Abstract
The ectoparasitic mite Varroa destructor remains a great threat for the beekeeping industry, for example contributing to excessive winter colony loss in Canada. For decades, beekeepers have sequentially used the registered synthetic varroacides tau-fluvalinate, coumaphos, amitraz, and flumethrin, leading to the risk of resistance evolution in the mites. In addition to the widespread resistance to coumaphos and pyrethroids, a decline in amitraz efficacy has recently been reported in numerous beekeeping regions in Canada. The goals of this study were to assess the evolution of resistance to amitraz in Canadian mite populations and to evaluate the presence and incidence of mutations previously associated with resistance to amitraz and pyrethroids in V. destructor. Our bioassay results confirmed the presence of amitraz-resistant mites in the population of Alberta. These phenotypic results were complemented by targeted genotyping of the octopamine receptor gene Octβ2R which revealed the presence of the mutation Y215H in 90% of tested apiaries with local allele frequencies ranging from 5 to 95%. The phenotypic resistance showed a significant correlation with the presence of this mutation across apiaries. In parallel, the L925I and L925M mutations in the voltage-gated sodium channel were identified in 100% of the tested apiaries with frequencies ranging from 33 to 97%, suggesting that resistance to pyrethroids remains widespread. These results support the notion that the practice of relying on a single treatment for a prolonged period can increase rates of resistance to current varroacides. Our findings suggest the need for large-scale resistance monitoring via genotyping to provide timely information to beekeepers and regulators. This will enable them to make an effective management plan, including rotation of available treatments to suppress or at least delay the evolution of resistance in V. destructor populations.
Similar content being viewed by others
Introduction
Among a broad diversity of plant pollinators, the honey bee, Apis mellifera L., plays an important role in pollinating wild plants and agricultural crops. Honey bees pollinate one third of the plants that are associated with human and animal food1,2 and produce several hive products. As one of the largest honey producing countries in the world, Canada produces over 41 × 106 kg honey per year, from approximately 810,000 honey bee colonies3. Alberta’s apicultural industry alone operates 317,500 colonies and produces approximately 16 × 106 kg of honey annually, representing the largest share of professional beekeepers and honey produce in Canada3.
Managed honey bees face many problems across the globe and beekeepers have been battling numerous honey bee health challenges to remain profitable. Like other managed agricultural species, particularly those maintained in higher densities than would be seen in the wild, honey bees face several infectious diseases caused by parasites and pathogens. Canadian beekeepers, in particular, experience an average of 15–46% colony winter losses, much of which is attributed to the impact of the ectoparasitic mite, Varroa destructor (Anderson and Trueman)4,5. As an obligate ectoparasite, V. destructor feeds on bees’ hemolymph and fat body for survival and reproduction, concurrently transmitting honey bee diseases6,7,8. It reproduces inside honey bee brood cells and quickly builds up its population, creating unsustainable conditions inside the honey bee colony. At the colony level, V. destructor imbalances honey bee winter thermoregulation and homeostasis leading to winter losses9.
A variety of cultural, biological, mechanical, and chemotherapeutic approaches are available for controlling V. destructor10,11. However, only a few of the currently available management tools are effective and practical, particularly for large-scale commercial beekeepers. Among the chemotherapeutic options, synthetic varroacides (e.g., amitraz) are only recommended as a last resort in an Integrated Pest Management (IPM) program. Instead, they are often the first and / or only management practice utilized. Despite studies warning about their overuse, chemical treatments against V. destructor have been implemented as a routine part of apicultural practice, because most other control methods are too labor-intensive of ineffective. Resulting from the overuse of the few available varroacides, mite populations worldwide have evolved resistance against these varroacides12,13,14,15,16.
Organophosphate, pyrethroid, and formamidine acaricide classes have been widely used to manage V. destructor populations. Beekeepers have only a few synthetic varroacides available, such as tau-fluvalinate, coumaphos, amitraz, and flumethrin. Since the 1990s, Canadian beekeepers have controlled V. destructor population using tau-fluvalinate or coumaphos. However, due to well-reported V. destructor resistance to these compounds, these products are no longer used to the same extent5,17. Currently, beekeepers are relying primarily on amitraz-based products that are considered relatively safe for bees and have historically demonstrated high efficacy5,18. Although previous Canadian studies between 2014 and 2022 confirmed an acceptable efficacy for amitraz in V. destructor control19,20,21,22,23, recent reports in 2023 have indicated that the efficacy of the formamidine Apivar and the pyrethroids Apistan and Bayvarol are declining in Canadian beekeeping operations5,24.
