Abstract
This research successfully synthesized semiconductive magnesioferrite (MgFe2O4) nanomaterials using a green chemistry method that utilizes the natural extract of Moringa olefeira serving as both a reducing and oxidizing agent. The optical characteristics and crystalline structure of the MgFe2O4 nanomaterials were analysed using photoluminescence, diffuse reflectance spectroscopy, and X-ray diffraction. Additionally, Fourier transform infrared spectroscopy provided valuable insights into the chemical bonding and composition. High-resolution transmission electron microscopy was employed to obtain extensive information on crystalline size and distribution. Furthermore, the electrochemical properties were assessed through cyclic voltammetry and electrochemical impedance spectroscopy, revealing an excellent voltametric response and pseudo-capacitive behaviour associated with faradaic reactions, as well as outstanding conductivity linked to the unique charge transport mechanisms present in the MgFe2O4 structure. The effectiveness of the MgFe2O4 nanomaterials in the photodegradation of methylene blue from aqueous solutions was evaluated under visible light irradiation. Photocatalytic experiments measured the influence of various parameters, including catalyst loading, dye concentration, and pH. The MgFe2O4 nanomaterials exhibited impressive photocatalytic degradation efficiency, achieving an 81% degradation rate at pH 5.0 within 120 min. Kinetic studies indicated that the degradation process adhered to a pseudo-first-order model, with a rate constant of 0.01533 min−1, signifying a rapid reaction under optimal conditions. This study provides a thorough understanding of the electrochemical properties and enhanced photocatalytic capabilities of MgFe2O4 nanomaterials, thereby advancing green nanotechnology for environmental remediation.
Similar content being viewed by others
Introduction
Nanomaterials, especially metal oxide nanoparticles, have attracted significant interest due to their distinctive characteristics, which include sensing capabilities, magnetism, electrical conductivity, capacitance, and catalytic properties1,2,3. Recent developments in green nanotechnology highlight the promise of biogenic methods for the sustainable production of nanomaterials, presenting a viable alternative to conventional techniques that often lead to environmental degradation. These environmentally friendly methods not only reduce environmental damage but also promote the development of innovative materials and composites essential for renewable energy generation, undertaking environmental issues, and enhancing quality of life4,5. Recent research by Saher and colleagues successfully synthesized Nd2Sn2O7 based nanostructured materials and cerium dioxide nanomaterials using greener methods for applications in photocatalysis and cancer therapy4,6. In contrast, biological synthesis has emerged as a viable, eco-friendly approach that efficiently produces magnetic ferrites while reducing harmful environmental effects. Studies have shown the effective utilization of various natural extracts to synthesize different binary metal oxides, including Nd2Sn2O7, ZnMn2O4, La2Zr2O7, CuFe2O4, MgFe2O4, Pr2CeO3, CoFe2O4, CoFe2O4@SiO2@Dy2Ce2O7, due to their favorable oxidation states and excellent electronic conductivity, making them suitable for applications in water treatment, energy storage, and medical fields5,6,7,8,9,10,11,12,13. Furthermore, nanostructured catalysts produced through green synthesis methods are increasingly being investigated for hydrogen production via water splitting, which is a promising pathway to clean energy. Nanocomposite materials, particularly those doped with rare-earth elements, along with V2O5, are well-regarded for their impressive properties across various applications, including photocatalysis, electrochemistry, and antibacterial functions14,15,16. A notable study published in Applied Surface Science Advances by Karthik Kannan and colleagues successfully synthesized a mixed metal oxide nanocomposite co-doped with Y3+ and Sm3+, highlighting its structural, electrochemical, photocatalytic, and antibacterial attributes14. Additionally, research by K. Chinnaiah and co-authors demonstrated that green-synthesized silver nanoparticles, when used as an electrode, achieved a specific capacitance of 825 F/g at a current density of 3 mA/g in a 1 M KOH electrolyte solution, maintaining a cyclic stability of approximately 88.35% after 5000 cycles at a current density of 5 mA/g15. Among these materials, spinel ferrites, especially