. gambiae, Interceptor G2 outperformed a standard pyrethroid-only in just one measure of bednet performance. In contrast, Olyset Plus demonstrated superiority in three performance metrics against a standard net in both vectors. For An. funestus populations, Royal Guard and PermaNet 3.0 each excelled in two performance metrics. In An. gambiae populations, besides Olyset Plus, only Royal Guard surpassed the standard net in two metrics, while PermaNet 3.0 and Interceptor G2 each were superior in only one metric.
Impact of resistance markers on efficacy of bednets
The impact of resistance or its escalation was assessed using two markers; 4.3 Kb-SV and G454A-Cyp9K1 markers point mutations. The efficacy of the three different types of bednets were evaluated using two critical entomological parameters: mortality and blood feeding rates.
The impact of the 4.3 Kb-SV and G454A-Cyp9K1 markers were vividly evident. In terms of mortality (Supplementary Fig. 1), there was a significant association between homozygous mutant alleles (RR) for the 4.3 Kb-SV marker and survival to only PermaNet 2.0 exposure [OR = 13.0 (1.27 – 133.29), P = 0.0221)], with PermaNet 3.0 being marginal. Generally, up to 68.4% of the mosquitoes with RR genotype for 4.3 Kb-SV marker survived exposure to PermaNet 2.0 compared to 42.9% and 50.0% for PermaNet 3.0 and Interceptor G2, respectively (Fig. 6 a-f). Contrastingly, the G454A-Cyp9K1 marker was only significantly associated with PermaNet 3.0 survival but in the heterozygote form (RS) since RR genotype was not detected in the hybrids [OR = 5.23 (1.22 – 22.45), P = 0.0235)]. In mosquitoes exposed to PermaNet 3.0, 48.0% having RS genotype for the G454A-Cyp9K1 marker survived exposure compared to only 15.0% homozygous wild-type genotype (SS), while there was no significant difference in survival with mosquitoes exposed to PermaNet 2.0 and Interceptor G2 (Fig. 6 g-l).
Impact of resistance markers on bednet-induced mortality. Panels (a, c, e) and (g, i, k) are stacked bars that illustrate the distribution of 4.3 Kb-SV and G454A-Cyp9K1 genotypes, respectively, among mosquitoes that either died or survived after exposure to PermaNet 2.0, PermaNet 3.0 and Interceptor G2 LLINs. Panels (b, d, f) and (h, j, l) are line plots that depict the association between frequency of 4.3 Kb-SV and 454A-Cyp9K1 R and S alleles, respectively, and the mosquitoes’ ability to survive exposure to these LLINs. The data reveal distinct trends: mosquitoes with RR genotype for 4.3 Kb-SV and RS genotype for 454A-Cyp9K1 markers exhibit increased survival against PermaNet 2.0 compared to SS genotypes (4.3 Kb-SV RR Alive = 68.42% vs. SS Alive = 14.29% and 454A-Cyp9K1 RS Alive = 58.62% vs. SS Alive = 46.43%), with only 4.3 Kb-SV being significant. For PermaNet 3.0, only the RS genotype of 454A-Cyp9K1 marker is significantly linked to higher survival rates (RS Alive = 48.0% vs. SS Alive = 15.0%). In contrast, RR genotype for 4.3 Kb-SV is relatively higher in dead than alive mosquitoes exposed to PermaNet 3.0 (Alive = 42.86%, Dead = 57.14%) and balanced in Interceptor G2 (Alive/Dead = 50.0%). The numbers in the brackets are the total number of mosquitoes screened.
The 4.3 Kb-SV marker was significantly associated with blood-feeding success on PermaNet 2.0, with both RR (P = 0. 0.00261) and RS (P = 0.0025) genotypes showing higher feeding rates than SS. At the allele level, the resistance allele (R) increased feeding odds [OR = 2.5, 95% CI:1.16–5.41; P = 0.0211] (Supplementary Table 1). Strikingly, no RR genotypes for the 4.3 Kb-SV marker were detected among unfed mosquitoes exposed to PermaNet 2.0, contrasting with PermaNet 3.0 (4.3 Kb-SV Unfed RR = 88.9%) and Interceptor G2 (4.3 Kb-SV Unfed RR = 80.0%) – though these differences were non-significant (Fig. 7).
Impact of resistance markers on bednet-induced blood feeding. Panels (a, c, e) and (g, i, k) are stacked bars that illustrate the distribution of 4.3 Kb-SV and G454A-Cyp9K1 genotypes, respectively, among mosquitoes that either blood fed or failed to blood feed after exposure to PermaNet 2.0, PermaNet 3.0 and Interceptor G2 LLINs. Panels (b, d, f) and (h, j, l) are line plots that depict the association between frequency of 4.3 Kb-SV and 454A-Cyp9K1 R and S alleles, respectively, and the mosquitoes’ ability to blood feed in the presence of these LLINs. The data reveals RR genotype can increase chances of mosquitoes with 4.3 Kb-SV to blood feed in presence of the LLINs and this was true and significant only for PermaNet 2.0 (4.3 Kb-SV RR Fed = 68.42% vs. SS Fed = 0.0%) but not with 454A-Cyp9K1 marker or other net types. The numbers in the brackets are the total number of mosquitoes screened.
