Abstract
We have developed a novel S-scheme mechanism to expand the photoresponse range of Bi2SiO5. This study reports the successful creation of a CN/BS heterojunction photocatalyst, which is composed of g-C3N5 and Bi2SiO5. The synthesis was achieved through a simple two-step procedure, involving hydrothermal treatment and subsequent calcination. The 10% CN/BS exhibits superior photocatalytic efficiency. When exposed to visible light, the CN/BS heterojunction photocatalyst achieves a removal rate of 98.8% regarding the breakdown of Rhodamine B (RhB), outperforming Bi2SiO5 by a factor of 5 and g-C3N5 by a factor of 3. Furthermore, the removal rate for Ciprofloxacin (CIP) reaches 96.0%, which is double that of Bi2SiO5 and 14 times higher than that of g-C3N5. It is evident that the photodegradation efficiency of 10% CN/BS towards organic pollutants significantly surpasses that of the precursor composite materials. The improved photocatalytic performance is likely due to the larger specific surface area, more efficient light harvesting, and the construction of an heterojunction. Crucially, the proposition of an S-scheme hypothesis for charge transport within the CN/BS heterojunction photocatalyst marks a pivotal advancement. This concept is of substantial importance for both the theoretical exploration and the practical deployment of photocatalytic materials.
Similar content being viewed by others
Introduction
Due to the rapid industrial growth, freshwater resources are under severe strain, leading to environmental contamination and posing a significant risk to human health. RhB, a synthetic dye, is known for its high solubility and stability, which makes it difficult to degrade and poses a threat to the aquatic ecosystem. CIP, a fluoroquinolone antibiotic, is widely detected in wastewater due to its extensive use, and its presence can lead to antibiotic resistance in bacterial populations. Not only that, RhB has been shown to be toxic to the human liver and kidneys, while CIP can cause allergic reactions and has been linked to the development of antibiotic-resistant bacteria, which poses a significant public health challenge1,2. The technology of photocatalytic degradation of organic pollutants, including RhB and CIP, is gaining increasing attention due to its advantages, such as the use of sunlight, environmental sustainability, and low energy consumption. Nevertheless, the shortcomings of single-component photocatalysts are evident, particularly their inadequate absorption of visible light and the swift recombining of electron-hole pairs3, restrict their broader application. Therefore, designing stable and efficient photocatalysts is crucial for advancing research and facilitating large-scale application of photocatalytic degradation reactions.
A variety of approaches have been proposed to enhance photocatalytic activity, including elemental doping4, morphological manipulation5, and the deposition of noble metals6. Creating semiconductor heterojunctions has been demonstrated as a viable strategy to address the limitations inherent in single-component photocatalysts. For instance, Chen et al. synthesized a Bi12SiO20/g-C3N4 heterojunction that efficiently degrades gentian violet under visible light irradiation7. In binary semiconductor systems, the energy levels facilitate the transfer of photo-induced carriers from one semiconductor to another, leading to efficient and sustained charge separation. Lu et al. reported that the calcined p-n heterojunction of Bi2O3/Bi2SiO5 enhances its photocatalytic activity, likely stem from the effective separation of photo-generated electron-hole carriers at the interface of the heterojunction, phase transitions in the metal oxides, and an increase in specific surface area8. Not limited to binary semiconductors, it also includes ternary9 and even multicomponent materials10. Jabbar et al. demonstrated the fabrication of a ternary S-scheme g-C3N4/Ag2WO4/Bi2S3 heterojunction that achieved 98% Congo red degradation in 60 min under LED light, attributing the enhancement to the improved charge separation efficiency, increased surface area, and dual S-scheme electron-hole migration pathways11. Without a doubt, forming heterojunctions by combining photocatalytic units is a crucial tactic to boost the efficiency of photocatalysis.
In recent years, bismuth-based photocatalysts such as BiOX (X = Cl, Br, I)12, Bi2O2CO313, Bi2Ti2O714, etc. have been utilized in wastewater photocatalysis due to their high chemical stability, visible light response, distinctive layered structures, suitable band energies, non-toxicity, and excellent electronic properties. Within the Bi2O3-SiO2 binary system, a novel compound has been identified: Bismuth silicate (Bi2SiO5). This discovery expands the Aurivillius family of compounds, which also includes Bi12SiO20 and Bi4Si3O12. It is acknowledged that Bi2SiO5 exhibits a layered structure that alternates between two-dimensional layers of (Bi2O2)2+ and (SiO3)2,3,4,5,6,7,8. Research indicates that materials with similar unique layered structures can enhance the separation and migration of photogenerated charge carriers by establishing an internal electric field (IEF) between layers of opposite charge characteristics15. Unfortunately, several challenges have hindered the photocatalytic applications of Bi2SiO5. These include a weak response to visible light, a high bandgap energy level of about 3.5 eV, rapid attraction between charge carriers, and a reduced diffusion rate of photocarriers16,17. Efforts have been made to enhance the catalytic activity of Bi2SiO5 by combining it with semiconductors that have narrower bandgaps. The goal of this strategy is to facilitate electron transport at the heterojunction, thereby generating a large amount of reactive species to drive the photocatalytic reaction18,19.
Graphitic carbon nitride, a metal-free organic polymer, is widely used in photocatalytic degradation. Its effectiveness stems from its ideal energy gap, adaptable structure, stability when exposed to visible light, and simple production process. g-C3N5, a nitrogen-enriched version of carbon nitride, has a lower bandgap20. This is due to the denser electron cloud around the heptazine core, a result of the extended conjugation from the overlap of azo nitrogens, which enhances its electronic performance and photocatalytic capability. However, similar to g-C3N4, its small surface area and quick charge recombination rate limit its wider use. Recent studies have demonstrated that the photocatalytic efficiency of both g-C3N421,22,23 and g-C3N54,24 can be significantly improved by forming heterojunctions. There is an urgent need to develop g-C3N5 photocatalysts that separate charge carriers efficiently and have outstanding redox properties.
Building on the aforementioned discussions, in this work, we developed an efficient S-scheme mechanism for RhB degradation by integrating g-C3N5 with Bi2SiO5 using a hydrothermal process to create an heterojunction photocatalyst. The structure of Bi2SiO5 facilitates the dispersion of g-C3N5, minimizes particle size, and curbs particle clumping. This reduced particle agglomeration is anticipated to enhance the catalyst’s surface area, thereby boosting its photocatalytic efficiency16. Furthermore, the heterojunction formation not only effectively prevent the recombination of photogenerated charge carriers, spatially separating oxidation and reduction sites but also enhances visible light absorption25. This research’s creation of CN/BS paves a novel path for the advancement of a distinctive S-scheme photocatalytic composite, intended for a broad spectrum of applications in both photocatalysis and electrocatalysis.
