Abstract
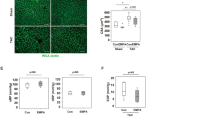
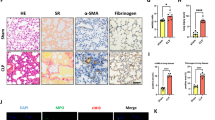
Acute pulmonary embolism (APE) is a major cardiovascular cause of death, characterized by inflammation and oxidative lung injury, for which targeted therapies are lacking. This study investigated the therapeutic potential of Sivelestat sodium, a neutrophil elastase (NE) inhibitor, in a rabbit APE model. APE rabbits were treated with Sivelestat sodium. Cardiopulmonary function was assessed via echocardiography and blood gas analysis. Histopathology, molecular assays (Western blot, RT-qPCR, immunofluorescence), and bioinformatics analysis were used to evaluate injury, inflammation, fibrosis, and the role of secreted phosphoprotein 1 (SPP1). Sivelestat sodium significantly improved cardiopulmonary function and reduced lung damage, neutrophil infiltration, and NETosis. It decreased levels of NE, proinflammatory cytokines (IL-1β, TNF-α, IL-6, IL-8), and markers of coagulation. Treatment also attenuated fibrosis and endothelial barrier injury. Bioinformatics identified SPP1 as a key ECM-related gene. Sivelestat inhibited NE-mediated upregulation of SPP1, subsequently restoring the expression of antioxidant genes (GSTM2, GCLC, GPX1, GPX7) and reducing oxidative stress and collagen deposition. Sivelestat sodium alleviates APE by inhibiting the NE-SPP1 axis, thereby reducing inflammation, oxidative stress, fibrosis, and endothelial injury. This identifies SPP1 as a novel therapeutic target for APE treatment.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are shown within this article.
References
Guarnieri, G. et al. Contemporary management of acute pulmonary embolism. Int. J. Cardiol. 436, 133422 (2025).
Smulders, Y. M. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc. Res. 48, 23–33 (2000).
Yang, J. et al. Oxidative stress in acute pulmonary embolism: emerging roles and therapeutic implications. Thromb. J. 22, 9 (2024).
Agnelli, G. & Becattini, C. Anticoagulant treatment for acute pulmonary embolism: a pathophysiology-based clinical approach. Eur. Respir J. 45, 1142–1149 (2015).
Elshahaat, H. A. et al. Role of serum biomarkers in predicting management strategies for acute pulmonary embolism. Heliyon 9, e21068 (2023).
Watts, J. A., Zagorski, J., Gellar, M. A., Stevinson, B. G. & Kline, J. A. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J. Mol. Cell. Cardiol. 41, 296–307 (2006).
Imiela, A. M., Mikolajczyk, T. P. & Pruszczyk, P. Novel insight into inflammatory pathways in acute pulmonary embolism in humans. Arch Immunol. Ther. Exp. (Warsz) 72 (2024).
Domon, H. et al. Streptococcus pneumoniae disrupts pulmonary immune defence via elastase release following pneumolysin-dependent neutrophil Lysis. Sci. Rep. 6, 38013 (2016).
Peng, R. et al. Neutrophil levels upon admission for the assessment of acute pulmonary embolism with intermediate- and high-risk: an indicator of thrombosis and inflammation. Thromb. J. 21, 28 (2023).
Sahebnasagh, A. et al. Neutrophil elastase inhibitor (sivelestat) May be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J. Clin. Pharm. Ther. 45, 1515–1519 (2020).
Suzuki, K. et al. Neutrophil elastase damages the pulmonary endothelial glycocalyx in Lipopolysaccharide-Induced experimental endotoxemia. Am. J. Pathol. 189, 1526–1535 (2019).
Xie, S. Z. et al. Targeting SPP1-orchestrated neutrophil extracellular traps-dominant pre-metastatic niche reduced HCC lung metastasis. Exp. Hematol. Oncol. 13, 111 (2024).
Wang, Y. et al. ACKR1(hi)ECs promote aortic dissection through adjusting macrophage behavior. Circ. Res. 136, 211–228 (2025).
Folco, E. J. et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through Interleukin-1alpha and cathepsin G. Arterioscler. Thromb. Vasc Biol. 38, 1901–1912 (2018).
Narasaraju, T. et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 179, 199–210 (2011).