The combined effect of both markers showed that possessing RR/RS genotype had an additive advantage to surviving exposure to both PermaNet 2.0 (P = 0.0256) and PermaNet 3.0 (P = 0.05) but not Interceptor G2 (Supplementary Table 2). Generally, the survival rate was higher in individuals that possessed RR/RS, RS/RS, RR/SS genotypes compared to SS/SS genotypes (Supplementary Table 2, Fig. 8 a, b, c, d, e, f). Similarly, individuals possessing RR/RS genotypes had an added advantage to successfully take a blood meal against only PermaNet 2.0 (P = 0.011) and not the other type of bednets (Supplementary Table 3, Fig. 8 g, h, I, j, k, l).
Combined Impact of resistance markers on bednet-induced mortality and blood feeding. Panels (a, c, e) and (g, i, k) are stacked bars that illustrate the distribution of combined genotypes of both markers, evaluated in terms of mortality and blood feeding success, respectively, after exposure to PermaNet 2.0, PermaNet 3.0 and Interceptor G2 LLINs. Panels (b, d, f) and (h, j, l) are line plots that depict the association between frequency of R and S alleles from the combination of both markers with mortality and blood feeding success, respectively. The results indicate that mosquitoes with RR/RS genotype combination exhibit a modestly increased likelihood of survival, which was statistically significant when compared to only SS/SS genotypes in mosquitoes exposed to PermaNet 2.0 (RS/RR Alive = 72.7% vs. SS/SS Alive = 0.0%) and PermaNet 3.0 (RS/RR Alive = 53.8 vs. SS/SS Alive = 0.0%). Additionally, this RR/RS genotype combination significantly enhances blood-feeding success against PermaNet 2.0 compared to SS/SS genotypes (RS/RR Fed = 81.8% vs. SS/SS Fed = 0.0%).) and, to a lesser but opposite extent (not significant), for Interceptor G2 (RS/RR Fed = 62.5% vs. SS/SS Fed = 100.0%). The numbers in the brackets are the total number of mosquitoes screened.
Discussion
The deployment of long-lasting insecticidal nets (LLINs) represents a cornerstone in malaria control efforts across endemic regions. With over 20 distinct LLIN types currently available from various manufacturers and distributed globally57, their effectiveness is increasingly challenged by multiple factors, notably the rapid escalation of insecticide resistance in sub-Saharan Africa. To address this, our study evaluated the efficacy of major prequalified LLINs against highly resistant populations of An. funestus and An. gambiae in Uganda, aligning with the country’s recent large-scale LLIN distribution efforts. These investigations are critical to understanding the practical impact of next-generation bednets in regions grappling with intense resistance pressures.
Efficacy of PBO LLINs against highly resistant malaria vectors
This study revealed significant variation in the efficacy of long-lasting insecticidal nets (LLINs) between An. funestus and An. gambiae populations in Uganda. PermaNet 3.0 and Olyset Plus elicited diminished mortality in An. funestus compared to An. gambiae. PermaNet 3.0 generally surpassed Olyset Plus in terms of mortality, except for An. funestus, where the difference was less pronounced. Conversely, Olyset Plus exhibited marginally superior blood-feeding inhibition in both species, surpassing pyrethroid-only bednets and PermaNet 3.0, likely attributable to permethrin’s potent excito-repellent attributes relative to deltamethrin or alpha-cypermethrin58. The observed elevated deterrence and exophily rates with Olyset Plus underscore its repellent prowess against resistant vectors.
Experimental hut trials yielded consistently lower mortality than WHO cone assays15,24,37, suggesting that tarsal contact mortality alone cannot encapsulate field-relevant outcomes. Behavioural circumvention in resistant mosquitoes, provoked by PBO nets’ irritancy59, likely modulates probing and biting behaviours in natural environs. The reduced PBO nets’ efficacy against An. funestus in Uganda, also observed in other geographical settings5,18, likely stems from its aggravated pyrethroid resistance status compared to An. gambiae37,60.
While experimental hut trials offer critical insights, low mortality rates should not be viewed as a definitive measure of poor LLIN performance in community settings. Instead, they highlight the broader impact of insecticide resistance on LLIN efficacy, as demonstrated in previous studies5,38,61. Despite the reduced mortality, PBO LLINs conferred substantial personal protection, particularly against An. gambiae. Cluster-randomised trials in Uganda and Tanzania corroborated PBO nets’ superiority over pyrethroid-only nets in both clinical and entomological metrics9,12,13,62. However, in Uganda, An. funestus density was largely unaffected and surged two years post-intervention, contrasting with a decline in An. gambiae numbers35. This trend mirrors a review by Msugupakulya et al. that showed the ascendancy of An. funestus as a dominant vector in eastern and southern Africa from 2010 to 2022 coinciding with large-scale distribution of LLINs63.
Our study indicates that PBO LLINs may have limited efficacy in areas dominated by highly resistant An. funestus compared to An. gambiae-prevalent areas. These outcomes can however be affected by resistance dynamics, underlying mechanisms, and net usage patterns. Although our study did not include PermaNet 2.0 or Olyset 2.0 for direct comparison with PBO nets, we evaluated Interceptor, a pyrethroid-only LLIN. As expected, Interceptor underperformed relative to PBO nets, reinforcing the superior efficacy of PBO nets, and aligning with findings from Tanzania27. Notably, Interceptor’s performance appears to have declined since 2006, when mosquito populations were more susceptible to pyrethroids64, highlighting the impact of escalating resistance on the effectiveness of pyrethroid-only LLINs.