Mutations in target sites and changes in the expression of detoxification enzymes have been proposed as mechanisms for the evolution of miticides resistance in V. destructor populations16,25,26,27,28. Resistance to pyrethroids in V. destructor has been attributed to mutations in the target site of the Voltage-Gated Sodium Channel (VGSC) that prevent the intended lethal hyperstimulation of the nervous system29, called knockdown resistance (kdr) or super-kdr30,31. Although, amino acid substitutions at positions 918 (M918L) and 925 (L925V, I, M) have been associated with resistance to pyrethroids in V. destructor populations25,26,27,32, other substitutions such as F1528P, L1596P, I1752P and M1823I, located in regions of the VGSC not usually associated with resistance, have also been reported25,33. Mutations at position 925 have a wide distribution25,26,27,34,35, while mutation M918L has so far only been reported in Spanish V. destructor populations and seems to be linked to L925V27. A thorough screening of samples collected across the USA only found L925I and L925M to be present26. Additional, more current information is needed to assess the potential for pyrethroid use against V. destructor, particularly in Canada where no survey has been conduced so far.
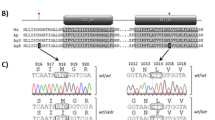
Amitraz acts as an octopamine receptor agonist that has been used against a variety of agricultural and veterinary pests including ticks, mites, and insects. It targets the octopamine and tyramine receptors which mediate numerous functions of octopamine and tyramine in the arthropod nervous systems36,37,38. It causes hyperactivity and ectoparasites become rapidly dislodged from their host39. Specific amino acid substitutions, such as N87S and Y215H, in the β2-adrenergic-like octopamine receptor (Octβ2R) have been associated with reduced efficacy of amitraz in V. destructor populations collected in France and in the USA, respectively40,41. Recently, Hernández-Rodríguez et al.42 found the F290L mutation in Spanish V. destructor populations associated with amitraz resistance. Despite assessments of resistance evolution17,21,23,43, no previous study has evaluated or tracked the presence and prevalence of mutations associated with resistance to amitraz and pyrethroids in Canadian beekeeping operations. Therefore, this study aimed to detect such mutations in populations of V. destructor across the province of Alberta.
Results
Assessment of Apivar efficacy
On average, Apiarium cages contained 296 ± 7 bees and showed a V. destructor parasitization rate of 4.5 ± 0.3%. Non-significant differences were observed in the mite mortality rate (weighted statement total mite: F = 1.14; df = 5, 69; p = 0.3466), and knockdown rate (weighted statement total mite: F = 1.27; df = 5, 69; p = 0.2866) among control groups for all apiaries. For Apivar treatments, the apiary AB-92 showed a significantly higher mite mortality (weighted statement total mite: F = 7.49; df = 5, 76; p < 0.0001) and knockdown rate (weighted statement total mite: F = 6.47; df = 5, 76; p < 0.0001) in comparison to other apiaries (Table 1). When Varroa-parasitized bees were exposed to Apivar, a low overall efficacy (22–55%), with a high variability among apiaries, was observed for all locations except for one apiary (AB-92) that showed a significantly higher efficacy of 92% (weighted statement total mite: F = 5.08; df = 5, 62; p = 0.0006) (Table 1). Altogether, results showed a general reduction of Apivar efficacy among the six apiaries bioassayed in this study in compared to previous study22.
Detecting mutations associated with resistance to amitraz
Samples collected in Alberta apiaries in 2020 and 2022 were screened for the presence of mutations N87S and Y215H in the Oct β2R gene to assess genetic resistance to amitraz40. According to the genotyping results, none of the mites analyzed carried the S87 mutant allele. However, the majority of samples contained mites carrying the H215 mutant allele, and only two samples from Rocky View (AB-31) and Strathcona (AB-43) counties were without mutant mites (Table S1, Fig. 1). Given the recessive inheritance of the resistance mutation37,38, an expected frequency (Fig. S1) and geographic distribution (Fig. 2) of resistant phenotypes were determined based on mites that were homozygous for the mutant allele (H215). Our data indicate that mutation Y215H, associated with resistance to amitraz, is widely distributed across the province of Alberta, with homozygotes (predicted to be resistant) ranging from 20 to 95% of the mites in the samples analyzed (Table S1, Figs. 1 and 2).
A significant difference in the prevalence of the H215 allele was found between genotyped samples from both 2020 and 2022 years (X2 = 27.7378; df = 1; p < 0.0001). The H215 allele frequency was higher in 2022 (58.78 ± 10.82%) than 2020 (37.1 ± 10.26%). In samples tested for both years, no similar trend was observed in the prevalence of H215 allele among mite populations. For instance, samples like AB-31 (Rocky view) with 0% mutant mites and others such as AB-18 (Fairview) had 95% of mutant mites. On the other hand, the mites collected from some of the operations (e.g. from Brooks (AB-69), High River (AB-73), and Oyen (AB-77) counties) that had reported failures in the Apivar treatment and were bioassayed using the Apiarium test, showed a consistent prevalence of 95% or above individuals homozygous for the mutant allele (Table 1; Table S1; Fig. 1).