Experimental
Materials
Bismuth nitrate pentahydrate (Bi2(NO3)3·5H2O), sodium metasilicate nonahydrate (Na2SiO3·9H2O), 3-amino-1,2,4-triazole (3-AT), hexadecyl trimethyl ammonium bromide (CTAB), ethanol, rhodamine B (RhB), isopropanol (IPA), benzoquinone (BQ), concentrated sulfuric acid (H2SO4), ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), sodium hydroxide (NaOH), all the aforementioned reagents are of analytical grade purity.
Synthesis of g-C3N5
To synthesize g-C3N5, begin by dissolving 1.5 g of 3-AT in 30 mL of distilled water and stirring the solution at 80 °C using a magnetically heated stirrer until it becomes dry and crystallizes. Once this occurs, grind the resulting crystals into a fine powder. Subsequently, transfer the powder into a crucible and calcine it in a muffle furnace at a heating rate of 5 °C per minute, reaching a temperature of 520 °C and maintaining it for 180 min. After the tube furnace is cooled to room temperature, take out the porcelain boat and pour the material into a mortar for thorough grinding to obtain pure g-C3N5.
Preparation of Bi2SiO5
First, in a beaker with 10 mL of deionized water, 1.94 g of Bi(NO3)3·5H2O was dissolved, and then 0.05 g of CTAB was added and sonicated at a constant temperature for 30 min, yielding liquid A. Likewise, liquid B was created by mixing 0.57 g Na2SiO3·9H2O, 10 mL deionized water, and 0.05 g CTAB. Slowly add solution B to solution A, and the pH level of the resulting mixture was regulated to reach 10.0 using a 2 mol/L NaOH solution and stirred at a constant temperature for 30 min to produce a milky-white suspension. The milky-white suspension underwent hydrothermal treatment in a Teflon-lined autoclave, maintaining a temperature of 120 °C over a period of 12 h. Once the suspension had reached room temperature after cooling, it was filtered under reduced pressure, washed three times with anhydrous ethanol and deionized water. Ultimately, the sample was subjected to vacuum drying at 60 °C for a duration of 8 h. The white Bi2SiO5 material was obtained by grinding.
Preparation of CN/BS
Initially, 0.05 g of CTAB, 30 mL of ethylene glycol, and various quantities of g-C3N5 were placed in a 250 mL beaker, and then sonicate at constant temperature for 30 min. Add 0.3 g Bi2SiO5 to the above solution, continue constant temperature ultrasound for 30 min, and then transfer to a magnetic stirrer. Following a vigorous 30-minute stirring at a steady temperature, the mixture proceeded to hydrothermal processing within an autoclave, enduring a reaction at 150 °C over the course of 8 h. Subsequently, the product was subjected to filtration and triple washing with methanol and deionized water. Finally, it was dried at 60 °C, yielding a CN/BS composite catalyst. Adding different amounts of g-C3N5 can yield composite catalysts with different mass ratios of 5% CN/BS, 10% CN/BS, and 15% CN/BS. Figure 1 illustrates the preparation process for the CN/BS composite.
Evaluation of the photocatalytic activities
The prepared composites’ photocatalytic activities concerning CIP26 and RhB27 were tested using established methods from prior reports. The experimental setup for degradation involved positioning the reaction at a 5 cm distance from a 500 W xenon lamp (190.0 mV/cm2). A water circulation system was utilized to keep the temperature at a steady 20 °C throughout the process. Prior to initiating the degradation, 40 mg of the catalyst was introduced into either 40 mL of RhB solution or 40 mL of CIP solution, both prepared at a concentration of 10 mg L− 1 and adjusted to a pH of 7. These mixtures were stirred in the dark for a period of 30 min to allow for adsorption-desorption equilibrium to be established. The photocatalytic reaction commenced upon the activation of the xenon lamp, with 3 mL samples of the solution being collected at regular intervals throughout the degradation process. After the catalyst was removed by centrifugal separation, the supernatant concentrations of RhB and CIP were ascertained by measuring the absorption maxima at 554 nm and 273 nm, respectively, utilizing a UV-visible spectrophotometer (Labtech 9100B). Other related characterization techniques can be found in previous work26.
Result and discussion
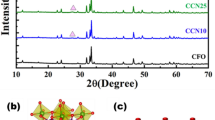
The phase structure and crystalline properties of g-C3N5, Bi2SiO5, and CN/BS were examined using X-ray diffraction (XRD), respectively (Fig. 2). As can be seen from the figure, the XRD curves of synthesized g-C3N5 and Bi2SiO5 are consistent with the information of the g-C3N5 (JCPDS 87-1526) and the tetrahedral Bi2SiO5 (PDF#36–0288), respectively. The characteristic peaks at 24.0°, 28.6°, 32.6°, 47.7°, 48.9° and 56.7° correspond to (101), (103), (110), (107), (116) and (213) crystal planes of Bi2SiO5 (PDF#36–0288), respectively28. Pure g-C3N5 can be observed to have a characteristic peak (002) at 27.4°, which is a result of the stacking of conjugated aromatic systems and has no other stray peaks, indicating that g-C3N5 has good crystallinity during the synthesis process29. After introducing Bi2SiO5, the signals of Bi2SiO5 can be observed in CN/BS composite, indicating the composite is successfully prepared by the hydrothermal method in our work.
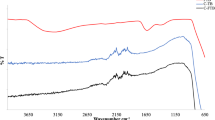
The FT-IR spectrum depicted in Fig. 3 was utilized to further investigate the functional groups of the pertinent photocatalytic materials. Comparable signature peaks, indicative of quintessential CN frameworks within a graphite matrix, were identified in both the pristine g-C3N5 and CN/BS. The observed IR absorption bands at wavelengths of 1238 cm− 1, 1317 cm− 1, 1404 cm− 1, 1552 cm− 1, and 1631 cm− 1 are indicative of the C-N stretching vibrations, denoted as νC-N, which are associated with the aromatic ring of heptazine (C6N7) present in g-C3N5, akin to those in g-C3N4. Stretching vibrations of N–H and O-H bonds account for the absorption peak between 3100 and 3300 cm− 130. The 806 cm− 1 peak distinctly observed is indicative of the triazine units’ breathing mode in g-C3N531, and this feature is prominently present in the CN/BS composite. Relative to pure Bi2SiO5, the broad peak observed between 850 and 1033 cm− 1 is likely a result of interference from adjacent spectral bands, corresponding to the stretching vibrational modes of Bi-O-Si at 859 cm− 1, (SiO5)6− at 995 cm− 1, and Si-O at 1033 cm− 1. Furthermore, the distinct peaks at 573 and 453 cm− 1 are respectively associated with the stretching vibrational modes of (SiO4)4− and Bi-O. The FTIR spectrum of the CN/BS composite material reveals an intense broadband around 3346 cm− 1, primarily due to the O-H stretching vibrations32. Compared to pristine g-C3N5, the incorporation of Bi2SiO5 notably augments the breadth of these O-H stretching vibrational bands. Comparing the three curves reveals that the characteristic peaks of the individual materials g-C3N5 and Bi2SiO5 are both evident in the composite CN/BS, further substantiating the successful fabrication of the composite material.