Efficacy of recently prequalified new generation dual ai LLINs against highly resistant mosquitoes, with focus on chlorfenapyr-based nets
Our study revealed that Interceptor G2 net achieved superior mortality against both An. funestus and An. gambiae in Uganda, with marked efficacy against highly resistant An. funestus. These findings align with other semi-field trials reporting high mortality for Interceptor G2 against both vectors24,25,26,27. African malaria vectors are largely susceptible to chlorfenapyr, though resistance was noted in An. gambiae from Cameroon, not yet in Uganda by 202142. Interceptor G2 exhibited lower blood-feeding inhibition and personal protection than pyrethroid-only or PBO nets, with greater efficacy against An. funestus than An. gambiae, likely due to chlorfenapyr negligible excito-repellent properties and alpha-cypermethrin reduced repellence compared to permethrin in Olyset Plus and to deltamethrin in PermaNet 3.058.
Interceptor G2 proved highly effective against resistant An. funestus, positioning it as the preferred LLIN in An. funestus-dominant regions. Against An. gambiae, its performance was comparable to PBO nets, only modestly surpassing pyrethroid-only nets. Cluster-randomised trials in Tanzania and Benin reported high efficacy of Interceptor G2 nets leading to reduced malaria infections62,65. Although low-to-moderate pyrethroid resistance was reported in the Tanzanian and Benin studies compared to an area like Mayuge with resistance escalation, the predominance of cytochrome P450-based mechanisms in malaria vectors especially An. funestus66,67,68,69,70, explains the high efficacy of chlorfenapyr nets in this vector compared to An. gambiae where resistance is driven by both metabolic and target-site mechanisms71,72. Nonetheless, modelling suggests pyrethroid-pyrrole LLINs could avert 65–75% of malaria cases, varying by epidemiological context73. The 2023 Interceptor G2 and PermaNet Dual LLIN distribution in Uganda particularly in high-resistance areas, awaits evaluation.
Our evaluation of Royal Guard, an LLIN combining PPF and pyrethroids, revealed no effect of PPF on oviposition rates (Supplementary Fig. 4) but revealed species-specific performance. Against An. funestus, Royal Guard outperformed the pyrethroid-only Interceptor in mortality and exit rates but not in blood-feeding inhibition or personal protection. In An. gambiae, it surpassed pyrethroid-only nets in mortality and blood-feeding inhibition, matching PBO nets and exceeding Olyset Plus in personal protection. These results align with a Ugandan trial showing comparable malaria outcomes for Royal Guard and PBO nets in An. gambiae-dominant areas28,30,35. Royal Guard was more effective against resistant An. gambiae than An. funestus, surpassing performance in Tanzania27, Benin74, and Cameroon24. These interspecies and geographic disparities likely reflect variations in resistance mechanisms, particularly the overexpression cytochrome P450s, with PPF and pyrethroids metabolised by shared cytochrome P450s75. This overlap may lead to synergistic or antagonistic effects across strains, species and regions since resistance mechanisms are geographically distinct72,76,77.
Impact of resistance escalation on performance of bednets
Numerous studies have explored the impact of insecticide resistance on bednet efficacy. Currently, the primary approach involves using diagnostic resistance markers associated with resistance genes as proxies to assess this impact. However, recent advancements in whole-genome sequencing78 are paving the way for more comprehensive methods to investigate resistance mechanisms and their effects on vector control tools.
In this study, both the 4.3 Kb-SV and G454A-CYP9K1 markers showed an association with survival to pyrethroid-only and PBO bednet respectively. Only the 4.3 Kb-SV marker showed a significant association with blood feeding. In Cameroon where both markers have spread to and were evaluated for the first time, similar results were obtained with 4.3 Kb-SV significantly associating with pyrethroid-only bednets52 and G454A-CYP9K1 associating with survival to only PBO nets but not Royal sentry (an alpha-cypermethrin-only) bednet in experimental hut trials51. Furthermore, we show for the first time that the combination of both markers significantly enhances mosquitoes’ ability to survive both pyrethroid-only and PBO nets but not chlorfenapyr nets, highlighting the impact of resistance escalation on vector control tools.
The 4.3 Kb-SV marker, first identified in eastern Uganda in 2014 and already fixed by that time52, was likely under strong selection from earlier interventions like standard LLINs and IRS between 2003 and 201479,80. This historical context may explain the absence of a clear association with survival hence cross-resistance with the newer bednets introduced post-2014. Furthermore, and consistent with prior studies5,38,50,61,81, we observed a significant association between the 4.3 Kb-SV marker and blood-feeding rates, suggesting a potential connection to probing and biting behaviours, a critical adaptation influencing vectorial capacity. In contrast, the selection of the G454A-Cyp9K1 marker was likely exacerbated by the distribution of next-generational PBO bednets compared to pyrethroid-only bednets, given its increase in frequency post 201451, reinforcing the role of metabolic resistance in diminishing the efficacy of bednets.