A significant difference of the frequency of the amitraz-resistance was observed among the three principal regions of Alberta over both years (X2 = 60.305; df = 2; p < 0.0001). The homozygous H215 allele in the Octβ2R gene was similarly frequent among regions in 2020 (F = 0.22; df = 2, 7; p = 0.8101). In contrast, the frequency varied significantly in 2022 (F = 5.91; df = 2, 6; p = 0.0382), with 95.67 ± 6.54% in the south, 57.5 ± 8.01% in the central, and 31.75 ± 5.66% in the northwestern part of the province (Table S1). Based on the V. destructor treatment history for spring and fall apiary treatments, mite samples were divided into three treatment regimes: (1) synthetic miticides only, (2) organic acids only, and (3) a combination of synthetic miticides and organic acids (Table S2). The frequency of individuals homozygous for the H215 mutant allele (resistance genotype) was significantly different among those groups (X2 = 64.33; df = 2; p < 0.0001), because Apivar exposed mites exhibiting higher frequencies of the resistant genotype than mites experiencing organic acid treatment only (Fig. 3).
Mutations associated with resistance to pyrethroids
Mites genotyped for amitraz-resistance were also investigated for the known alleles associated with pyrethroids-resistance25,26. A high prevalence (40–100%) of the mutations M925 and I925 was found in mites across beekeeping operations (Table S1, Fig. 4). Allele I925 was more prevalent than allele M925, averaging 62% and 9%, respectively. The mites homozygous for I925 were identified in 17 out of the 18 samples and represented the majority of the samples overall (averaging 53%), while the heterozygous L925M was only detected in four samples with a low average frequency of 1.3% (Table S1, Fig. 4).
Frequency (%) of different genotypes detected at position 925 of the VGSC protein in selected beekeeping operations from Alberta. Light orange, orange, dark orange, light blue, blue, and dark blue bars indicate the frequency of mites heterozygous I925M, homozygous M925, homozygous I925, heterozygous L925M, heterozygous L925I, and homozygous L925, respectively.
Susceptible and resistant phenotypes were predicted considering based on the genotyping results and the recessive inheritance of kdr-type resistance in V. destructor44. According to this estimation, resistance to pyrethroids is highly prevalent (60%) across the province of Alberta, with samples such as AB-18 (Fairview) and AB-77 (Oyen) having a predicted frequency of resistant mites as high as 93% and 97%, respectively (Table S1, Figs. 5 and S2). Analyses also revealed significant differences in the frequency of resistant or susceptible mites between 2020 and 2022 samples for the M925 (X2 = 22.2959; df = 1; p < 0.0001), but not for the I925 (X2 = 14.6981; df = 1; p = 0.2755) genotypes (Table S1, Figs. 5 and S2). V. destructor populations showed a significant difference in I925 frequency among the three principal regions of the province (X2 = 14.6981; df = 2; p = 0.0006), where I925 frequency was higher in southern (59.13 ± 8.93%) and central (52.96 ± 9.78%) regions than in the north-west (48.74 ± 8.27%). The M925 frequency in southern (9.43 ± 2.87%) Alberta was significantly higher than apiaries located in the north-west (0.36 ± 2.66%) of the province (X2 = 49.532; df = 2; p < 0.0001) (Table S1).
Discussion
Our study provides the first report of the detection and distribution of mutations associated with resistance to varroacides such as amitraz and pyrethroids in Canada. Previous Canadian studies showed a high efficacy (> 92%) for Apivar in beekeeping regions of Nova Scotia, Prince Edward Island, New Brunswick21, Ontario23, and Alberta18,22. However, decreasing Apivar efficacy has anecdotally been reported across the country5,24. Our study confirms the evolution of resistance to amitraz in the V. destructor population from Alberta at the genetic and phenotypic level and also confirms the presence of mutations associated with the resistance to pyrethroids.
The intensive use of pesticides favours the evolution of resistance in target organisms and amitraz is not an exception. Amitraz-resistance has already been reported in mites, ticks, and insects12,39,45. In the case of V. destructor, resistance to amitraz has been described in apiaries in the USA12,13,41, Mexico46, Argentina47, the Czech Republic48, France49, and Spain42. Correspondingly, our data revealed a low efficacy of amitraz in most of the samples collected from apiaries in Alberta. Five out of six tested apiaries showed less than 55% efficacy. Interestingly, one operation (AB-92, Clyde County) presented a 92% efficacy for Apivar in the Apiarium bioassay. However, the genetic assay found 53% of the individuals to be homozygous for the mutant allele H215 in AB-92 samples collected through the provincial inspection. This discrepancy may have been caused by unlikely but possible differences in the frequency of resistance genes between individual colonies that were separately sampled for the Apiarium bioassay and the genotyping. The information provided by the AB-92 beekeeper confirmed that there was no selection pressure from amitraz (i.e., Apivar) in this apiary for a few years, since only oxalic acid was applied in that period. An increase use of oxalic acid seems to be the prevailing trend, with the majority of beekeepers (59%) participating in our study disclosing that they applied oxalic acid to control mites in both spring and fall. However, amitraz continues to be extensively used throughout the province: 41% of beekeepers in this study treated their colonies with this varroacide at least once in the previous year. A smaller proportion (18%) opted for combinations of both varroacides or even added formic acid to the mixture (Table S2). These responses agree with a Canada-wide survey stating that miticides containing amitraz, oxalic or formic acids and thymol are the major varroacides currently used in Canadian operations5.