TEM and HRTEM analyses were conducted to elucidate the structure of the fabricated specimens. Examination of the CN/BS TEM images (Fig. 4a,b) reveals rod-like Bi2SiO5 nanoparticles interspersed among g-C3N5 nanosheets, confirming the successful integration of Bi2SiO5 and g-C3N5, which is a fundamental prerequisite for the fabrication of heterostructures. The HRTEM images (Fig. 4c) depict a lattice spacing of approximately 0.37 nm in Bi2SiO5, aligning with the (101) plane33. The TEM findings are congruent with the XRD data. Additionally, EDS was utilized to delineate the elemental composition of the CN/BS composite, as depicted in Fig. 4d and e. The findings indicate the presence of elements such as C, N, O, Si and Bi. The even distribution of C, N, Si, and Bi throughout the CN/BS sample suggests a uniform interlacing of Bi2SiO5 nanorods with g-C3N5 nanosheets, leading to the formation of numerous microscale heterojunctions.
The valence states and surface chemical composition of the CN/BS nanocomposite were determined via XPS, with results depicted in Fig. 5. The XPS survey spectrum confirmed the elemental constituents of O, N, C, Bi, and Si within the CN/BS nanocomposite, as illustrated in Fig. 5a, corroborating the energy-dispersive spectrometer (EDS) analysis presented in Fig. 3d. In the high-resolution XPS spectrum of the C 1s region depicted in Fig. 5b, two distinct peaks are observed: one at 284.8 eV, signifying C-C (sp3) bonds, and another at 287.9 eV, corresponding to N-C = N (sp2) bonds34. The sp3 carbon peak arises from residual unreacted precursors, amorphous carbon, and exogenous carbon, while the sp2 peak is attributed to the aromatic carbon atoms that form the heptazine units (C6N7) within the g-C3N5 matrix. In the high-resolution XPS (HR-XPS) spectra for the N 1s (Fig. 5c), peaks at 398.5 eV and 401.0 eV are attributed to the secondary nitrogen (C = N-C) and the C-N = N-C/C-NH2 groups within the g-C3N5 structure, respectively24. Additionally, a faint peak at 403.1 eV is observed, which is due to the collective effect of π-π electron delocalization35. The HR-XPS analysis of the CN/BS material within the O 1s energy range, as illustrated in Fig. 5d, disclosed two distinct peaks at binding energies of approximately 530.1 and 532.1 eV. The peak at the lower energy is indicative of the Bi-O bonds present in Bi2SiO5, whereas the peak at the higher energy is associated with both O2 and H2O molecules adsorbed on the surface36,37, which facilitate the production of ·O2− and ·OH with notable oxidative power, thus boosting the photocatalytic efficiency of the CN/BS composite8. Of course, the peak at 532.1 eV could also be attributed to the presence of external -OH groups, which are equally beneficial for photocatalytic degradation. The feeble peak observed at 534.4 eV is indicative of transitions associated with charge transfer38. In the Bi 4f spectrum of CN/BS, the Bi 4f5/2 and Bi 4f7/2 levels are detected at around 159.3 eV and 164.6 eV, respectively (Fig. 5e), confirm the predominant presence of Bi in the trivalent oxidation state39. As illustrated in Fig. 5f, the Si 2p spectrum is associated with the SiO56− group, manifesting a peak at a binding energy of 102.6 eV40.
Integrating g-C3N5 substantially boosts Bi2SiO5’s photocatalytic efficacy and durability during the degradation of Rhodamine B. To determine the source of this favorable outcome, our preliminary investigation focused on g-C3N5’s impact on light absorption, which are pivotal factors that could modulate the catalyst’s performance and reliability20. The absorption peak in Fig. 6a indicates that Bi2SiO5 can effectively capture ultraviolet light, while g-C3N5 demonstrates a robust capacity for light absorption within the visible spectrum. By combining g-C3N5 and Bi2SiO5, photocatalytic material CN/BS exhibits a broad absorption peak from ultraviolet to visible light, as shown in Fig. 6a. It combines the ultraviolet light absorption characteristics of Bi2SiO5 and the visible light absorption characteristics of g-C3N5, thereby exhibiting higher photoresponsivity in the full spectrum range. The Tauc plot analysis, as depicted in Fig. 6b, discloses bandgap widths of 2.18 eV for g-C3N5 and 3.81 eV for Bi2SiO5. Upon integration, composites with diverse g-C3N5 ratios exhibit significantly reduced bandgaps, with the 10% CN/BS and 15% CN/BS demonstrating marked enhancements in light absorption. These outcomes indicate that incorporating g-C3N5 to narrow the bandgap can effectively augment the light utilization efficiency of Bi2SiO5 in the full spectrum range.
Figure 7 delineates the photoluminescence spectrum (PL) excited at a wavelength of 389 nm, that is indirectly ascertaining the efficiency of photogenerated carriers migration based on the recombination degree of electron-hole pairs41. The pronounced fluorescence emission peak of g-C3N5 suggests a high rate of electron-hole recombination. Upon compositing with Bi2SiO5, the emission intensity of CN/BS’ PL is markedly diminished, indicating that the recombination of charge carriers in the heterogeneous photocatalyst is curtailed, thereby effectively facilitating the migration and separation of carriers34. Notably, the 10% CN/BS exhibited the weakest PL intensity, indicative of the most effective separation of photogenerated electron-hole pairs. The reason might be that the proportion of g-C3N5 in the complex influences the intimate structure between g-C3N5 and Bi2SiO5, thereby affecting the quantity of electron-highways formed.
Figure 8a indicates that the photocurrent generated by CN/BS upon irradiation is significantly stronger than that produced by g-C3N5 and Bi2SiO5. This is attributed to Bi2SiO5’s ability to effectively capture photoinduced electrons generated by the excitation of g-C3N5, thereby promoting the separation and migration of photogenerated charge carriers42.