Jamil, A. et al. Current interventional therapies in acute pulmonary embolism. Prog Cardiovasc. Dis. 69, 54–61 (2021).
Yang, T. et al. Combined effects of a neutrophil elastase inhibitor (sivelestat sodium) and a free radical scavenger (edaravone) on lipopolysaccharide-induced acute lung injury in rats. Inflamm. Res. 61, 563–569 (2012).
Weng, J. et al. Sivelestat sodium alleviated lipopolysaccharide-induced acute lung injury by improving Endoplasmic reticulum stress. Gene 884, 147702 (2023).
Martinez Licha, C. R., McCurdy, C. M., Maldonado, S. M. & Lee, L. S. Current management of acute pulmonary embolism. Ann. Thorac. Cardiovasc. Surg. 26, 65–71 (2020).
von Nussbaum, F. & Li, V. M. Neutrophil elastase inhibitors for the treatment of (cardio)pulmonary diseases: into clinical testing with pre-adaptive pharmacophores. Bioorg. Med. Chem. Lett. 25, 4370–4381 (2015).
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Sagcan, G. et al. Importance of oxidative stress in the evaluation of acute pulmonary embolism severity. BMC Pulm Med. 22, 382 (2022).
Genschmer, K. R. et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell 176, 113–126e115 (2019).
Kumar, A. et al. Inhibition of PDIA3 in club cells attenuates osteopontin production and lung fibrosis. Thorax 77, 669–678 (2022).
Henkin, S., Ujueta, F., Sato, A. & Piazza, G. Acute pulmonary embolism: Evidence, Innovation, and horizons. Curr. Cardiol. Rep. 26, 1249–1264 (2024).
Miyahara, T., Kubo, K., Koizumi, T., Kobayashi, T. & Sekiguchi, M. Specific neutrophil elastase inhibitor does not attenuate acute lung injury induced by air embolism in awake sheep. Exp. Lung Res. 22, 613–625 (1996).
Becattini, C. & Agnelli, G. Risk stratification and management of acute pulmonary embolism. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 404–412 (2016).
LeVarge, B. L., Wright, C. D. & Rodriguez-Lopez, J. M. Surgical management of acute and chronic pulmonary embolism. Clin. Chest Med. 39, 659–667 (2018).
Roy, P. M., Douillet, D. & Penaloza, A. Contemporary management of acute pulmonary embolism. Trends Cardiovasc. Med. 32, 259–268 (2022).
Yang, J., Madani, M. M., Mahmud, E. & Kim, N. H. Evaluation and management of chronic thromboembolic pulmonary hypertension. Chest 164, 490–502 (2023).
Valerio, L. et al. Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: the FOCUS study. Eur. Heart J. 43, 3387–3398 (2022).
Ushakumari, C. J. et al. Neutrophil elastase increases vascular permeability and leukocyte transmigration in cultured endothelial cells and obese mice. Cells 11 (2022).
Konstam, M. A. et al. Evaluation and management of Right-Sided heart failure: A scientific statement from the American heart association. Circulation 137, e578–e622 (2018).
Huisman, M. V. et al. Pulmonary embolism. Nat. Rev. Dis. Primers. 4, 18028 (2018).
Vyas, V., Sankari, A. & Goyal, A. in StatPearls (2025).
Alerhand, S., Sundaram, T. & Gottlieb, M. What are the echocardiographic findings of acute right ventricular strain that suggest pulmonary embolism? Anaesth. Crit. Care Pain Med. 40, 100852 (2021).
Brittain, E. L., Janz, D. R., Monahan, K. J. & Sevin, C. M. Acute improvement in right ventricular function after treatment of presumed massive pulmonary embolism with thrombolytics. Pulm Circ. 2, 522–524 (2012).
Perrier, A. et al. D-dimer testing for suspected pulmonary embolism in outpatients. Am. J. Respir Crit. Care Med. 156, 492–496 (1997).
Cade, J., Hirsh, J. & Regoeczi, E. Mechanisms for elevated fibrin/fibrinogen degradation products in acute experimental pulmonary embolism. Blood 45, 563–568 (1975).
