Abstract
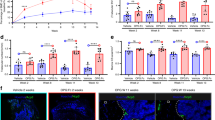
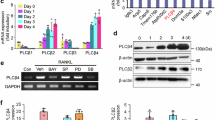
Osteocytes regulate bone remodeling by interacting with osteoblasts and osteoclasts. Hypoxia influences osteocyte function and has been linked to increased osteoclastogenesis in pathological conditions such as orthodontic tooth movement (OTM) and bone metabolic diseases; however, the molecular mechanisms underlying these effects remain unclear. This study aimed to identify hypoxia-responsive genes in osteocytes and investigate their effects on osteoclastogenesis. Transcriptome analysis of murine long bone osteocyte-Y4 (MLO-Y4) osteocytes cultured under hypoxia (2% O2) revealed that lipocalin-2 (Lcn2) was the most significantly upregulated gene. Real-time RT-PCR confirmed increased Lcn2 expression and an elevated Rankl/osteoprotegerin (Opg) ratio. Primary osteocytes were purified from DMP1-Topaz mice showed same hypoxic response. Functional analysis demonstrated that Lcn2 did not directly affect osteoclast precursors. However, it enhanced osteoclastogenesis via osteocytes in co-culture experiments. Western blot analysis demonstrated that LCN2 activated the MAPK signaling pathway in osteocytes. Furthermore, immunohistochemical analysis of hypoxic osteocytes on the compression side of OTM exhibited increased LCN2 expression. These findings suggest that LCN2 is upregulated in osteocytes under hypoxia and promotes osteoclastogenesis by increasing RANKL expression. This study provides new insights into the molecular mechanisms of bone resorption under hypoxic conditions and suggests Lcn2 as a potential therapeutic target for bone metabolic diseases.
Similar content being viewed by others
Data availability
The RNA-seq datasets generated and analyzed during this study are available in the NCBI Gene Expression Omnibus (GEO) under accession number GSE298138. All other data supporting the findings of this study are included in the article and its Supplementary Information files.
References
Bonewald, L. F. The amazing osteocyte. J. Bone Min. Res. 26 (2), 229–238. https://doi.org/10.1002/jbmr.320 (2011).
Marahleh, A., Kitaura, H., Ohori, F., Noguchi, T. & Mizoguchi, I. The osteocyte and its osteoclastogenic potential. Front. Endocrinol. (Lausanne). 14, 1121727. https://doi.org/10.3389/fendo.2023.1121727 (2023).
Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell … and more. Endocr. Rev. 34 (5), 658–690. https://doi.org/10.1210/er.2012-1026 (2013). Epub 2013 Apr 23.
Tatsumi, S. et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell. Metab. 5 (6), 464–475. https://doi.org/10.1016/j.cmet.2007.05.001 (2007).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17 (10), 1231–1234. https://doi.org/10.1038/nm.2452 (2011).
Xiong, J. & O’Brien, C. A. Osteocyte RANKL: new insights into the control of bone remodeling. J. Bone Min. Res. 27 (3), 499–505. https://doi.org/10.1002/jbmr.1547 (2012).
Robling, A. G. & Bonewald, L. F. The osteocyte: new insights. Annu. Rev. Physiol. 82, 485–506. https://doi.org/10.1146/annurev-physiol-021119-034332 (2020).
Xiong, J. et al. Osteocytes, not osteoblasts or lining Cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One. 10 (9), e0138189. https://doi.org/10.1371/journal.pone.0138189 (2015).
Gaber, T., Dziurla, R., Tripmacher, R., Burmester, G. R. & Buttgereit, F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann. Rheum. Dis. 64 (7), 971–980. https://doi.org/10.1136/ard.2004.031641 (2005). Epub 2005 Mar 30.
Pouyssegur, J. & López-Barneo, J. Hypoxia in health and disease. Mol. Aspects Med. 47–48, 1–2. https://doi.org/10.1016/j.mam.2016.02.001 (2016).