The heterogeneity of phenotypic resistance and frequency of resistance alleles among apiaries that are sometimes in close proximity suggest that the individual apicultural practices of each operation influence resistance evolution. This argument is further supported by the significant differences in homozygous H215 frequency among beekeepers with different V. destructor treatment practices (Fig. 3). Our results thus confirm the value of replacing the use of a single synthetic varroacide with multiple control measures in the context of an IPM plan. These approaches can involve cultural (e.g. monitoring, promoting colony strength, and brood interruption), biological (e.g. resistant or tolerant bee stocks), and chemical (e.g. rotating synthetic miticides with organic compounds) practices11,50. In light of the recent predominance of amitraz-based V. destructor control, our finding of amitraz resistance in Alberta suggests that regular monitoring of resistance23 to adapt management strategies are key components of successful and sustainable V. destructor mite control.
So far, the resistance to amitraz and pyrethroids in V. destructor has been associated with amino acid substitutions in their respective target molecules25,26,40. Although it is possible that other mechanisms, including the increased detoxification of the active ingredients, may be contributing to the resistant phenotype in certain populations, there is no conclusive evidence supporting such association and therefore it was not studied here. The frequency of mutations in the target sites was determined in some of the samples bioassayed in this study, as well as in samples collected from routine inspections in 2020 and 2022. The genotyping of single mites showed that these mutations were present in the population as early as 2020. Even though we sampled for two years and at multiple locations, we have a very limited picture of the temporal and spatial dynamics of resistance in V. destructor populations and the resistant alleles might have been present in Alberta much earlier. Furthermore, we only examined known mutations and cannot exclude the evolution of novel resistance alleles in Alberta mites. In the case of amitraz-resistant mutations, we only detected Y215H, which likely due to the geographical proximity of Canada and the USA where Y215H has been found repeatedly41. However, the population origins of V. destructor in Canada are not clear and further investigations may be conducted to determine the actual origin of the resistant alleles detected in Canada. The presence of N87S or another unidentified mutations associated with resistance cannot be ruled out, since Canada imports honey bees from a variety of countries and resistance evolution is a dynamic process.
The frequency of the mutant alleles to amitraz in the samples analysed showed high variability, with only two samples (Rocky view, AB-31, and Strathcona, AB-43) being free of mutant mites. Since apiary AB-43 was a relatively mite-free operation, we were able to collect only seven mites for genotyping analysis. Consequently, the conclusion that AB-43 is an operation with a fully susceptible V. destructor population relies on a small sample size. Several other factors may contribute to the observed variability, with the treatment scheme being the most important. Once a mutation associated with resistance is present in the mite population, each treatment with amitraz selects for mites carrying the mutation over those with the wild type. Thus, a high frequency of mutants is often correlated with mites recently treated with amitraz. Our results align with this scenario because apiaries with high amitraz usage, such as AB-18 (Fairview), AB-20 (Smoky River), AB-69 (Brooks), AB-73 (High River), and AB-77 (Oyen), showed a higher prevalence in the frequency of mutant mites. On the other hand, the rate at which a resistance mutation disappears from the population once amitraz is removed from treatments will depend on the fitness cost imposed by the mutation. Samples collected in Tofield (AB-32), Bezanson (AB-39), Wanham (AB-87, AB-88, AB-89, and AB-90), and Clyde (AB-92) carried H215 mutants even though beekeepers reported using oxalic acid as the predominant treatment during the last few years. In these cases, frequent amitraz applications may have led to a build-up of resistance alleles in the past and current counter selection in the absence of amitraz has not been strong enough to eliminate the resistance alleles from these populations. The alternative of amitraz residues in wax and other hive components continuing to select for resistance across multiple years seems unlikely51.
In Canada, the use of tau-fluvalinate and flumethrin17,18 has been reduced for years due to the evolution of resistance5. Nevertheless, our results show that mutations associated with resistance to pyrethroids are still present at considerable frequencies in the evaluated colonies. The mutations L925I and L925M have already been detected in many locations of the USA26,27. Mutations L925V and M918L, which are also associated with resistance to pyrethroids but have been geographically restricted so far to European countries27,30, were not found in this study. Our data indicates that L925I and L925M were present in 2020, but it is likely that these mutations existed in Canadian apiaries long before 2020 because resistance to pyrethroids has been common in Canada since 200117. The inbreeding reproduction system of Varroa might help the persistence of resistance mutations in mite populations even at the absence of chemical use52. The mites carrying resistance against pyrethroids suffer a fitness cost32,53, which should lead to the loss of resistance. However, tau-fluvalinate and flumethrin can persist and accumulate in bees wax and other colony products54,55,56,57, maintaining selection in favour of the resistance alleles. In this study, we were not able to simultaneously evaluate the phenotypic resistance to pyrethroids along with the tests of Apivar, but given sufficient mite samples, the Apiarium technique can be adapted to evaluate the phenotypic resistance to pyrethroids as well.
