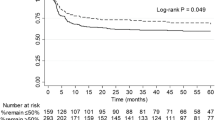
Abstract
Partial nephrectomy has become the gold standard treatment for small renal masses. This study aimed to assess the impact of soft coagulation hemostasis on parenchymal volume reduction of the operated kidney after an open partial nephrectomy. We retrospectively reviewed 94 patients with small renal tumors who underwent open partial nephrectomy with soft-coagulation hemostasis at our institution. We measured the preoperative and postoperative renal volumes by computed tomography (CT) and calculated the renal volume reduction ratio as postoperative volume/preoperative volume. We performed multivariate analysis to identify the predictors of renal volume reduction. The median renal volume ratio was 0.75, and the median renal volume reduction rate was − 2.49 cm3/month (IQR, − 3.33 to − 1.59). The RENAL score was inversely associated with ipsilateral renal volume reduction. In multivariate analysis, RENAL score, and tumor size were independent predictors of postoperative renal volume reduction. Soft coagulation hemostasis may influence the postoperative renal volume after partial nephrectomy, especially in patients with complex tumors.
Similar content being viewed by others
Introduction
Partial nephrectomy (PN) is the gold standard surgical treatment for renal tumors smaller than 7 cm in diameter1,2,3. One of the main objectives of PN is to preserve renal function, and the effect of different surgical techniques, including renal artery clamping and renorrhaphy, on postoperative renal function is still debated.
The common methods of hemostasis after tumor excision are soft-coagulation or parenchymal suture and renorrhaphy of the renal cortex4,5,6,7. Some studies have reported that soft coagulation is superior to renorrhaphy in preserving renal function, but this is inconclusive6. Our previous report investigated the preservation of the estimated glomerular filtration ratio (eGFR) one and three months after surgery using soft coagulation. The eGFR preservation rates were 91.0% and 90.7%, respectively5.
While soft coagulation is effective for achieving hemostasis, it can also lead to denaturing and necrosis of renal parenchyma8. However, the impact of soft coagulation on kidney volume reduction after partial nephrectomy remains poorly understood. In this retrospective study, we aimed to assess the chronological changes in kidney volume following surgery and identify predictive factors associated with kidney volume reduction.
The decline in postoperative renal function after PN is attributed, in part, to the excision of functioning nephrons adjacent to the tumor and renorrhaphy, which results in focal devascularization9. Our institute performs PN using a soft coagulation system for hemostasis, omitting renal artery clamping and renorrhaphy. Despite this approach, we observed a trend toward volume decline in the operated kidney postoperatively. Various factors may contribute to this decline, including hemostasis methods. Our study aimed to shed light on the impact of soft coagulation hemostasis on parenchymal volume reduction in partial nephrectomy. We can optimize surgical techniques and improve patient outcomes by understanding these factors.
Results
Patient characteristics are listed in Table 1. The median age and median tumor size were 65 years (Interquartile range (IQR), 56–69.75) and 28 mm (IQR, 20.0–40.0), respectively. The median RENAL nephrometry score was 7 (IQR, 6–8). The surgical results are shown in Table 2. The median operative time and estimated blood loss were 124 min (IQR, 102–152) and 170 ml (IQR, 70–407), respectively. The median postoperative follow-up period was 14 months (IQR, 12–15).
Volume reduction of the operated kidney was observed at a median ratio of 0.77, while the contralateral renal volume did not change at one-year follow-up (Fig. 1A). The median ipsilateral renal volume reduction rate was − 2.49 cm3/month (IQR, − 3.33 to − 1.59), and the volume reduction correlated with estimated glomerular filtration rate (eGFR) decline (r = 0.63) (Fig. 1B).
The RENAL score negatively correlated with volume reduction of the ipsilateral kidney (Fig. 2A). Notably, the RENAL score did not correlate with the resected renal parenchymal volume (Fig. 2B). Univariate analysis revealed tumor size, RENAL score, operation time, and estimated blood loss correlated with renal volume reduction (Table 3). Further, multivariate analysis revealed that preoperative eGFR, RENAL score, and the estimated blood loss were the independent predictors of the postoperative volume reduction with an unstandardized regression coefficient B (95% CI) of − 0.026 (− 0.046, − 0.007), − 0.24 (− 0.45, − 0.032), and − 0.0017 (− 0.003, − 0.000), respectively (p-value, 0.007, 0.024 and 0.022, respectively) (Table 3).