We show for the first time that when both markers are present, their combined effect significantly boosts mosquito survival against PBO and pyrethroid-only bednets, with a more pronounced impact on pyrethroid-only nets. This synergy likely reflects complementary resistance mechanisms responding to mixed selection pressures, explaining the persistence of An. funestus populations despite deployment of earlier next-generation bednets63. Our results indicate that the molecular basis of these resistance mechanisms further elucidates the impact of resistance escalation to vector control. For instance, the 4.3 Kb-SV marker is associated with the overexpression of CYP6P9A and CYP6P9b genes52, which have been linked to intense pyrethroid resistance36,37. Similarly, the CYP9K1 gene has also been shown to be involved in resistance escalation36,37 and the G454A-Cyp9K1 marker confers this resistance by accelerating the metabolism of pyrethroids, rather than increasing enzyme overexpression51.
The absence of a clear impact of both markers on Interceptor G2 bednet indicates that they are specific to pyrethroid resistance, with no impact on the chlorfenapyr which has a distinct mode of action. Chlorfenapyr distinct mode action likely masks the killing effect of pyrethroids in the dual ai Interceptor G2 and PermaNet Dual nets. In An. funestus, resistance is predominantly driven by overexpression of cytochrome P450 enzymes66,67,69,70,76,82,83,84, which are induced by pyrethroids that may in turn activate chlorfenapyr, thereby enhancing its toxicity. Consequently, pyrethroid resistance escalation may not compromise Interceptor G2 and PermaNet Dual nets, explaining the high performance of these nets against An. funestus populations in this study.
Efficacy LLINs against Culex mosquitoes
Our study also assessed the performance of LLINs against Culex species in Uganda, a vector often overlooked in experimental hut trials due to its limited role in major vector-borne disease transmission in sub-Saharan Africa. Mortality rates were low, like those observed for An. funestus, except for Interceptor G2, which exhibited higher efficacy. Most LLINs provided robust personal protection against Culex species, consistent with findings from Tanzania15. This highlights the efficacy of insecticide-treated nets (ITNs) in reducing Culex biting nuisance and potential pathogen transmission, including Japanese encephalitis (virus), lymphatic filariasis (parasite) and West Nile fever (virus)85. While viral disease risk is minimal in Uganda, Culex mosquitoes alongside Anopheles, may contribute to lymphatic filariasis transmission in Northern Uganda and Lake Kyoga basin86,87. However, mass drug administration effectively controls lymphatic filariasis88, rendering Culex mosquitoes of minor public health significance in Uganda. Continued surveillance remains essential in lymphatic filariasis-endemic regions to address potential emerging risks.
Limitations
The study had a few limitations. Firstly, in the An. gambiae data, the total number of mosquito collections were relatively low for the treatment arms (ranging from 1.1 to 1.7 mosquitoes per night). We did not break down the species composition of An. gambiae s.l. since resistance profiles and behaviour can be significantly different. Therefore, the An. gambiae data should be interpreted with caution. Secondly, in the genetic crossing experiment, the use of hybrid mosquitoes can have some genetic variability leading to unpredictable phenotypic plasticity such as altered fitness affecting survival and recapture rates, changes in probing and biting behaviours, altered genetic diversity affecting resistance-associated genes48,49,50,89. However, our genotype–phenotype results align closely with prior studies involving free-flying mosquitoes51,52, further confirming the validity of our findings. Thirdly, the experimental design of the study did not assess the impact of net washing which might not accurately reflect real-world conditions where nets are washed and used over an extended period. Evaluating LLINs in both unwashed and washed states is recommended by WHO guidelines and would have provided a more comprehensive understanding of their efficacy and durability in practical use. However, this would not have affected the final goal of the study, which was to examine the performance of new bednets, as washing typically diminishes the insecticidal effectiveness. Lastly, another limitation of this study is that Interceptor, the pyrethroid-only comparator, differs from PBO and PPF nets in both fabric type and pyrethroid component, making it a suboptimal comparator according to WHO guidelines for superiority trials. Consequently, the reported superiority should be interpreted with caution, although these differences are unlikely to have materially affected the outcomes, as previous experimental hut trials have shown little or no difference in performance among pyrethroid-only nets against resistant vector populations90.
Conclusion
Insecticide resistance has profoundly undermined the efficacy of LLINs in sub-Saharan Africa, with Uganda standing as a compelling case study. Our investigation unveiled striking disparities in LLIN performance across two major vector species, with Interceptor G2 net demonstrating superior efficacy especially in An. funestus, albeit showing reduced performance in An. gambiae. In contrast, PBO LLINs and Royal Guard exhibited commendable performance against An. gambiae but not An. funestus. The heterogeneity in LLIN performance underscores the urgency for precise vector mapping and a nuanced, possibly rotational, LLIN deployment strategy. Moreover, our findings reveal that resistance mechanisms in An. funestus significantly compromise the performance of pyrethroid-only and PBO nets, while Interceptor G2 net remain largely unaffected. Hence, novel resistance mechanisms could emerge from the selection pressure to counter chlorfenapyr-treated nets given their recent nationwide rollout in Uganda in 2023, necessitating vigilant resistance surveillance. Ultimately, these insights reinforce the imperative for adaptive, evidence-informed vector control strategies to safeguard the long-term gains in malaria control amidst an evolving insecticide resistance landscape.
Data availability
All datasets generated or analysed during this study are included in this published article and its supplementary files.
References
WHO. World Malaria Report 2022. (World Health Organisation, 2023).
Churcher, T. S., Lissenden, N., Griffin, J. T., Worrall, E. & Ranson, H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife 5, 1–26 (2016).