Moreover, as shown in the EIS plot (Fig. 8b). Compared with g-C3N5, the CN/BS material exhibits an enhanced charge transfer rate capability, which is confirmed by its smaller arc radius. Correspondingly, CN/BS exhibits the highest electronic conductivity, further confirming that there is a sufficiently tight interface between g-C3N5 and Bi2SiO5 for high-speed electron transfer. The photoelectrochemical analysis demonstrated that the integration of g-C3N5 and Bi2SiO5 significantly enhances the transfer of electrons at the interface. This leads to the production of a substantial number of electron-hole pairs, which in turn efficiently activate RhB. This results in a significant enhancement of the system’s overall catalytic efficiency.
Photocatalytic performance
As depicted in Fig. 9a, the photocatalytic performance of the unique photoactive carriers in the composite system was evaluated using dye (RhB) degradation. The process began with the dye solution being mixed with the photocatalyst in the absence of light for a duration of 30 min to establish an adsorption-desorption equilibrium. As shown in Fig. 9a for Bi2SiO5, g-C3N5, 5% CN/BS, 10% CN/BS, and 15% CN/BS, distinct absorption characteristics were observed upon the addition of various catalysts. All composite photocatalytic materials exhibited higher absorption activity compared to the individual precursors Bi2SiO5 and g-C3N5, with 10% CN/BS showing the highest activity. Based on the composite material’s superior physical adsorption capabilities, this study further endowed it with chemical processing functionality by creating a CN/BS heterojunction, effectively disrupting the molecular structure of RhB. The pristine Bi2SiO5 (18.65%) and pure g-C3N5 (34.43%) both showed poor photodegradation efficiency under 105 min of irradiation. This is attributed to the relatively low proportion of effective photo-induced electron-hole pairs in the individual systems of Bi2SiO5 and g-C3N5. fortunately, the composite photocatalysts demonstrated a markedly improved performance. The 10% CN/BS composite material demonstrated the highest RhB degradation efficiency of 98.53% under 105 min of irradiation, surpassing the degradation efficiencies of 5% CN/BS (79.85%) and 15% CN/BS (63.87%). This is consistent with the PL and EIS results analysis (Figs. 6 and 7), indicating that the 10% CN/BS composite material facilitates the transfer of carriers in photocatalysis. Figure 9b shows that the the catalyst’s RhB degradation time correlates with -ln(C/C0) in a proportional manner, conforming to the first-order kinetic equation43,44. The 10% CN/BS composite material possesses the highest kinetic rate of 0.01778 min− 1, surpassing g-C3N5 by a factor of 6 and Bi2SiO5 by a factor of 13. To delve deeper into the photocatalytic properties of 10% CN/BS, an investigation was conducted to examine the effect of the amount of catalyst in the RhB solution (Fig. 9c). The degradation efficiency was observed to increase progressively with the catalyst dosage, reaching a peak at 40 mg, beyond which it decreased. The role of a photocatalyst is to convert light energy into chemical energy; hence, increasing the amount of photocatalyst can boost the quantity of photo-induced reactive species, thereby enhancing photocatalytic activity. However, when the photocatalyst dosage in the reaction solution exceeds 40 mg, the increased concentration may raise the likelihood of particle collisions, potentially leading to the quenching of photogenerated carriers on the catalyst surface, which could decrease photocatalytic activity45. It is also possible that the capture of light by the photocatalyst is hindered due to particle aggregation from collisions, resulting in reduced photocatalytic efficiency46. The maximum degradation efficiency of 98.53% is achieved at a catalyst dosage of 40 mg.
As depicted in Fig. 9d, the photocatalytic performance of 10% CN/BS was further evaluated using antibiotic (CIP) as a model pollutant. Compared to their individual counterparts, Composite materials have much better CIP adsorption. Meanwhile, CN/BS have great CIP degradation rates, especially with 10% CN/BS achieving a degradation rate of 96.0% within 120 min. Its kinetic rate is 0.0200 min− 1, exceeding that of g-C3N5 by 6 times and that of Bi2SiO5 by 13 times (Fig. 9e). Consequently, the heterojunction between Bi2SiO5 and g-C3N5 was successfully formed, and the synergistic effect of the two promotes the photoreaction process. The 10% CN/BS’s mineralization capability was evaluated through the observation of TOC decrease. As depicted in Fig. 9f, the 10% CN/BS reached a 67.86% reduction in TOC after a duration of 120 min. This result indicates that the photocatalyst is quite effective in breaking down CIP molecules.
Finally, the influence of external factors such as inorganic ions, pH, and pollutant concentration on degradation activity was explored. Ions in water significantly impact the elimination of pollutants47. As shown in Fig. 9g for cations and Fig. 9h for anions, common cations such as Na+, Ca2+, K+, and Mg2+, as well as anions like Cl−, Ac−, and SO42−, hardly affect the photodegradation of CIP. However, Al3+, PO43−, SiO32−, and CO32− have significant inhibitory effects on CIP photodegradation, which may be due to ion quenching of photoactive species and interference with CIP adsorption48,49,50. The pH range of wastewater significantly affects the degradation of organic matter51. Figure 9i shows that the CIP removal rate is high in neutral to acidic solutions, with little pH-dependent difference, reaching around 96%. Figure 9j shows that CIP degradation efficiency declines with increasing initial concentrations. This is due to competition for active sites on the catalyst and reduced light absorption and utilization on the catalyst surface, leading to a decrease in hydroxyl radicals and photocatalytic efficiency52. In summary, CN/BS maintains efficient photocatalytic degradation ability for CIP under different external conditions, demonstrating good applicability and anti-interference properties.
Photodegradation of RhB and CIP over the as-prepared composite photocatalysts (10 mg L− 1, except for j): (a) time-dependent degradation of RhB; (b) First-order kinetics for RhB; (c) degradation efficiency for RhB at different catalyst dosages; (d) time-dependent degradation of CIP; (e) First-order kinetics for CIP; (f) TOC comparison with and without CN/BS; (g) Effects of different cations on photodegradation; (h) Effects of different anions on photodegradation; (i) Influence of initial solution pH on degradation; (j) Impact of CIP concentration on degradation efficiency.