Dunst, J., Ahrens, S., Paulussen, M., Burdach, S. & Jürgens, H. Prognostic impact of tumor perfusion in MR-imaging studies in ewing tumors. Strahlenther Onkol. 177 (3), 153–159. https://doi.org/10.1007/s00066-001-0804-8 (2001).
Chen, W. et al. HIF-1α regulates bone homeostasis and Angiogenesis, participating in the occurrence of bone metabolic diseases. Cells 11 (22), 3552. https://doi.org/10.3390/cells11223552 (2022).
Usategui-Martín, R. et al. Molecular mechanisms involved in Hypoxia-Induced alterations in bone remodeling. Int. J. Mol. Sci. 23 (6), 3233. https://doi.org/10.3390/ijms23063233 (2022).
Zhu, J. et al. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J. Cell. Physiol. 234 (11), 21182–21192. https://doi.org/10.1002/jcp.28721 (2019). Epub 2019 Apr 29.
Villalvilla, A. et al. The adipokine lipocalin-2 in the context of the osteoarthritic osteochondral junction. Sci. Rep. 6 29243. https://doi.org/10.1038/srep29243 (2016).
Xiao, X., Yeoh, B. S. & Vijay-Kumar, M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu. Rev. Nutr. 37, 103–130. https://doi.org/10.1146/annurev-nutr-071816-064559 (2017). Epub 2017 Jun 19.
Abella, V. et al. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 20 (8), 565–571 (2015). Epub 2015 Dec 15.
Chu, S. T., Lin, H. J., Huang, H. L. & Chen, Y. H. The hydrophobic pocket of 24p3 protein from mouse uterine luminal fluid: fatty acid and retinol binding activity and predicted structural similarity to lipocalins. J. Pept. Res. 52 (5), 390–397. https://doi.org/10.1111/j.1399-3011.1998.tb00663.x (1998).
Devireddy, L. R., Gazin, C., Zhu, X. & Green, M. R. A cell-surface receptor for Lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123 (7), 1293–1305. https://doi.org/10.1016/j.cell.2005.10.027 (2005).
Rucci, N. et al. Lipocalin 2: a new mechanoresponding gene regulating bone homeostasis. J. Bone Min. Res. 30 (2), 357–368. https://doi.org/10.1002/jbmr.2341 (2015).
Capulli, M. et al. A complex role for Lipocalin 2 in bone metabolism: global ablation in mice induces osteopenia caused by an altered energy metabolism. J. Bone Min. Res. 33 (6), 1141–1153. https://doi.org/10.1002/jbmr.3406 (2018). Epub 2018 Mar 24.
Mera, P., Ferron, M. & Mosialou, I. Regulation of energy metabolism by Bone-Derived hormones. Cold Spring Harb Perspect. Med. 8 (6), a031666. https://doi.org/10.1101/cshperspect.a031666 (2018).
Arnett, T. R. et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. 196 (1), 2–8. https://doi.org/10.1002/jcp.10321 (2003).
Orriss, I. R. et al. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell. Physiol. 220 (1), 155–162. https://doi.org/10.1002/jcp.21745 (2009).
Sun, K. T. et al. MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation. Bone 73, 145 – 53. (2015).
Song, X. et al. HIF-1α induces hypoxic apoptosis of MLO-Y4 osteocytes via JNK/caspase-3 pathway and the apoptotic-osteocyte-mediated osteoclastogenesis in vitro. Tissue Cell. 67, 101402. https://doi.org/10.1016/j.tice.2020.101402 (2020). Epub 2020 Jun 29.
Niklas, A., Proff, P., Gosau, M. & Römer, P. The role of hypoxia in orthodontic tooth movement. Int. J. Dent. 2013, 841840. https://doi.org/10.1155/2013/841840 (2013). Epub 2013 Oct 21.