Discussion
Renal function in the operated kidney occasionally experiences a decline of approximately 20% due to incomplete recovery from ischemia or nephron loss following partial nephrectomy. Factors such as excision of renal parenchyma and damage from reconstruction also play a critical role in postoperative renal function impairment9.
In our study, we performed partial nephrectomies using soft coagulation, avoiding renal hilar clamping and renal reconstruction, as reported previously4,5. Remarkably, renal function at one-year follow-up remained similar to that at three months postoperatively. The eGFR preservation rate was 89.0% at three months postoperatively and 86.9% at 12 months postoperatively4,5. However, the benefit of the off-clamp technique in preserving renal function after PN remains controversial. While some studies have reported less decline in renal function with off-clamp surgery than on-clamp surgery10, several other studies have failed to demonstrate the advantage of off-clamp surgery in eGFR preservation over on-clamp surgery in the setting of a pneumoperitoneum11,12,13,14.
The impact of renorrhaphy on postoperative renal function also remains a topic of debate. Omitting cortical renorrhaphy may contribute to eGFR preservation in the short postoperative period14,15,16,17. However, renorrhaphy can damage intraparenchymal vessels, which may lead to renal artery pseudoaneurysms. Notably, a propensity score-matched analysis of open partial nephrectomy found that renal artery pseudoaneurysms were more frequent in the renorrhaphy group18. Conversely, the non-renorrhaphy technique has not demonstrated benefits in preserving the vascularized parenchymal mass of the operated kidney and global renal function, especially for T1b renal tumors18. It is worth noting that in the study mentioned, soft coagulation hemostasis was applied exclusively to the non-renorrhaphy group19.
However, using soft coagulation for hemostasis can adversely affect renal function. Aggressively applying soft coagulation to control bleeding may damage the renal parenchyma, impairing renal function. For instance, when soft coagulation was employed until hemostasis was achieved during partial nephrectomy, denaturation of renal parenchyma was observed up to 4.6 mm from the surface, regardless of the cauterization time in vivo8,20. Fujisaki et al. reported in their pig experiments that the renal parenchymal temperature increased by 15.6 °C at a depth of 5 mm and 8.8 °C at 10 mm when the surface of the kidney was cauterized with soft-coag over a period of 2, 5 and 10 s8. Despite these concerns, off-clamp partial nephrectomy using a soft coagulation system is gaining recognition as a promising surgical technique due to its positive impact on postoperative renal function11,21.
Our study identified tumor size and the RENAL score as independent predictors for postoperative parenchymal volume reduction. Additionally, reports suggest that lower parenchymal mass preservation is associated with larger tumor size, greater tumor complexity, and longer ischemia time22,23. Complex tumors often necessitate aggressive soft coagulation to control bleeding, but this approach must be carefully balanced to avoid compromising renal parenchyma and causing renal dysfunction and volume loss.
This study did not assess the split renal function using 99 m Tc dimercaptosuccinic acid (DMSA) renography. Although previous reports have suggested that CT volumetry can serve as an alternative to DMSA renography for calculating split renal function, incorporating DMSA could have provided additional insights into our study24,25,26. Other limitations of our study include a small patient cohort and a relatively short follow-up period. Future investigation into the long-term effects of soft coagulation on renal volume and function is anticipated. Hemostasis, in partial nephrectomy, using a soft coagulation system that may impact postoperative renal volume, particularly in patients with complex tumors.
Methods
Patients
Of the 220 patients with renal tumors who underwent off-clamp non-nephrectomy partial nephrectomy at our institution from 2013 to 2020, 95 were included in this retrospective cohort study. Patients without computed tomography (CT) volumetry data, with multiple partial nephrectomies, bilateral tumors, and a horseshoe kidney were excluded. Patients who underwent a single unilateral partial nephrectomy and were followed up by CT scans for at least one year were included. We finally analyzed the clinical records of 94 patients.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of NTT Medical Center, Tokyo (ID: 21-3). Informed consent was obtained in the form of opt-out on the website. Those who were rejected were excluded. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013).