Stability is a critical metric for assessing a material’s capacity to degrade organic dyes53. Consequently, the stability of the CN/BS composite was evaluated through successive cycles of RhB photodegradation tests. The findings revealed that the photocatalytic efficiency of CN/BS remained impressively high at 89.93% even after five cycles, with only minor variations within a 2.5% on average range every time, as depicted in Fig. 10a, indicating the consistent performance of its photocatalytic properties. The XRD patterns in Fig. 10b compare the crystalline structure of the catalyst before and after the reaction. The nearly identical diffraction peaks suggest that the CN/BS composite’s crystal structure remains largely unchanged, further confirming the catalyst’s excellent cyclic stability. It was observed that the photodegradation efficiency for RhB by the CN/BS composite slightly decreased after multiple uses, potentially due to the reduction in catalyst surface area from repeated washing and prolonged utilization, which may have limited the contact between RhB and active sites. Nonetheless, the CN/BS material’s stability and reusability in photocatalytic degradation processes remain commendable.
Proposed degradation pathway
To pinpoint the principal reactive agents, experiments involving free radical scavengers were additionally implemented. Specifically, EDTA-2Na, IPA, and p-BQ were introduced to trap h+ (holes), ·OH (hydroxyl radicals), and ·O2− (superoxide radicals) respectively26,54.
Referring to Fig. 11, the RhB degradation efficiency reached 98.5% in the absence of a trapping agent; however, it sharply declined to 58.9% upon the introduction of EDTA-2Na, suggesting that holes (h+) are the predominant factor in the degradation process. Following the addition of isopropanol (IPA), the RhB degradation efficiency was reduced from 98.5 to 63.6%, signifying that hydroxyl radicals (·OH) also significantly influence the photocatalytic breakdown. In addition, the degradation efficiency of RhB was similarly reduced by the addition of p-BQ solution, from 98.5 to 76.5%, suggesting that superoxide radicals (·O2−) were one of the reactive species involved in the RhB degradation process. The findings suggest that holes (h+) are the key active species in the photocatalytic degradation reaction, hydroxyl radicals (·OH) exert a substantial impact, and superoxide radicals (·O2−) contribute to a certain extent.
Figure 12 displays the Mott-Schottky plots for the prepared g-C3N5 and Bi2SiO5 samples. Notably, both samples showed positive slopes, a trait typical of n-type semiconductors55,56. The measurement of flat band potentials for g-C3N5 and Bi2SiO5 yields values of -0.56 V and − 0.05 V, respectively, when referenced to Ag/AgCl electrode. These values correspond to -0.36 V and 0.15 V when referenced to the normal hydrogen electrode (NHE)57. That is, g-C3N5 and Bi2SiO5 had conduction band potentials of -0.46 V and 0.05 V, respectively58. As shown in Fig. 5b, g-C3N5 and Bi2SiO5 exhibit band gaps of 2.18 eV and 3.81 eV, respectively. Consequently, the valence band potentials for these materials are 1.72 V for g-C3N5 and 3.86 V for Bi2SiO5.
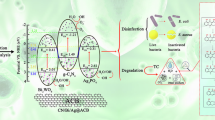
A hypothetical mechanism is suggested (Fig. 13). The conduction band potentials (CB) for g-C3N5 and Bi2SiO5 are − 0.46 and 0.05 V, respectively, and the valence band potentials (VB) are 1.72 and 3.86 V, respectively. The potential for g-C3N5 to create a type II or an S-scheme heterojunction with Bi2SiO5 is contingent upon its CB and VB potentials being more negative than Bi2SiO5’s. The precise configuration of this junction is largely determined by the particular mechanisms of electron transfer that are operative.
Given that the flat band potential of n-type Semiconductors typically indexes their Fermi energy levels59, upon contact, electrons moving from g-C3N5’s higher Fermi level to Bi2SiO5’s lower Fermi level, achieving equilibrium at the interface between the two60. This equilibrium results in an upward bend of g-C3N5’s energy bands and the downward bend of Bi2SiO5’s at the interface. Furthermore, it also induces the formation of accumulation and depletion zones at the interface, culminating in the generation of an IEF oriented from g-C3N5 towards Bi2SiO561.
Upon irradiation, should a standard type II heterojunction be formed (illustrated in Fig. 13, left), it is seen that the transport of e− and h+ is severely hindered by the energy barriers and the IEF. Additionally, the VB potential of g-C3N5, standing at 1.72 V, is insufficient to meet the redox potentials necessary for the generation of hydroxyl radicals (·OH) from H2O/·OH (2.27 V relative to NHE at pH = 7) and OH-/·OH (1.99 V relative to NHE at pH = 7)30, thereby precluding the emergence of ·OH. Similarly, the CB potential of Bi2SiO5 cannot generate superoxide radicals (·O2−). This appears to contradict the findings from the active species analysis, which indicate that h+, ·OH and ·O2− are the primary high-energy species. Therefore, the formation of type II heterojunctions in the CN/BS composites can be discounted.
Figure 13, right depicts the S-type heterojunction mechanism that facilitates the migration of charge carriers. Under the promotion of band bending, the IEF, and an electron-highways, a significant concentration of e− and holes h+ with robust redox properties emerge on the CB of g-C3N5 and the VB of Bi2SiO5, respectively. It is apparent that the h+ exhibit sufficient positive charge to oxidize H2O and HO− to ·OH, while the e− possess ample negative charge to reduce O2 to ·O2− (-0.33 V relative to NHE at pH = 7)5. In conclusion, the excited species h+, ·OH, and ·O2− play a pivotal role in the degradation process of RhB and its intermediates.
The above analysis aligns with the findings from the experiments that scavenge active species (Fig. 11), thus determining that S-type heterojunctions were created in the CN/BS composites. CN/BS composite materials can achieve full spectral range photoresponse and prevent quenching of e−/h+pairs to improve the photocatalytic degradation efficiency of RhB.
Conclusion
To summarize, we successfully synthesized the CN/BS photocatalyst, which features efficient electron pathways. The combination of g-C3N5 and Bi2SiO5 forms a hybrid matrix that induces energy band bending, creating an internal electric field that effectively drives the movement of photogenerated carriers. Extensive analysis confirmed that these electron pathways greatly reduce the quenching of electron-hole pairs, thereby increasing the availability of redox species, mainly h+, ·OH, and ·O2−. In photocatalytic degradation tests, CN/BS composite materials significantly enhance the degradation rates of RhB and CIP. The kinetics constant for RhB degradation by the optimized 10% CN/BS heterojunction photocatalyst is 13 times greater than that of Bi2SiO5 and 6 times greater than that of g-C3N5. Furthermore, the kinetics constant for CIP degradation is 4 times greater than that of Bi2SiO5 and an impressive 40 times greater than that of g-C3N5. This enhanced activity is due to the formation of an S-scheme heterojunction and the introduction of electron pathways, which is is pivotal for deepening the comprehension and application of such materials in photocatalysis.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Ahmad, M. et al. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of rhodamine B. J. Environ. Chem. Eng. 9, 104725. https://doi.org/10.1016/j.jece.2020.104725 (2021).