Costa, D. et al. Altered bone development and turnover in Transgenic mice over-expressing lipocalin-2 in bone. J. Cell. Physiol. 228 (11), 2210–2221. https://doi.org/10.1002/jcp.24391 (2013).
Ranjbar Taklimie, F. et al. Hypoxia induces Astrocyte-Derived Lipocalin-2 in ischemic stroke. Int. J. Mol. Sci. 20 (6), 1271. https://doi.org/10.3390/ijms20061271 (2019).
Xiong, H. et al. Up-regulation of miR-138 inhibits hypoxia-induced cardiomyocyte apoptosis via down-regulating lipocalin-2 expression. Exp. Biol. Med. (Maywood). 241 (1), 25–30 (2016). Epub 2015 Jun 30.
Metzger, C. E. & Narayanan, S. A. The role of osteocytes in inflammatory bone loss. Front. Endocrinol. (Lausanne). 10, 285. https://doi.org/10.3389/fendo.2019.00285 (2019).
Metzger, C. E., Gong, S., Aceves, M., Bloomfield, S. A. & Hook, M. A. Osteocytes reflect a pro-inflammatory state following spinal cord injury in a rodent model. Bone 120, 465–475. https://doi.org/10.1016/j.bone.2018.12.007 (2019). Epub 2018 Dec 11.
Ormsby, R. T. et al. Osteocytes respond to particles of clinically-relevant conventional and cross-linked polyethylene and metal alloys by up-regulation of resorptive and inflammatory pathways. Acta Biomater. 87, 296–306 (2019). Epub 2019 Jan 25.
Marahleh, A. et al. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front. Immunol. 10, 2925. https://doi.org/10.3389/fimmu.2019.02925 (2019).
Netzer, N. et al. Oxidative Stress Fat. Biomolecules ;5(2):1143–1150. doi: https://doi.org/10.3390/biom5021143. (2015).
Kim, H., Oh, B. & Park-Min, K. H. Regulation of osteoclast differentiation and activity by lipid metabolism. Cells 10 (1), 89. https://doi.org/10.3390/cells10010089 (2021).
Kong, X. et al. FATP2 regulates osteoclastogenesis by increasing lipid metabolism and ROS production. J. Bone Min. Res. 39 (6), 737–752. https://doi.org/10.1093/jbmr/zjae034 (2024).
Ye, D. et al. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J. Hepatol. 65 (5), 988–997. https://doi.org/10.1016/j.jhep.2016.05.041 (2016).
An, H. S. et al. Lipocalin-2 promotes acute lung inflammation and oxidative stress by enhancing macrophage iron accumulation. Int. J. Biol. Sci. 19 (4), 1163–1177. https://doi.org/10.7150/ijbs.79915 (2023).
Zhang, Y. et al. Lipocalin 2 expression and secretion is highly regulated by metabolic stress, cytokines, and nutrients in adipocytes. PLoS One. 9 (5), e96997. https://doi.org/10.1371/journal.pone.0096997 (2014).
Xu, G. et al. Lipocalin-2 induces cardiomyocyte apoptosis by increasing intracellular iron accumulation. J. Biol. Chem. 287 (7), 4808–4817. https://doi.org/10.1074/jbc.M111.275719 (2012). Epub 2011 Nov 22.
Peng, X. et al. The interplay between HIF-1α and noncoding RNAs in cancer. J. Exp. Clin. Cancer Res. 39 (1), 27. https://doi.org/10.1186/s13046-020-1535-y (2020).
Guo, C. et al. Hypoxia-Responsive Golgi-Targeted prodrug assembled with anthracycline for improved antitumor and antimetastasis efficacy. ACS Nano. 17 (24), 24972–24987. https://doi.org/10.1021/acsnano.3c07183 (2023). Epub 2023 Dec 13.
Morten, K. J., Badder, L. & Knowles, H. J. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J. Pathol. 229 (5), 755–764. https://doi.org/10.1002/path.4159 (2013).