Genetic testing has the advantage that the same individuals can be assayed for many different loci and we thus could confirm that many individuals carried mutations associated with the resistance to amitraz and to pyrethroids, likely conferring some level of resistance to both synthetic miticide classes. The accumulation of multiple resistances reduces the spectrum of treatments that beekeepers can use for V. destructor control. Once established, resistance alleles can spread quickly through populations, making proper pesticide stewardship and IPM to avoid the establishment of resistance therefore crucial from the very beginning of the use of a novel compound. In regards to the varroacides currently used by beekeepers, our study along with others that show spatial and temporal variability of resistances16,27 indicate that individual beekeeper practices matter and it is not possible to generalise regarding the situation of the resistance in an area, province or country. Consequently, timely and specific tests are required to have trustworthy and reliable information regarding the frequency of mutants in a particular apiary, which can then allow beekeepers to make an informed decision when considering the best possible management for their operation.
Materials and methods
Mite collection samples
V. destructor mite samples were collected during summer and fall (August-October) from apiaries in three regions of Alberta (north west, central, and south) before beekeepers had applied fall miticide treatments. One set of mites was randomly collected from operations that underwent provincial regulatory inspection in 2020 and 2022 (Table S1). A second group of mites was collected from six apiaries whose owners reported a loss of Apivar efficacy under the provincial monitoring program in 2022 (Table 1). A sample of approximately 1000 adult bees were collected from brood frames of colonies with a mite infestation exceeding the fall economic threshold (> 3%) and transported live in a shipping box to the laboratory for further analysis. The efficacy of Apivar was evaluated using the Apiarium test with live mites43. For genetic assays, all mite samples from inspections and Apiarium tests were placed in 1.5 mL centrifuge tubes (Fisher brand, Canada), preserved at −20 °C in 96% ethanol, and subsequently shipped for genotyping to the University of Valencia (Spain). The history of V. destructor treatment in each operation was collected through a questionnaire (Table S2).
Apivar efficacy assay
Following previous protocols43, Apiarium cages were treated with small pieces of Apivar strips (3 cm long). Strips were placed into the incision of the cages and hung using a binder clips (1 ¼″, Staples, Canada). Each colony was studied in three replicate cages containing an Apivar strip and three negative control cages with a piece of inert plastic strip (1 × 2.54 cm) without chemical. Each cage replicate received a quarter cup (60 mL) of bees from a colony’s live shipping box, and was provided with two sugar cubes. Each Apiarium was placed on a piece of circular sticky board (Contech Inc., BC, Canada) in Petri dishes (15 cm diameter, Fisher brand, Canada) and incubated for 4 h at 25 ± 1 °C and 60 ± 5% RH in the dark. After the exposure period (4 h), all live and dead V. destructor that dropped from the bee cluster were counted and removed from sticky boards and the Apiarium cage’s body. At the end of the trials, all cages were placed in the freezer at −20 °C for 2–3 h, and the alcohol wash technique was used to collect the mites that remained on the bee cluster58.
Mortality was assessed using a fine-tipped paint brush by gently probing immobile mites. Mites with no appendage movement were considered dead. The number of mites remaining on the bees was determined by a subsequent alcohol wash. The total number of mites was calculated by summing all mites dropping (live and dead) during the trials and the remaining mites collected from the bee cluster after the alcohol wash. The knockdown rate was defined as percentage of live and dead mites that dropped among all mites. Mite mortality rate was analyzed based on the dead mite counts43. The efficacy of Apivar was assessed based on reduction in the mean abundance of V. destructor for the pre-exposure (before) and post-exposure (after) in treatments and control groups using the following equation59: Efficacy (%) = [1 − ((Ta × Cb)/(Tb × Ca))] × 100. Where Tb and Ta indicate the mean abundance of mites before and after exposure to Apivar, respectively; and Cb and Ca indicate the mean abundance of mites for the negative control at the same time of exposure (before and after, respectively). The effective threshold for each operation was calculated based on the pre-treatment mean abundance of mites43. At the end of the trials, the rates of knockdown, mite mortality, and efficacy were calculated at 4 h post-exposure.