Surgical techniques
All patients underwent retroperitoneal open partial nephrectomy as described elsewhere4. Briefly, partial nephrectomy was performed by blunt separation and sharp cutting followed by hemostasis with monopolar SOFT COAG (VIO300D, ERBE, Germany). To minimize blood loss, tumor resection was advanced by millimeters at a time. The renal pedicle was not secured or clamped, and cortical renorrhaphy was omitted. Resection beds were sutured with 4-0 VICRYL® only when the collecting system was opened. Urine leakage was ruled out by intravenous injection of indigo carmine solution. TachoSil® was placed on the resection surface to ensure hemostasis.
Assessment of renal function and perioperative reduction in renal function
The eGFR was calculated using the equation:
Perioperative eGFR preservation at five days, one month, three months, and 12 months after surgery was calculated as:
Assessment of renal volume
Renal volume (RV) measurements were performed with the SYNAPSE SAI viewer (FUJIFILM, Tokyo, Japan). The images reconstructed from non-contrast CT (5 mm slice) were analyzed, and a radiologist supervised renal volumetry. The preoperative ipsilateral renal volume was calculated as (preoperative renal volume − tumor volume). The renal volume reduction rate was calculated as follows:
and the renal volume ratio was calculated as:
Statistical analysis
Friedmann’s test was performed for multi-group comparison. For post-hoc two-group analysis, the Mann–Whitney test was performed. Univariate and multivariate regression analyses were performed to identify postoperative renal volume reduction predictors. Statistical significance was set at p < 0.05. All statistical analyses were performed using the SPSS version 24 ((IBM co. ltd., Tokyo, Japan).
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Grivas, N. et al. Robot-assisted versus open partial nephrectomy: Comparison of outcomes. A systematic review. Minerva Urol. Nefrol. 712, 113–120. https://doi.org/10.23736/s0393-2249.19.03391-5 (2019).
Sri, D. et al. Robotic-assisted partial nephrectomy (RAPN) and standardization of outcome reporting: A prospective, observational study on reaching the “Trifecta and Pentafecta”. J. Robot. Surg. 15, 571–577. https://doi.org/10.1007/s11701-020-01141-z (2020).
Furukawa, J. et al. “Trifecta” outcomes of robot-assisted partial nephrectomy: A large Japanese multicenter study. Int. J. Clin. Oncol. 25, 347–353. https://doi.org/10.1007/s10147-019-01565-0 (2020).
Nakamura, M. et al. Assessment of surgical outcomes of off-clamp open partial nephrectomy without renorrhaphy for ≥T1b renal tumours. Int. J. Clin. Oncol. 26, 1955–1960. https://doi.org/10.1007/s10147-021-01966-0 (2021).
Nakamura, M. et al. Predictive factors for postoperative renal function after off-clamp, non-renorrhaphy partial nephrectomy. Transl. Androl. Urol. 11, 1226–1233. https://doi.org/10.21037/tau-22-321 (2022).
Nakamura, K. et al. Soft coagulation in robot-assisted partial nephrectomy without renorrhaphy: Comparison with standard suture. Int. J. Urol. 274, 352–354. https://doi.org/10.1111/iju.14195 (2020).
Tohi, Y. et al. Comparison of perioperative outcomes of robot-assisted partial nephrectomy without renorrhaphy: Comparative outcomes of cT1a versus cT1b renal tumors. Int. J. Urol. 26(9), 885–889. https://doi.org/10.1111/iju.14046 (2019).
Fujisaki, A. et al. Histological and radiological evaluation of thermal denaturation depth using soft coagulation during partial nephrectomy in living pigs. Int. J. Urol. 28, 1274–1280. https://doi.org/10.1111/iju.14672 (2021).
Mir, M. C. et al. Parenchymal volume preservation and ischemia during partial nephrectomy: Functional and volumetric analysis. Urology 82(2), 263–268. https://doi.org/10.1016/j.urology.2013.03.068 (2013).
Deng, W., Liu, X., Hu, J., Chen, L. & Fu, B. Off-clamp partial nephrectomy has a positive impact on short- and long-term renal function: A systematic review and meta-analysis. BMC Nephrol. 19, 188. https://doi.org/10.1186/s12882-018-0993-3 (2018).
Antonelli, A. et al. On-clamp versus off-clamp robotic partial nephrectomy: A systematic review and meta-analysis. Urologia 86, 52–62. https://doi.org/10.1177/0391560319847847 (2019).
Anderson, B. G. et al. Comparing Off-clamp and on-clamp robot-assisted partial nephrectomy: A prospective randomized trial. Urology 126, 102–109. https://doi.org/10.1016/j.urology.2018.11.053 (2019).