Priya, P. S. et al. Rhodamine B, an organic environmental pollutant induces reproductive toxicity in parental and teratogenicity in F1 generation in vivo. Comp. Biochem. Physiol. C-Toxicol Pharmacol. 280 https://doi.org/10.1016/j.cbpc.2024.109898 (2024).
Jabbar, Z. H. et al. A critical review describes wastewater photocatalytic detoxification over Bi5O7I-based heterojunction photocatalysts: characterizations, mechanism insight, and DFT calculations. J. Environ. Chem. Eng. 12, 112241. https://doi.org/10.1016/j.jece.2024.112241 (2024).
Jia, J. et al. A novel S-scheme ZnO/Ce-g-C3N5 heterojunctions with enhanced photocatalytic activity. J. Sol-Gel Sci. Technol. 111, 819–833. https://doi.org/10.1007/s10971-024-06491-w (2024).
Jabbar, Z. H. et al. Rational design of novel 0D/0D Bi2Sn2O7/CeO2 in the core-shell nanostructure for boosting the photocatalytic decomposition of antibiotics in wastewater: S-type-based mechanism. Mater. Sci. Semicond. Process. 173, 108165. https://doi.org/10.1016/j.mssp.2024.108165 (2024).
Hajjaji, M. A. et al. Platinum nanoparticles decorated TiO2 nanotubes for VOCs and bacteria removal in simulated real condition: Effect of the deposition method on the photocatalytic degradation process efficiency. J. Photochem. Photobiol A. 458 https://doi.org/10.1016/j.jphotochem.2024.115975 (2025).
Chen, C. C., Chen, T. T., Shaya, J., Wu, C. L. & Lu, C. S. Bi12SiO20/g-C3N4 heterojunctions: Synthesis, characterization, photocatalytic activity for organic pollutant degradation, and mechanism. J. Taiwan Inst. Chem. Eng. 123, 228–244. https://doi.org/10.1016/j.jtice.2021.05.042 (2021).
Lu, H. et al. A high-performance Bi2O3/Bi2SiO5 p-n heterojunction photocatalyst induced by phase transition of Bi2O3. Appl. Catal. B-Environ. 237, 59–67. https://doi.org/10.1016/j.apcatb.2018.05.069 (2018).
Graimed, B. H., Okab, A. A., Jabbar, Z. H., Issa, M. A. & Ammar, S. H. Highly stable β-Bi2O3/Ag decorated nanosilica as an efficient Schottky heterojunction for ciprofloxacin photodegradation in wastewater under LED illumination. Mater. Sci. Semicond. Process. 156, 107303. https://doi.org/10.1016/j.mssp.2022.107303 (2023).
Okab, A. A. & Alwared, A. I. Photodegradation of tetracycline antibiotic by ternary recyclable Z-scheme g-C3N4/Fe3O4/Bi2WO6/Bi2S3 photocatalyst with improved charge separation efficiency: Characterization and mechanism studies. Environ. Nanotechnol. Monit. Manage. 19, 100767. https://doi.org/10.1016/j.enmm.2022.100767 (2023).
Jabbar, Z. H., Okab, A. A., Graimed, B. H., Issa, M. A. & Ammar, S. H. Photocatalytic destruction of Congo red dye in wastewater using a novel Ag2WO4/Bi2S3 nanocomposite decorated g-C3N4 nanosheet as ternary S-scheme heterojunction: Improving the charge transfer efficiency. Diam. Relat. Mater. 133, 109711. https://doi.org/10.1016/j.diamond.2023.109711 (2023).
Chawla, A. et al. Bi-rich BixOyBrz-based photocatalysts for energy conversion and environmental remediation: A review. Coord. Chem. Rev. 491 https://doi.org/10.1016/j.ccr.2023.215246 (2023).
Li, Y. C., Peng, B. & Peng, Y. Rational engineering β-Bi2O3/Bi2O2CO3 heterojunction photocatalyst from tetragonal BiOCl microrods displaying high photocatalytic performance. Mater. Sci. Eng. B-Adv Funct. Solid-State Mater. 307 https://doi.org/10.1016/j.mseb.2024.117501 (2024).
Jiao, J. et al. Cost effective glass-ceramics containing Bi2Ti2O7 nanocrystals for photocatalytic degradation of RhB. J. Non-Cryst Solids 646 https://doi.org/10.1016/j.jnoncrysol.2024.123229 (2024).
Tu, S. et al. Flower-like Bi4O5I2/Bi5O7I nanocomposite: Facile hydrothermal synthesis and efficient photocatalytic degradation of propylparaben under visible-light irradiation. RSC Adv. 6, 44552–44560. https://doi.org/10.1039/C6RA03988J (2016).
Chen, A. et al. Z-scheme Bi2SiO5/Ni-doped Ag6Si2O7 heterojunction with remarkable photocatalytic performance for MO degradation with enhanced light absorption ability: Adjustment of electron transfer path and energy band structure. Appl. Surf. Sci. 642, 158465. https://doi.org/10.1016/j.apsusc.2023.158465 (2024).
Xie, Y. L., Xue, R. C., Chen, P. Y., Shen, C. C. & Yu, L. P. Construction of oxygen-vacancy-rich Z-scheme polypyrrole/Bi2SiO5 heterojunction for efficient degradation of antibiotics under simulated sunlight. J. Solid State Chem. 328, 124342. https://doi.org/10.1016/j.jssc.2023.124342 (2023).
Yu, Z. et al. One-pot hydrothermal preparation of rich-oxygen vacant Bi2SiO5/CuBi2O4 Z-Scheme heterojunction for visible light-driven photocatalytic removal of antibiotic-resistant bacteria. Chem. Eng. J. 478, 147353. https://doi.org/10.1016/j.cej.2023.147353 (2023).
Zhang, M. et al. In situ co-crystallization synthesis of the direct Z-scheme Bi5O7I/Bi2SiO5 heterojunction for enhanced photocatalytic rhodamine B and ciprofloxacin degradation. Inorg. Chem. Commun. 159, 111729. https://doi.org/10.1016/j.inoche.2023.111729 (2024).
Kumar, P. et al. C3N5: A low Bandgap Semiconductor containing an azo-linked carbon nitride framework for photocatalytic, photovoltaic and adsorbent applications. J. Am. Chem. Soc. 141, 5415–5436. https://doi.org/10.1021/jacs.9b00144 (2019).
Abdul kader, H. D. et al. Enhancing visible-light-based photodegradation of antibiotics over facile constructed BiVO4/S-doped g-C3N4 heterojunctions in an airlift photocatalytic reactor. J. Water Process. Eng. 65, 105900. https://doi.org/10.1016/j.jwpe.2024.105900 (2024).