Farnham, A. W. The mode of action of piperonyl butoxide with reference to studying pesticide resistance. Piperonyl Butoxide https://doi.org/10.1016/b978-012286975-4/50014-0 (1999).
Snoeck, S. et al. The effect of insecticide synergist treatment on genome-wide gene expression in a polyphagous pest. Sci. Rep. 7, 13440 (2017).
Menze, B. D. et al. An experimental hut evaluation of PBO-based and pyrethroid-only nets against the malaria vector anopheles funestus reveals a loss of bed nets efficacy associated with GSTe2 metabolic resistance. Genes (Basel) 11, 143 (2020).
Ketoh, G. K. et al. Efficacy of two PBO long lasting insecticidal nets against natural populations of Anopheles gambiae s.l. in experimental huts, Kolokopé, Togo. PLoS ONE 13, e0192492 (2018).
Okia, M. et al. Bioefficacy of long-lasting insecticidal nets against pyrethroid-resistant populations of Anopheles gambiae s.s. from different malaria transmission zones in Uganda. Parasit. Vectors 6, 1–11 (2013).
Oruni, A. et al. Pyrethroid resistance and gene expression profile of a new resistant An. gambiae colony from Uganda reveals multiple resistance mechanisms and overexpression of Glutathione-S-Transferases linked to survival of PBO-pyrethroid combination. Wellcome Open Res. 9, 13 (2024).
Protopopoff, N. et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two factorial design trial. Lancet 391, 1577–1588 (2018).
WHO Global Malaria Programme. Achieving and maintaining universal coverage with long-lasting insecticidal nets for malaria control. Who 4 (2017).
Staedke, S. G. et al. LLIN Evaluation in Uganda Project (LLINEUP) - Impact of long-lasting insecticidal nets with, and without, piperonyl butoxide on malaria indicators in Uganda: Study protocol for a cluster-randomised trial. Trials 20, 1–13 (2019).
Maiteki-Sebuguzi, C. et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): Final results of a cluster-randomised trial embedded in a national distribution campaign. Lancet Infect. Dis. 23, 247–258 (2023).
Staedke, S. G. et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): A pragmatic, cluster-randomised trial embedded in a national LLIN distribution campaign. Lancet 395, 1292–1303 (2020).
Gleave, K., Lissenden, N., Chaplin, M., Choi, L. & Ranson, H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide- treated nets to prevent malaria in Africa. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD012776.pub3 (2021).
Tungu, P. et al. Evaluation of PermaNet 3.0 a deltamethrin-PBO combination net against Anopheles gambiae and pyrethroid resistant Culex quinquefasciatus mosquitoes: An experimental hut trial in Tanzania. Malar. J. 9, 21 (2010).
Koudou, B. G., Koffi, A. A. & MaloneHemingway, D. J. Efficacy of PermaNet® 2.0 and PermaNet® 3.0 against insecticide-resistant Anopheles gambiae in experimental huts in Côte d’Ivoire. Malar. J. 10, 172 (2011).
Pennetier, C. et al. Efficacy of Olyset® plus, a new long-lasting insecticidal net incorporating permethrin and piperonil-butoxide against multi-resistant malaria vectors. PLoS ONE 8, e75134 (2013).
Ngufor, C. et al. Comparative efficacy of two pyrethroid-piperonyl butoxide nets (Olyset Plus and PermaNet 3.0) against pyrethroid resistant malaria vectors: A non-inferiority assessment. Malar. J. 21, 20 (2022).
Akoton, R. et al. Experimental huts trial of the efficacy of pyrethroids/piperonyl butoxide (Pbo) net treatments for controlling multi-resistant populations of anopheles funestus s.s. in kpomè, Southern Benin. Wellcome Open Res. 3, 71 (2018).
WHO. Prequalified lists: vector control products (website). Geneva: World Health Organization 2021. World Health Organisation https://extranet.who.int/prequal/vector-control-products/prequalified-product-list# (2021).
Raghavendra, K. et al. Chlorfenapyr: A new insecticide with novel mode of action can control pyrethroid resistant malaria vectors. Malar. J. 10, 16 (2011).
Huang, P. et al. A comprehensive review of the current knowledge of chlorfenapyr: Synthesis, mode of action, resistance, and environmental toxicology. Molecules 28, 7673. https://doi.org/10.3390/molecules28227673 (2023).
Tchouakui, M. et al. Comparative study of the effect of solvents on the efficacy of neonicotinoid insecticides against malaria vector populations across Africa. Infect. Dis. Poverty 11, 23–31 (2022).
Tchouakui, M. et al. High efficacy of chlorfenapyr-based net Interceptor® G2 against pyrethroid-resistant malaria vectors from Cameroon. Infect. Dis. Poverty 12, 81 (2023).
Camara, S. et al. Efficacy of Interceptor® G2, a new long-lasting insecticidal net against wild pyrethroid-resistant Anopheles gambiae s.s. from Côte d’Ivoire: A semi-field trial. Parasite 25, 42 (2018).
Tungu, P. K., Michael, E., Sudi, W., Kisinza, W. W. & Rowland, M. Efficacy of interceptor® G2, a long-lasting insecticide mixture net treated with chlorfenapyr and alpha-cypermethrin against Anopheles funestus: Experimental hut trials in north-eastern Tanzania. Malar. J. 20, 180 (2021).