Genotyping of mites
The presence of mutant alleles associated with resistance to pyrethroids or amitraz were detected by specific high-throughput genotyping based on TaqMan™ assays. A detailed description of the methodology, as well as the sequences of specific probes and primers for detecting each mutation (Table S3), can be found in González-Cabrera et al.26 and in Hernández‑Rodríguez et al.40 for the mutations associated with pyrethroids or amitraz resistance, respectively. Briefly, the mites were placed in a 96-well plate (one mite per well) and incubated at room temperature for 3 min in 20 µl of 0.25 M NaOH. Then they were ground with a plastic homogeniser and the solution was neutralised adding 10 µl of 0.25 M HCl, 5 µl of 0.5 M Tris-HCl and 5 µl of 2% Triton X-100. The plate was further incubated as above and then spun at 32,000×g for 5 min. The supernatant containing the genomic DNA was stored at − 20 °C until used. For the TaqMan™ assay, 1.5 µl of genomic DNA solution extracted from a single mite was mixed with 7.5 µl of 2× TaqMan Fast Advanced Master mix (Thermo Fisher Scientific, Madrid, Spain), 0.9 mM of each primer, and 0.2 mM of each probe in a total reaction volume of 15 µl. Cycling conditions were set as follows: 2 min at 50 °C, 10 min at 95 °C followed by 40 cycles of 15 s at 95 °C and 45 s at 60 °C. The increase of fluorescence was monitored in real time by acquiring each cycle on the relevant filter of a StepOne Real-Time PCR System (Thermo Fisher Scientific, Madrid, Spain). Each genotype was assigned according to the fluorescence profile recorded along the run. Genotyping data processing was carried out using the dedicated software StepOne Software ver. 2.3 (Thermo Fisher Scientific, Madrid, Spain).
Statistical analysis
Mixed model ANOVAs were applied to analyze the knockdown and mite mortality rates, and efficacy. In the Apiarium test, apiaries were analyzed as treatments and cages were replicates and treated as random effects60. The Pearson’s Chi-squared test and mixed model ANOVA were used to test whether the mutations frequency differed among years, regions, and treatment regimes. The normality of the variables was tested using Shapiro–Wilk tests. Variables that represented proportions and differed significantly from a normal distribution were arcsine transformed prior to analyses. The Bonferroni post-hoc test was used to compare mean values of the mite mortality rate, knockdown rate, and efficacy. Since the total number of V. destructor was not equal in all replicates in the Apiarium test, a weighted statement (i.e. weight total mite) was applied in the corresponding analyses.
Data availability
Data pertaining to this study can be obtained from the corresponding author upon request.
References
Holden, C. Report warns of looming pollination crisis in North America. Science 134, 397–397 (2006).
Calderone, N. W. Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS One. 7, e37235 (2012).
Statistic & Canada Production and value of honey (2022). https://agriculture.canada.ca/sites/default/files/documents/2023-08/HoneyReport_2022-eng.pdf. Accessed May 2024.
Guzmán-Novoa, E. et al. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41, 443–450 (2010).
Canadian Association of Professional Apiculturists (CAPA). Statement on Honey Bee Wintering Losses in Canada (2023). https://capabees.com/shared/CAPA-Statement-on-Colony-Losses-2022-2023_final.pdf. Accessed May 2024.
Di Prisco, G. et al. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 92, 151–155 (2010).
Posada-Florez, F. et al. Deformed wing virus type A, a major honey bee pathogen, is vectored by the mite Varroa destructor in a non-propagative manner. Sci. Rep. 9, 12445 (2019).
Han, B. et al. Life-history stage determines the diet of ectoparasitic mites on their honey bee hosts. Nat. Commun. 15, 725 (2024).
Schafer, M. O. et al. Concurrent parasitism alters thermoregulation in honey bee (Hymenoptera: Apidae) winter clusters. Ann. Entomol. Soc. Am. 104, 476–482 (2011).
van der Steen, J. & Flemming, V. Varroa conarol: A brief overview of available methods. Bee World. 98, 50–56 (2021).
Jack, C. J. & Ellis, J. D. Integrated pest management control of Varroa destructor (Acari: Varroidae), the most damaging pest of (Apis mellifera L. (Hymenoptera: Apidae)) colonies. J. Insect Sci. 21, 6 (2021).
Elzen, P. J. et al. Detection of resistance in US Varroa jacobsoni Oud. (Mesostigmata: Varroidae) to the acaricide fluvalinate. Apidologie 30, 13–17 (1999).
Elzen, P. J. et al. Control of Varroa jacobsoni Oud. Resistant to fluvalinate and amitraz using coumaphos. Apidologie 31, 437–441 (2000).
Millán-Leiva, A., Hernández-Rodríguez, C. S. & González-Cabrera, J. New PCR–RFLP diagnostics methodology for detecting Varroa destructor resistant to synthetic pyrethroids. J. Pest Sci. 91, 937–941 (2018).
Stara, J. et al. Detection of tau-fluvalinate resistance in the mite Varroa destructor based on the comparison of vial test and PCR–RFLP of kdr mutation in sodium channel gene. Exp. Appl. Acarol. 77, 161–171 (2019).
Hernández-Rodríguez, C. S. et al. Large-scale monitoring of resistance to coumaphos, amitraz, and pyrethroids in Varroa destructor. Insects 12, 27 (2021).