Ammar, S. H., Abdulmajeed, Y. R., Khadim, H. J., Jabbar, Z. H. & Khudhair, E. M. Rational assembly of Z-scheme FeTiO3/Fe-doped g-C3N4 photocatalytic heterojunctions: Photodegradation behavior and mechanism insight. J. Water Process. Eng. 61, 105275. https://doi.org/10.1016/j.jwpe.2024.105275 (2024).
Ali, F. D., Ammar, S. H., Rashed, M. K., Jabbar, Z. H. & Hadi, H. J. Boosting visible-light-promoted photodegradation of norfloxacin by S-doped g-C3N4 grafted by NiS as robust photocatalytic heterojunctions. J. Mol. Struct. 1312, 138611. https://doi.org/10.1016/j.molstruc.2024.138611 (2024).
Zhang, W. et al. Embedding In2.77S4 into NCDs-(g-C3N5) hybrid materials to enhance photocatalytic hydrogen production and rhodamine B degradation by constructing electron-highways. Surf. Interfaces 46, 103955. https://doi.org/10.1016/j.surfin.2024.103955 (2024).
Zhang, H. et al. 2D/2D Bi2WO6/C3N5 S-scheme heterojunction for highly selective production of CH4 by photocatalytic CO2 reduction under visible light. Appl. Catal. A 686 https://doi.org/10.1016/j.apcata.2024.119914 (2024).
Zhang, G. et al. Synergistic enhancement of Ce–ZnO/g-C3N4 photocatalytic performance using N,O-bis-vacancy induction and S-scheme heterojunctions. J. Rare Earths. 42, 817–826. https://doi.org/10.1016/j.jre.2023.08.002 (2024).
Li, P. B. et al. Preparation of large-faced flower-like Bi2WO6 using carbon as a template to enhanced photocatalytic activity under visible light. J. Phys. Chem. Solids. 171 https://doi.org/10.1016/j.jpcs.2022.110968 (2022).
Ren, Y. et al. BiPO4/Ag3PO4/Bi2SiO5 heterojunction: Controllable synthesis and its enhancement of visible light catalytic activity. J. Mater. Sci. Mater. Electron. 34, 1200. https://doi.org/10.1007/s10854-023-10563-y (2023).
Li, K. et al. Boron doped C3N5 for photocatalytic nitrogen fixation to ammonia: the key role of boron in nitrogen activation and mechanism. Chem. Eng. J. 435 https://doi.org/10.1016/j.cej.2022.135017 (2022).
Jabbar, Z. H., Graimed, B. H., hamzah Najm, H., Ammar, S. H. & Taher, A. G. Reasonable decoration of CuO/Cd0.5Zn0.5S nanoparticles onto flower-like Bi5O7I as boosted step-scheme photocatalyst for reinforced photodecomposition of bisphenol A and cr(VI) reduction in wastewater. J. Environ. Manage. 348, 119302. https://doi.org/10.1016/j.jenvman.2023.119302 (2023).
Jabbar, Z. H. et al. Building a robust S-scheme BiOCl/CuBi2O4 system for photocatalytic oxidation of sulfamethoxazole under solar light irradiation. Sol. Energy. 275, 112640. https://doi.org/10.1016/j.solener.2024.112640 (2024).
Bordun, O. M. Vibrational spectra of thin eulytine films. J. Appl. Spectrosc. 64, 476–479. https://doi.org/10.1007/BF02683889 (1997).
Ren, Y., Wang, X., Liu, X., Li, H. & Gao, S. Synthesis and visible light catalytic activity of Ag3PO4/Bi2SiO5 nanocomposites. J. Solid State Chem. 317, 123708. https://doi.org/10.1016/j.jssc.2022.123708 (2023).
Alam, K. M. et al. Photocatalytic mechanism control and study of carrier dynamics in CdS@C3N5 core–shell nanowires. ACS Appl. Mater. Interfaces 13, 47418–47439. https://doi.org/10.1021/acsami.1c08550 (2021).
Li, S. et al. Designing oxygen vacancy mediated bismuth molybdate (Bi2MoO6)/N-rich carbon nitride (C3N5) S-scheme heterojunctions for boosted photocatalytic removal of tetracycline antibiotic and cr(VI): Intermediate toxicity and mechanism insight. J. Colloid Interface Sci. 624, 219–232. https://doi.org/10.1016/j.jcis.2022.05.151 (2022).
Song, H., Wu, R., Yang, J., Dong, J. & Ji, G. Fabrication of CeO2 nanoparticles decorated three-dimensional flower-like BiOI composites to build p-n heterojunction with highly enhanced visible-light photocatalytic performance. J. Colloid Interface Sci. 512, 325–334. https://doi.org/10.1016/j.jcis.2017.10.080 (2018).
Liu, J. S. et al. Construction of AgBr/Bi2SiO5 composite with Z-scheme structure and its visible light photocatalytic performance. J. Mater. Sci. Mater. Electron. 34 https://doi.org/10.1007/s10854-023-10432-8 (2023).
Chermette, H., Pertosa, P. & Michel-Calendini, F. M. Molecular orbital study of satellites in XPS spwctra of BaTiO3 and TiO2. Chem. Phys. Lett. 69, 240–245. https://doi.org/10.1016/0009-2614(80)85055-X (1980).
Dou, L. et al. One-pot solvothermal fabrication of S-scheme OVs-Bi2O3/Bi2SiO5 microsphere heterojunctions with enhanced photocatalytic performance toward decontamination of organic pollutants. Appl. Surf. Sci. 527, 146775. https://doi.org/10.1016/j.apsusc.2020.146775 (2020).
Dou, L., Zhong, J., Li, J., Pandian, R. & Burda, C. In-situ construction of 3D nanoflower-like BiOI/Bi2SiO5 heterojunctions with enhanced photocatalytic performance for removal of decontaminants originated from a step-scheme mechanism. Appl. Surf. Sci. 544, 148883. https://doi.org/10.1016/j.apsusc.2020.148883 (2021).
Li, S. J. et al. In situ construction of a C3N5 nanosheet/Bi2WO6 nanodot S-scheme heterojunction with enhanced structural defects for the efficient photocatalytic removal of tetracycline and cr(vi). Inorg. Chem. Front. 9, 2479–2497. https://doi.org/10.1039/d2qi00317a (2022).
Zhao, F. et al. Photocatalytic degradation of tetracycline by N-CQDs modified S-g-C3N4 nanotubes and its product toxicity evaluation. Sep. Purif. Technol. 314, 123533. https://doi.org/10.1016/j.seppur.2023.123533 (2023).