Kim, H. J. et al. Lipocalin-2 inhibits osteoclast formation by suppressing the proliferation and differentiation of osteoclast lineage cells. Exp. Cell. Res. 334 (2), 301–309 (2015). Epub 2015 Mar 23.
Müller, H. D., Caballé-Serrano, J., Lussi, A. & Gruber, R. Inhibitory effect of saliva on osteoclastogenesis in vitro requires toll-like receptor 4 signaling. Clin. Oral Investig. 21 (8), 2445–2452. https://doi.org/10.1007/s00784-016-2041-7 (2017). Epub 2017 Jan 18.
Wei, L. et al. Lipocalin-2 regulates hippocampal microglial activation in poststroke depression. Front. Aging Neurosci. 13, 798335. https://doi.org/10.3389/fnagi.2021.798335 (2021).
Mine, Y., Makihira, S., Yamaguchi, Y., Tanaka, H. & Nikawa, H. Involvement of ERK and p38 MAPK pathways on Interleukin-33-induced RANKL expression in osteoblastic cells. Cell. Biol. Int. 38 (5), 655–662. https://doi.org/10.1002/cbin.10249 (2014). Epub 2014 Jan 31.
Rossa, C., Ehmann, K., Liu, M., Patil, C. & Kirkwood, K. L. MKK3/6-p38 MAPK signaling is required for IL-1beta and TNF-alpha-induced RANKL expression in bone marrow stromal cells. J. Interferon Cytokine Res. 26 (10), 719–729. https://doi.org/10.1089/jir.2006.26.719 (2006).
Kitaura, H. et al. Role of the interaction of tumor necrosis factor-α and tumor necrosis factor receptors 1 and 2 in Bone-Related cells. Int. J. Mol. Sci. 23 (3), 1481. https://doi.org/10.3390/ijms23031481 (2022).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28 (11), 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51 (D1), D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Marahleh, A. et al. Effect of TNF-α on osteocyte RANKL expression during orthodontic tooth movement. J. Dent. Sci. 16 (4), 1191–1197. https://doi.org/10.1016/j.jds.2021.03.006 (2021).
Kanou, K. et al. Effect of age on orthodontic tooth movement in mice. J. Dent. Sci. 19 (2), 828–836. https://doi.org/10.1016/j.jds.2023.09.016 (2024).
Noguchi, T. et al. TNF-α stimulates the expression of RANK during orthodontic tooth movement. Arch. Oral Biol. 117, 104796. https://doi.org/10.1016/j.archoralbio.2020.104796 (2020).
Fan, Z. et al. Exacerbating orthodontic tooth movement in mice with salt-sensitive hypertension. J. Dent. Sci. 20 (2), 764–769. https://doi.org/10.1016/j.jds.2024.10.020 (2025).
Acknowledgements
A part of this study was supported by a support system for young researchers who use research equipment, instruments, and devices at Tohoku University. We thank the Biomedical Research Core of Tohoku University Graduate School of Medicine for supporting fluorescence-activated cell sorting (FACS).
Funding
This work was supported by JST SPRING, Grant Number JPMJSP2114. JSPS KAKENHI Grant Numbers JP21K10178 and JP22K17244 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
K.N., H.K., and F.O. contributed to designing this study. K.N., H.K., F.O., J.R., A.M., J.M., A.L., Z.F., and K.M. performed the experiments. K.N., H.K., and F.O. analyzed the data and confirmed the results. K.N., H.K., and F.O. drafted the manuscript. H.K. supervised the project. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were performed in accordance with the ARRIVE guidelines and were approved by the Regulations for Animal Experiments and Related Activities at Tohoku University (2018DnA-028-06).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Narita, K., Ohori, F., Marahleh, A. et al. Lipocalin-2 upregulation in hypoxic murine osteocytes enhances RANKL-induced osteoclastogenesis. Sci Rep (2026). https://doi.org/10.1038/s41598-025-34575-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-34575-2