Currie, R. W., Pernal, S. F. & Guzmán-Novoa, E. Honey bee colony losses in Canada. J. Apicult. Res. 49, 104–106 (2010).
Bahreini, R. Honey bee pest control products registered for use in Canada. Alberta Bee News 10–13 (Alberta Beekeepers Commission, 2023).
Vandervalk, L. P., Nasr, M. E. & Dosdall, L. M. New miticides for integrated pest management of Varroa destructor (Acari: Varroidae) in honey bee colonies on the Canadian prairies. J. Econ. Entomol. 107, 2030–2036 (2014).
Al Naggar, Y. et al. Effects of treatments with Apivar and Thymovar on V. destructor populations, virus infections and indoor winter survival of Canadian honey bee (Apis mellifera L.) colonies. J. Apicult. Res. 54, 548–554 (2015).
Olmstead, S. et al. Apivar and Bayvarol suppress varroa mites in honey bee colonies in Canadian maritime provinces. J. Acad. Entomol. Soc. 15, 46–49 (2019).
Bahreini, R. et al. Miticidal activity of fenazaquin and fenpyroximate against Varroa destructor, an ectoparasite of Apis mellifera. Pest Manag. Sci. 78, 1686–1697 (2022).
Morfin, N. et al. Surveillance of synthetic acaricide efficacy against Varroa destructor in Ontario, Canada. Can. Entomol. 154, e17 (2022).
Muirhead, S. Varroa resistance in Alberta. Alberta Bee News (Alberta Beekeepers Commission, 2023).
Gonzalez-Cabrera, J. et al. An amino acid substitution (L925V) associated with resistance to pyrethroids in Varroa destructor. PLoS One 8, e82941 (2013).
González-Cabrera, J. et al. Novel mutations in the voltage-gated sodium channel of pyrethroid-resistant Varroa destructor populations from the Southeastern USA. PLoS One 11, e0155332 (2016).
Millán-Leiva, A. et al. Mutations associated with pyrethroid resistance in Varroa mite, a parasite of honey bees, are widespread across the United States. Pest Manag. Sci. 77, 3241–3249 (2021).
Vlogiannitis, S. et al. Reduced proinsecticide activation by cytochrome P450 confers coumaphos resistance in the major bee parasite Varroa destructor. Proc. Natl. Acad. Sci. 118, e2020380118 (2021).
Yu, S. J. The Toxicology and Biochemistry of Insecticides (CRC Press, 2008).
Dong, K. et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 50, 1–17 (2014).
De Rouck, S., Inak, E., Dermauw, W. & Van Leeuwen, T. A review of the molecular mechanisms of acaricide resistance in mites and ticks. Insect Biochem. Mol. Biol. 28, 103981 (2023).
González-Cabrera, J. et al. A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite, Varroa destructor. J. Pest Sci. 91, 1137–1144 (2018).
Wang, R. et al. Association of novel mutations in a sodium channel gene with fluvalinate resistance in the mite, Varroa destructor. J. Apicult. Res. 41, 17–25 (2002).
Alissandrakis, E., Ilias, A. & Tsagkarakou, A. Pyrethroid target site resistance in Greek populations of the honey bee parasite Varroa destructor (Acari: Varroidae). J. Apicult. Res. 56, 625–630 (2017).
Almecija, G. et al. Varroa destructor resistance to tau-fluvalinate: relationship between in vitro phenotypic test and VGSC L925V mutation. Pest Manag. Sci. 78, 5097–5105 (2022).
Evans, P. D. & Gee, J. D. Action of formamidine pesticides on octopamine receptors. Nature 287, 60–62 (1980).
Hollingworth, R. M. & Lund, A. E. Biological and Neurotoxic Effects of Amidine Pesticides (FAO, 1982).
Dudai, Y. et al. Formamidines interact with Drosophila octopamine receptors, alter the flies’ behavior and reduce their learning ability. J. Comp. Physiol. A 161, 739–746 (1987).
Takata, M. et al. A point mutation in the β-adrenergic‐like octopamine receptor: possible association with amitraz resistance. Pest Manag. Sci. 76, 3720–3728 (2020).
Hernández-Rodríguez, C. S. et al. Resistance to amitraz in the parasitic honey bee mite Varroa destructor is associated with mutations in the β-adrenergic-like octopamine receptor. J. Pest Sci., 1–17 (2022).
Rinkevich, F. D. et al. Confirmation of the Y215H mutation in the β2-octopamine receptor in Varroa destructor is associated with contemporary cases of amitraz resistance in the United States. Pest Manag. Sci. 79, 2840–2845 (2023).
Hernández-Rodríguez, C. S., Moreno‐Martí, S., Emilova‐Kirilova, K. & González‐Cabrera, J. A new mutation in the octopamine receptor associated with amitraz resistance in Varroa destructor. Pest Manag. Sci. (2024).