Martin, J. L. et al. Bio-efficacy of field aged novel class of long-lasting insecticidal nets, against pyrethroid-resistant malaria vectors in Tanzania: A series of experimental hut trials. MedRxiv https://doi.org/10.1101/2023.10.21.23297289 (2024).
Gonahasa, S. et al. LLIN Evaluation in Uganda Project (LLINEUP2) – Effect of long-lasting insecticidal nets (LLINs) treated with pyrethroid plus pyriproxyfen vs LLINs treated with pyrethroid plus piperonyl butoxide in Uganda: A cluster-randomised trial. PLOS Glob. Public Health 5, e0003558 (2025).
Ngufor, C. et al. Evaluating the attrition, fabric integrity and insecticidal durability of two dual active ingredient nets (Interceptor® G2 and Royal® Guard): Methodology for a prospective study embedded in a cluster randomized controlled trial in Benin. Malar. J. 22, 276 (2023).
Lynd, A. et al. LLIN Evaluation in Uganda Project (LLINEUP): A cross-sectional survey of species diversity and insecticide resistance in 48 districts of Uganda. Parasit. Vectors. 12, 94. https://doi.org/10.1186/s13071-019-3353-7 (2019).
Kamya, M. R. et al. Dramatic resurgence of malaria after 7 years of intensive vector control interventions in Eastern Uganda. PLOS Glob. Public Health 4, e0003254 (2024).
Krezanoski, P. et al. Adjusting vector surveillance for human behaviors reveals Anopheles funestus drove a resurgence in malaria despite IRS with clothianidin in Uganda. Sci. Rep. 15, 17728 (2025).
Mawejje, H. D. et al. Impact of seasonality and malaria control interventions on Anopheles density and species composition from three areas of Uganda with differing malaria endemicity. Malar. J. 20, 138 (2021).
Musiime, A. K. et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar. J. 18, 445 (2019).
Lynd, A. et al. LLIN Evaluation in Uganda Project (LLINEUP)–effects of a vector control trial on Plasmodium infection prevalence and genotypic markers of insecticide resistance in Anopheles vectors from 48 districts of Uganda. Sci. Rep. 14, 14488 (2024).
Oruni, A. et al. Temporal evolution of insecticide resistance and bionomics in Anopheles funestus, a key malaria vector in Uganda. Sci. Rep. 14, 32027 (2024).
Tchouakui, M. et al. Pyrethroid resistance aggravation in ugandan malaria vectors is reducing bednet efficacy. Pathogens 10, 415 (2021).
Weedall, G. D. et al. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Transl. Med. 11, eaat7386 (2019).
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes Second edition. (2016).
Ministry of Health, U. The republic of uganda ministry of health on the road to a Malaria-free Uganda - Second Universal Coverage Mosquito Net distribution Campaign offers hope to Uganda Table Of Contents. 1–20 (2018).
Assatse, T. et al. Anopheles funestus populations across africa are broadly susceptible to neonicotinoids but with signals of possible cross-resistance from the GSTe2 gene. Trop. Med. Infect. Dis. 8, 244 (2023).
Tchouakui, M. et al. Detection of a reduced susceptibility to chlorfenapyr in the malaria vector Anopheles gambiae contrasts with full susceptibility in Anopheles funestus across Africa. Sci. Rep. 13, 2363 (2023).
World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Mr4 1–30 ISBN 978 92 4 150515 4. (2013).
Morgan, J. C., Irving, H., Okedi, L. M., Steven, A. & Wondji, C. S. Pyrethroid resistance in an anopheles funestus population from uganda. PLoS ONE 5, e11872 (2010).
Gillies, M. T. & Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region). South African Inst. Med. Res. 55, 1–146 (1987).
WHO/CDS/GMP. Data Requirements and Protocol for Determining Non-Inferiority of Insecticide-Treated Net and Indoor Residual Spraying Products within an Established WHO Intervention Class Global Malaria Programme. http://www.who.int/malaria (2019).
Challenger, J. D. et al. Assessing the variability in experimental hut trials evaluating insecticide-treated nets against malaria vectors. Curr. Res. Parasitol. Vector-Borne Dis. 3, 100115 (2023).
Tchouakui, M. et al. Substrate promiscuity of key resistance P450s confers clothianidin resistance whilst increasing chlorfenapyr potency in malaria vectors. Cell Rep. 43, 114566 (2024).
Mugenzi, L. M. J. et al. The duplicated P450s CYP6P9a/b drive carbamates and pyrethroids cross-resistance in the major African malaria vector Anopheles funestus. PLoS Genet. 19, e1010678 (2023).
Mugenzi, L. M. J. et al. Cis-regulatory CYP6P9b P450 variants associated with loss of insecticide-treated bed net efficacy against Anopheles funestus. Nat. Commun. 10, 4652 (2019).
Djoko Tagne, C. S. et al. A single mutation G454A in the P450 CYP9K1 drives pyrethroid resistance in the major malaria vector Anopheles funestus reducing bed net efficacy. Genetics https://doi.org/10.1093/genetics/iyae181 (2024).
Mugenzi, L. M. J. et al. Association of a rapidly selected 4.3kb transposon-containing structural variation with a P450-based resistance to pyrethroids in the African malaria vector Anopheles funestus. PLoS Genet. 20, e1011344 (2024).