Moresco, R. N., Vargas, L. C., Voegeli, C. F. & Santos, R. C. D-dimer and its relationship to fibrinogen/fibrin degradation products (FDPs) in disorders associated with activation of coagulation or fibrinolytic systems. J. Clin. Lab. Anal. 17, 77–79 (2003).
Kapoor, S., Opneja, A. & Nayak, L. The role of neutrophils in thrombosis. Thromb. Res. 170, 87–96 (2018).
Vazquez-Garza, E., Jerjes-Sanchez, C., Navarrete, A., Joya-Harrison, J. & Rodriguez, D. Venous thromboembolism: thrombosis, inflammation, and immunothrombosis for clinicians. J. Thromb. Thrombolysis. 44, 377–385 (2017).
Swystun, L. L. & Liaw, P. C. The role of leukocytes in thrombosis. Blood 128, 753–762 (2016).
Darbousset, R. et al. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 120, 2133–2143 (2012).
Noubouossie, D. F. et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood 129, 1021–1029 (2017).
Gould, T. J. et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler. Thromb. Vasc Biol. 34, 1977–1984 (2014).
Engelmann, B. & Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13, 34–45 (2013).
Ginzberg, H. H. et al. Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G705–717 (2001).
Vorobjeva, N. V. & Pinegin, B. V. Neutrophil extracellular traps: mechanisms of formation and role in health and disease. Biochem. (Mosc). 79, 1286–1296 (2014).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Zhou, Y. et al. The emerging role of neutrophil extracellular traps in Arterial, venous and Cancer-Associated thrombosis. Front. Cardiovasc. Med. 8, 786387 (2021).
Zabczyk, M. et al. Elevated lactate levels in acute pulmonary embolism are associated with prothrombotic fibrin clot properties: contribution of NETs formation. J Clin. Med 9 (2020).
Sun, G. et al. Elevated serum levels of neutrophil elastase in patients with influenza virus-associated encephalopathy. J. Neurol. Sci. 349, 190–195 (2015).
Fuchs, T. A. et al. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U S A. 107, 15880–15885 (2010).
Li, T. et al. Lysophosphatidic acid promotes thrombus stability by inducing rapid formation of neutrophil extracellular traps: A new mechanism of thrombosis. J. Thromb. Haemost. 18, 1952–1964 (2020).
Xu, J., Zhang, C., Wu, K., Qian, Y. & Hu, W. A comparative analysis of Sivelestat sodium hydrate and Ulinastatin combination therapy in the treatment of sepsis with acute respiratory distress syndrome. BMC Pulm Med. 24, 283 (2024).
Yue, B. et al. SPP1 induces idiopathic pulmonary fibrosis and NSCLC progression via the PI3K/Akt/mTOR pathway. Respir Res. 25, 362 (2024).
Yu, D. et al. Acute beneficial effects of sodium Nitroprusside in a rabbit model of massive pulmonary embolism associated with circulatory shock. Am. J. Pathol. 188, 1768–1778 (2018).
Funding
This work was supported by the Zhejiang Public Welfare Technology Application Research Project (grant number LTGD23H010002), the Zhejiang Province Medical and Health Science and Technology Plan Project (grant number 2024KY1706), and the Shaoxing Health Science and the Technology Plan Project (grant number 2022KY013).
Author information
Authors and Affiliations
Contributions
Peng Xu, Jing Chen: Conceptualization; Data curation; Investigation; Methodology; Writing – original draft. Weizhong Feng: Conceptualization; Data curation; Investigation. Weizhong Feng: Formal analysis; Methodology. Haixia Xu: Data curation; Methodology. Jianfeng Xu, Junqing Zhou: Conceptualization; Investigation; Methodology; Writing – review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All the rabbit studies were carried out in accordance with the ARRIVE guidelines. The animal experiments in this study were carried out by the ‘’Guiding Principles in the Care and Use of Animals’’ (China) and approved by the Laboratory Animal Management and Ethics Committee of Shaoxing Central Hospital (No. 2023Z060).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, J., Xu, P., Feng, W. et al. Sivelestat sodium mitigates lung injury post-acute pulmonary embolism by inhibiting NE-SPP1 neutrophil recruitment and restoring redox balance. Sci Rep (2026). https://doi.org/10.1038/s41598-025-34824-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-34824-4