Bahreini, R. et al. Comparing the efficacy of synthetic varroacides and Varroa destructor phenotypic resistance using Apiarium and Mason jar bioassay techniques. Pest Manag. Sci. 80, 1577–1592 (2024).
Davies, T. G., Field, L. M., Usherwood, P. N. & Williamson, M. S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life. 59, 151–162 (2007).
Jonsson, N. N. & Hope, M. Progress in the epidemiology and diagnosis of amitraz resistance in the cattle tick Boophilus microplus. Vet. Parasitol. 146, 193–198 (2007).
Rodríguez-Dehaibes, S. R. et al. Resistance to amitraz and flumethrin in Varroa destructor populations from Veracruz, Mexico. J. Apicult. Res. 44, 124–125 (2005).
Maggi, M. D. et al. Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. Parasitol. Res. 107, 1189–1192 (2010).
Kamler, M. et al. Comparison of tau-fluvalinate, acrinathrin, and amitraz effects on susceptible and resistant populations of Varroa destructor in a vial test. Exp. Appl. Acarol. 69, 1–9 (2016).
Almecija, G. et al. Inventory of Varroa destructor susceptibility to amitraz and tau-fluvalinate in France. Exp. Appl. Acarol. 82, 1–16 (2020).
Vilarem, C. et al. Varroa destructor from the laboratory to the field: control, biocontrol and IPM perspectives—A review. Insects 12, 800 (2021).
Chaimanee, V., Johnson, J. & Pettis, J. S. Determination of amitraz and its metabolites residue in honey and beeswax after apivar treatment in honey bee (Apis mellifera) colonies. J. Apicult. Res. 61, 213–218 (2022).
Lester, P. J. Integrated resistance management for acaricide use on Varroa destructor. Front. Bee Sci. 1, 1297326 (2023).
Milani, N. & Della Vedova, G. Decline in the proportion of mites resistant to fluvalinate in a population of Varroa destructor not treated with pyrethroids. Apidologie 33, 417–422 (2002).
Mullin, C. A. et al. High levels of miticides and agrochemicals in north American apiaries: implications for honey bee health. PLoS One. 5, e9754 (2010).
Kiljanek, T. et al. Multiple pesticide residues in live and poisoned honeybees–preliminary exposure assessment. Chemosphere 175, 36–44 (2017).
Calatayud-Vernich, P. et al. Pesticide residues in honey bees, pollen and beeswax: assessing beehive exposure. Environ. Pollut. 241, 106–114 (2018).
Végh, R. et al. Pesticide residues in bee bread, propolis, beeswax and royal jelly—a review of the literature and dietary risk assessment. Food Chem. Toxicol. 176, 113806 (2023).
Bahreini, R. et al. Evaluation of potential miticide toxicity to Varroa destructor and honey bees, Apis mellifera, under laboratory conditions. Sci. Rep. 10, 21529 (2020).
Henderson, C. F. & Tilton, E.W. Acaricides tested against the brown wheat mite. J. Econ. Entomol. 48, 157–161 (1955).
SAS Inc. SAS/STATVR 9.3 User’s Guide (SAS Institute Inc., 2012).
Acknowledgements
We thank Cassandra Docherty, Emily Olson, Nicole McCormick, Ali Panasiku, Andrew Nagy, Anton Grechukha, Wyatt Carson, and Olivia de Herdt for their technical support. Furthermore, we acknowledge the financial support of RDAR (Results Driven Agriculture Research) and the Alberta Beekeepers Commission. The work at the Universitat de València was funded by MICIU/AEI/ 10.13039/501100011033 and by FEDER/EU, grant PID2022-140432OB-I00. Sara Moreno Martí was recipient of a PhD grant (PRE2019-090417) funded by MICIU/AEI /10.13039/501100011033 and by ESF+.
Author information
Authors and Affiliations
Contributions
Conceptualization and Design: R.B, J.G.C Sample analysis: R.B, J.G.C, C.S.H, S.M.M, S.M, R.L Methodology and Data Collection: R.B, J.G.C, C.S.H, S.M.M, S.M, R.L; Data Analysis: R.B, J.G.C, C.S.H, S.M.M; Data Curation and interpretation: R.B, J.G.C, C.S.H, S.M.M; Wrote First Draft: R.B, J.G.C, C.S.H, S.M.M; Revised Manuscript: R.B, J.G.C, C.S.H, S.M.M, S.M, R.L, O.R; All authors have read and approved the content of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bahreini, R., González-Cabrera, J., Hernández-Rodríguez, C.S. et al. Arising amitraz and pyrethroids resistance mutations in the ectoparasitic Varroa destructor mite in Canada. Sci Rep 15, 1587 (2025). https://doi.org/10.1038/s41598-025-85279-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85279-6
Keywords
This article is cited by
-
Field trials of the novel varroacide, 1-allyloxy-4-propoxybenzene, against Varroa destructor in Western Canada
Scientific Reports (2025)
-
Behavioral and molecular disruptions in honey bees induced by lithium chloride exposure
Scientific Reports (2025)