Anderson, B. G., Potretzke, A. M., Du, K., Vetter, J. & Figenshau, R. S. Off-clamp robot-assisted partial nephrectomy does not benefit short-term renal function: A matched cohort analysis. J. Robot. Surg. 12, 401–407. https://doi.org/10.1007/s11701-017-0745-6 (2018).
Kreshover, J. E., Kavoussi, L. R. & Richstone, L. Hilar clamping versus off-clamp laparoscopic partial nephrectomy for T1b tumors. Curr. Opin. Urol. 23, 399–402. https://doi.org/10.1097/MOU.0b013e3283632115 (2013).
Bahler, C. D. et al. Feasibility of omitting cortical renorrhaphy during robot-assisted partial nephrectomy: A matched analysis. J. Endourol. 29, 548–555. https://doi.org/10.1089/end.2014.0763 (2015).
Bertolo, R. et al. Suture techniques during laparoscopic and robot-assisted partial nephrectomy: A systematic review and quantitative synthesis of peri-operative outcomes. BJU Int. 123, 923–946. https://doi.org/10.1111/bju.14537 (2019).
Bertolo, R. et al. Systematic review and pooled analysis of the impact of renorrhaphy techniques on renal functional outcome after partial nephrectomy. Eur. Urol. Oncol. 2, 572–575. https://doi.org/10.1016/j.euo.2018.11.008 (2019).
Tachibana, H., Takagi, T., Kondo, T., Ishida, H. & Tanabe, K. Comparison of perioperative outcomes with or without renorrhaphy during open partial nephrectomy: A propensity score-matched analysis. Int. Braz. J. Urol. 44, 467–474. https://doi.org/10.1590/s1677-5538.ibju.2016.0581 (2018).
Takagi, T. et al. Assessment of surgical outcomes of the non-renorrhaphy technique in open partial nephrectomy for ≥T1b renal tumors. Urology 86, 529–533. https://doi.org/10.1016/j.urology.2015.05.018 (2015).
Ota, T. et al. Soft coagulation in partial nephrectomy without renorrhaphy: Feasibility of a new technique and early outcomes. Int. J. Urol. 21, 244–247. https://doi.org/10.1111/iju.12276 (2014).
Hongo, F. et al. Laparoscopic off-clamp partial nephrectomy using soft coagulation. Int. J. Urol. 22, 731–734. https://doi.org/10.1111/iju.12808 (2015).
Wu, J. et al. Vascularized parenchymal mass preserved with partial nephrectomy: Functional impact and predictive factors. Eur. Urol. Oncol. 2, 97–103. https://doi.org/10.1016/j.euo.2018.06.009 (2019).
Beksac, A. T. et al. Measuring volumetric segmentation changes in the ipsilateral and contralateral kidney postpartial nephrectomy. Urol. Oncol. 38, 798.e1-798.e7. https://doi.org/10.1016/j.urolonc.2020.05.016 (2020).
Knox, M. K., Rivers-Bowerman, M. D., Bardgett, H. P. & Cowan, N. C. Multidetector computed tomography with triple-bolus contrast medium administration protocol for preoperative anatomical and functional assessment of potential living renal donors. Eur. Radiol. 20, 2590–2599. https://doi.org/10.1007/s00330-010-1855-y (2010).
Habbous, S., Garcia-Ochoa, C., Brahm, G., Nguan, C. & Garg, A. X. Can split renal volume assessment by computed tomography replace nuclear split renal function in living kidney donor evaluations? A systematic review and meta-analysis. Can. J. Kidney Health Dis. 6, 2054358119875459. https://doi.org/10.1177/2054358119875459 (2019).
Lal, H. et al. Determination of split renal function in voluntary renal donors by multidetector computed tomography and nuclear renography: How well do they correlate?. SA J. Radiol. 25, 2009. https://doi.org/10.4102/sajr.v25i1.2009 (2021).
Funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
IT, MK, and MN wrote the original draft. IT, MK, TI, AO, YM, TM, and MN analyzed the data. HK, SK, YS, and MN supervised the research. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tsuru, I., Kusakabe, M., Izumi, T. et al. Impact of thermal denaturation on renal volume reduction after partial nephrectomy using soft coagulation hemostasis. Sci Rep 15, 1164 (2025). https://doi.org/10.1038/s41598-025-85362-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85362-y