Matos, R. et al. Design and photo-Fenton performance of Graphene/CuS/Fe3O4 tertiary nanocomposites for rhodamine B degradation. Catal. Today 418 https://doi.org/10.1016/j.cattod.2023.114132 (2023).
Sun, Z. W., Li, J. M., Qiu, X. Y., Wang, K. L. & Guo, L. One-step thermal polymerization synthesis of P and K co-doped two-dimensional porous g-C3N4 photocatalyst with enhanced visible light photocatalytic activity for RhB. Mater. Lett. 370 https://doi.org/10.1016/j.matlet.2024.136812 (2024).
Zheng, S. et al. One-step preparation of MoOx/ZnS/ZnO composite and its excellent performance in piezocatalytic degradation of rhodamine B under ultrasonic vibration. J. Environ. Sci. 125, 1–13. https://doi.org/10.1016/j.jes.2021.10.028 (2023).
Mirzaeifard, Z., Shariatinia, Z., Jourshabani, M. & Rezaei Darvishi, S. M. ZnO photocatalyst revisited: Effective photocatalytic degradation of emerging contaminants using S-Doped ZnO nanoparticles under visible light radiation. Ind. Eng. Chem. Res. 59, 15894–15911. https://doi.org/10.1021/acs.iecr.0c03192 (2020).
Ahmad, S. et al. The effect of mineral ions present in tap water on photodegradation of organic pollutants: Future perspectives. Water 15 https://doi.org/10.3390/w15010175 (2023).
Dugandzic, A. M. et al. Effect of inorganic ions, photosensitisers and scavengers on the photocatalytic degradation of nicosulfuron. J. Photochem. Photobiol. A 336, 146–155. https://doi.org/10.1016/j.jphotochem.2016.12.031 (2017).
Santiago, D. E. et al. Effect of inorganic ions on the photocatalytic treatment of agro-industrial wastewaters containing imazalil. Appl. Catal. B Environ. 156–157, 284–292. https://doi.org/10.1016/j.apcatb.2014.03.022 (2014).
Wen, X. J. et al. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 358, 141–154. https://doi.org/10.1016/j.jcat.2017.11.029 (2018).
Khan, N. A. et al. Recent trends in disposal and treatment technologies of emerging-pollutants: A critical review. TrAC Trends Anal. Chem. 122, 115744. https://doi.org/10.1016/j.trac.2019.115744 (2020).
Zhang, H. et al. Highly conductive Ti3C2 MXene reinforced g-C3N4 heterojunction photocatalytic for the degradation of ciprofloxacin: Mechanism insight. Sep. Purif. Technol. 330, 125520. https://doi.org/10.1016/j.seppur.2023.125520 (2024).
Xie, Y. L., Xue, R. C., Chen, P. Y., Shen, C. C. & Yu, L. P. Construction of oxygen-vacancy-rich Z-scheme polypyrrole/Bi2SiO5 heterojunction for efficient degradation of antibiotics under simulated sunlight. J. Solid State Chem. 328 https://doi.org/10.1016/j.jssc.2023.124342 (2023).
Zhang, S. et al. The synergetic enhancement of piezo catalytic performance to remove tetracycline by K2Ti6O13/TiO2 composite. J. Alloys Compd. 900 https://doi.org/10.1016/j.jallcom.2021.163492 (2022).
El-Bahy, Z. M. et al. Tuning the structural and optical parameters of Li1.2Ni0.4Fe2O4 for efficient degradation of dye and pharmaceutical compounds. J. Mol. Struct. 1321 https://doi.org/10.1016/j.molstruc.2024.139927 (2025).
Nurlaela, E. et al. Critical role of the semiconductor–electrolyte interface in photocatalytic performance for water-splitting reactions using Ta3N5 particles. Chem. Mater. 26, 4812–4825. https://doi.org/10.1021/cm502015q (2014).
Ismael, M. Facile synthesis of NiO-loaded g-C3N4 heterojunction photocatalyst for efficient photocatalytic degradation of 4-nitrophenol under visible light irradiation. J. Photochem. Photobiol. A 439, 114576. https://doi.org/10.1016/j.jphotochem.2023.114576 (2023).
Chang, F. et al. Boosted photocatalytic NO removal performance by S-scheme hierarchical composites WO3/Bi4O5Br2 prepared through a facile ball-milling protocol. Sep. Purif. Technol. 278, 119662. https://doi.org/10.1016/j.seppur.2021.119662 (2021).
Ren, J. et al. Bionic construction of a dual Z-scheme MoO3/ZnIn2S4/black phosphorus quantum dots heterojunction with expanded redox surfaces and photoelectron transfer ability for high-efficiency photocatalysis. Chem. Eng. J. 465, 142894. https://doi.org/10.1016/j.cej.2023.142894 (2023).
Wang, Q. et al. S-scheme towards interfacial charge transfer between POMs and MOFs for efficient visible-light photocatalytic cr (VI) reduction. Environ. Pollut. 347 https://doi.org/10.1016/j.envpol.2024.123707 (2024).
Wang, C. C., Rong, K., Liu, Y. P., Yang, F. & Li, S. J. Carbon quantum dots-modified tetra (4-carboxyphenyl) porphyrin/BiOBr S-scheme heterojunction for efficient photocatalytic antibiotic degradation. Sci. China Mater. 67, 562–572. https://doi.org/10.1007/s40843-023-2764-8 (2024).
Acknowledgements
The work was financially supported by the Bingtuan Science and technology Program (2022ZD099, 2024DA036) and the presidential research fund of Tarim University (TDZKCX202208).
Author information
Authors and Affiliations
Contributions
Shaowei Qin: Visualization; formal analysis; writing-review and editing. Lili Huang: Conceptualization; Visualization; formal analysis; writing-review and editing. Yuan Zhang: Conceptualization; data curation; methodology; project administration. Tao Zhang: Investigation; data curation. Mingxia Tian: Formal analysis; writing-review and editing. Jianhui Jiang: Conceptualization; funding acquisition; investigation; methodology; supervision; validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qin, S., Huang, L., Zhang, Y. et al. A high-performance g-C3N5/Bi2SiO5 heterojunction photocatalyst induced by constructing S-scheme electron-highways. Sci Rep 15, 787 (2025). https://doi.org/10.1038/s41598-025-85268-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85268-9
Keywords
This article is cited by
-
Self-assembled CdS/C3N5 heterostructure for photocatalytic CO2 reduction
Journal of Sol-Gel Science and Technology (2025)