Meyer, D., Zeileis, A., Kurt, H., Gerber, F. & Friendly, M. Package ‘vcd’: visualizing categorical data. Repo. CRAN https://doi.org/10.32614/CRAN.package.vcd (2024).
Aragon, T. J., Fay, M. P., Wollschlaeger, D. & Omidpanah, A. ‘Epitools: Epidemiology Tools’: Tools for training and practicing epidemiologists including methods for two-way and multi-way contingency tables. Repo. CRAN https://doi.org/10.32614/CRAN.package.epitools (2020).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Schratz, P. oddsratio: Odds Ratio Calculation for GAM(M)s; GLM(M)s. Repository CRAN Preprint at https://doi.org/10.32614/CRAN.package.oddsratio (2025).
UNICEF. Long-Lasting Insecticidal Nets-Market and Supply Update October 2022. https://www.unicef.org/supply/media/13951/file/LLIN-Market-and-Supply-Update-October-2022.pdf (2022).
Achee, N. L., Sardelis, M. R., Dusfour, I., Chauhan, K. R. & Grieco, J. P. Characterization of spatial repellent, contact irritant, and toxicant chemical actions of standard vector control compounds1. J. Am. Mosq. Control Assoc. 25, 156–167 (2009).
Reid, E. et al. Behavioural responses of Anopheles gambiae to standard pyrethroid and PBO-treated bednets of different operational ages. Curr. Res. Parasitol. Vector-Borne Dis. 6, 100227 (2024).
Menze, B. D. et al. Marked aggravation of pyrethroid resistance in major malaria vectors in Malawi between 2014 and 2021 is partly linked with increased expression of P450 alleles. BMC Infect. Dis. 22, 660 (2022).
Menze, B. D. et al. Experimental hut trials reveal that CYP6P9a/b P450 alleles are reducing the efficacy of pyrethroid-only olyset net against the malaria vector anopheles funestus but PBO-based olyset plus net remains effective. Pathogens 11, 638 (2022).
Mosha, J. F. et al. Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania: A four-arm, cluster-randomised trial. Lancet 399, 1227–1241 (2022).
Msugupakulya, B. J. et al. Changes in contributions of different Anopheles vector species to malaria transmission in east and southern Africa from 2000 to 2022. Parasit. Vectors 16, 408. https://doi.org/10.1186/s13071-023-06019-1 (2023).
Malima, R. et al. Evaluation of the Long-Lasting Insecticidal Net Interceptor LN: Laboratory and Experimental Hut Studies against Anopheline and Culicine Mosquitoes in Northeastern Tanzania. http://www.parasitesandvectors.com/content/6/1/296 (2013).
Accrombessi, M. et al. Efficacy of pyriproxyfen-pyrethroid long-lasting insecticidal nets (LLINs) and chlorfenapyr-pyrethroid LLINs compared with pyrethroid-only LLINs for malaria control in Benin: A cluster-randomised, superiority trial. Lancet 401, 435–446 (2023).
Wondji, C. S. et al. Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res. 19, 452–459 (2009).
Wondji, C. S., Hearn, J., Irving, H., Wondji, M. J. & Weedall, G. RNAseq-based gene expression profiling of the Anopheles funestus pyrethroid-resistant strain FUMOZ highlights the predominant role of the duplicated CYP6P9a/b cytochrome P450s. G3: Genes Genomes Genet. 12, jkab352 (2022).
Riveron, J. M. et al. Genome-wide transcription and functional analyses reveal heterogeneous molecular mechanisms driving pyrethroids resistance in the major malaria vector Anopheles funestus across Africa. G3: Genes, Genomes, Genet. 7, 1819–1832 (2017).
Irving, H., Riveron, J. M., Ibrahim, S. S., Lobo, N. F. & Wondji, C. S. Positional cloning of rp2 QTL associates the P450 genes CYP6Z1, CYP6Z3 and CYP6M7 with pyrethroid resistance in the malaria vector Anopheles funestus. Heredity (Edinb) 109, 383–392 (2012).
Ibrahim, S. S., Ndula, M., Riveron, J. M., Irving, H. & Wondji, C. S. The P450 CYP6Z1 confers carbamate/pyrethroid cross-resistance in a major African malaria vector beside a novel carbamate-insensitive N485I acetylcholinesterase-1 mutation. Mol. Ecol. 25, 3436–3452 (2016).
Hancock, P. A., Ochomo, E. & Messenger, L. A. Genetic surveillance of insecticide resistance in African Anopheles populations to inform malaria vector control. Trends Parasitol. 40, 604–618. https://doi.org/10.1016/j.pt.2024.04.016 (2024).
Donnelly, M. J., Isaacs, A. T. & Weetman, D. Identification, validation, and application of molecular diagnostics for insecticide resistance in malaria vectors. Trends Parasitol. 32, 197–206 (2016).
Churcher, T. S. et al. The epidemiological benefit of pyrethroid–pyrrole insecticide treated nets against malaria: An individual-based malaria transmission dynamics modelling study. Lancet Glob. Health 12, e1973–e1983 (2024).
Ngufor, C., Agbevo, A., Fagbohoun, J., Fongnikin, A. & Rowland, M. Efficacy of Royal Guard, a new alpha-cypermethrin and pyriproxyfen treated mosquito net, against pyrethroid-resistant malaria vectors. Sci. Rep. 10, 12227 (2020).
Yunta, C. et al. Pyriproxyfen is metabolized by P450s associated with pyrethroid resistance in An. gambiae. Insect. Biochem. Mol. Biol. 78, 50–57 (2016).
Weedall, G. D. et al. An Africa-wide genomic evolution of insecticide resistance in the malaria vector Anopheles funestus involves selective sweeps, copy number variations, gene conversion and transposons. PLoS Genet. 16, e1008822 (2020).
Riveron, J. M. et al. Insecticide Resistance in Malaria Vectors: An Update at a Global Scale (In Towards malaria elimination-a leap forward, IntechOpen, 2018).
Nagi, S. C. et al. Targeted genomic surveillance of insecticide resistance in African malaria vectors. BioRxiv https://doi.org/10.1101/2025.02.14.637727 (2025).
Kigozi, R. et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS ONE 7, e42857 (2012).
Namuganga, J. F. et al. The impact of stopping and starting indoor residual spraying on malaria burden in Uganda. Nat. Commun. 12, 2635 (2021).
Mugenzi, L. M. J. et al. A 6.5-kb intergenic structural variation enhances P450-mediated resistance to pyrethroids in malaria vectors lowering bed net efficacy. Mol. Ecol. 29, 4395–4411 (2020).
Tatchou-Nebangwa, N. M. T. et al. Two highly selected mutations in the tandemly duplicated CYP6P4a and CYP6P4b genes drive pyrethroid resistance in Anopheles funestus in West Africa. BMC Biol. 22, 286 (2024).
Sandeu, M. M., Mulamba, C., Weedall, G. D. & Wondji, C. S. A differential expression of pyrethroid resistance genes in the malaria vector Anopheles funestus across Uganda is associated with patterns of gene flow. PLoS ONE 15, e0240743 (2020).
Hearn, J. et al. Multi-omics analysis identifies a CYP9K1 haplotype conferring pyrethroid resistance in the malaria vector Anopheles funestus in East Africa. Mol. Ecol. 31, 3642–3657 (2022).
WHO. WHO Factsheet (Vector-Borne Diseases). https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (2020).
Stensgaard, A. S. et al. Ecological Drivers of Mansonella perstans Infection in Uganda and Patterns of Co-endemicity with Lymphatic Filariasis and Malaria. PLoS Negl. Trop. Dis. 10, e0004319 (2016).
Stensgaard, A. S. et al. Bayesian geostatistical modelling of malaria and lymphatic filariasis infections in Uganda: Predictors of risk and geographical patterns of co-endemicity. Malar. J. 10, 298 (2011).
Odongo-Aginya, E. I. et al. Wuchereria bancrofti infection at four primary schools and surrounding communities with no previous blood surveys in northern Uganda: The prevalence after mass drug administrations and a report on suspected non-filarial endemic elephantiasis. Trop. Med. Health 45, 20 (2017).
Chan, W. Y., van Hoffmann, A. A. & Oppen, M. J. H. Hybridization as a conservation management tool. Conserv. Lett. 12, e12652. https://doi.org/10.1111/conl.12652 (2019).
Nash, R. K. et al. Systematic review of the entomological impact of insecticide-treated nets evaluated using experimental hut trials in Africa. Curr. Res. Parasitol. Vector-Borne Dis. 1, 100047. https://doi.org/10.1016/j.crpvbd.2021.100047 (2021).
Acknowledgements
We extend our heartfelt gratitude to the Village Health Teams (VHTs) and assistants in Mayuge district for their invaluable support in recruiting volunteers. We deeply appreciate the volunteers who participated in the hut trial and assisted with mosquito collection. We also express our sincere thanks to the technicians and administration at the Centre for Research in Infectious Diseases (CRID) in Cameroon for their contributions to mosquito rearing and laboratory work, and to the Uganda Virus Research Institute (UVRI) in Entebbe for their efforts in organizing field activities and preparing samples for shipping.
Funding
This study was funded by BMGF (INV-006003) and Wellcome Trust (217188/Z/19/Z).
Author information
Authors and Affiliations
Contributions
C.S.W. conceived and designed the research with inputs from B.D.M, M.T and J.K. A.O carried out the resarch, field work, sample processing, laboratory analysis, data entry, data analysis and writing the first draft of the manuscript. A.O was assisted by B.D.M and R.F.T in the field and V.B.N-F in the laboratory. M.T., J.H, J.K and C.S.W supervised the study and revision of the first draft of the manuscript. All authors contributed to the writing of the final draft of the manuscript. All authors read, revised and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The protocol to conduct this study was approved by The Uganda Virus Research Institute Research Ethics Committee (UVRI REC) (Ref: GC/127/833) and Uganda National Council for Science and Technology (UNCST) (HS2063ES). Prior to trials, written, informed and signed consents were obtained from the volunteers (sleepers). All the volunteers involved in the study were supervised, followed up and treated when showing signs and symptoms of malaria. All methods in this trial were performed in accordance with the relevant guidelines and regulations.
Consent for publication
All authors have consented to publication of this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oruni, A., Menze, B.D., Fotso-Toguem, Y.G. et al. Chlorfenapyr bednets effectively overcome pyrethroid resistance escalation in highly resistant Anopheles malaria vectors in Uganda. Sci Rep 16, 4292 (2026). https://doi.org/10.1038/s41598-025-34493-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-34493-3