magnesium ferrite (MgFe2O4), have gained attention as promising candidates due to their excellent chemical stability, notable magneto crystalline structure, and outstanding electrochemical properties. MgFe2O4 nanomaterials are highly versatile, finding applications in lithium-ion batteries, photocatalysis, sensors, and hyperthermia treatments, making them a significant focus of research across multiple disciplines. Their unique pseudo-capacitive characteristics enhance electrochemical capacitance through Faradaic reactions, establishing them as a viable choice for supercapacitor applications17,18,19. The significant electrochemical properties of MgFe2O4 nanoparticles, particularly when combined with conductive materials like carbon black, enhance their applicability in batteries and supercapacitors14,15,20. The intrinsic conductivity and large surface area of these nanoparticles facilitate improved charge transport, thereby increasing energy storage capabilities. In comparison to basic metal oxides, spinel ferrites demonstrate superior electrochemical performance and conductivity, making them well-suited for advanced applications17,18,19. As a result, there is growing interest in developing alternative, efficient, and cost-effective methods for synthesizing magnesium ferrite nanomaterials. Traditional production methods, including both physical and chemical approaches, often encounter issues such as excessive waste, high energy demands, and environmental implications10,11,12,20. Moreover, the unique characteristics and capability of MgFe2O4 to efficiently harness light and facilitate charge separation positions it as an outstanding candidate for efficient photocatalytic applications. With an optimal band gap of approximately 1.7 to 2.0 eV, MgFe2O4 efficiently captures visible light, improving photocatalytic activity. Unlike TiO2, which predominantly absorbs UV light, MgFe2O4 can harness visible light for enhanced photocatalytic degradation, particularly relevant in sunlight. Its spinel configuration promotes enhanced charge carrier separation, increasing effectiveness in decomposing organic pollutants such as dyes. Additionally, its intrinsic magnetic properties facilitate easy catalyst recovery post-reaction, a crucial advantage in wastewater treatment scenarios. MgFe2O4 nanomaterials play a multifaceted role in photocatalytic methylene blue (MB) dye degradation, acting as effective catalysts that facilitate the generation of reactive species, allowing for efficient dye degradation with the added advantages of magnetic separability and stability. This makes MgFe2O4 a promising candidate for environmental remediation techniques specializing in dye removal. MgFe2O4 can be doped or modified with other materials (e.g., metal ions, carbon-based materials) to further enhance its photocatalytic performance14,15,16,17,18,19. This tuning process can focus on enhancing light absorption, boosting surface reactivity, or improving the catalyst’s stability under various conditions. Recent developments highlight the potential for creating MgFe2O4 nanomaterials through environmentally friendly methods, underscoring their ecological advantages. Research has shown that fine-tuning the physical and chemical properties of MgFe2O4 can significantly improve its photocatalytic performance, thereby accelerating the breakdown of pollutants. A study conducted by Saeid Taghavi Fardood and colleagues utilized the biosynthesis of magnesium ferrite for the photocatalytic degradation of malachite green dye under visible light, achieving nearly 98% removal14,15,21. The aim of the research is to investigates the electrochemical and physical properties of Magnesioferrite (MgFe2O4) nanomaterials, with a focus on their photocatalytic effectiveness in degrading methylene blue, while emphasizing eco-friendly synthesis methods to meet the growing demand for sustainable materials in environmental remediation. Future investigations will search deeper into the interaction of these processes to enhance dye degradation efficiency and promote the advancement of innovative water treatment technologies.
Materials and methods
Chemicals: All substances used were of analytical quality and were obtained from Merck.
Magnesium nitrate (Mg(NO3)2), with a purity of 98%, from the company Merck.
Iron(III) nitrate (Fe(NO3)3)—98% purity, Merck.
Deionized water (DI-H2O), Moringa oleifera leaves, Hot plate.
Stirring rod, Filter paper, pH indicator.
Dried leaves of Moringa oleifera were sourced from Burkina Faso, with Zeba Abinatou from the Forestry Department of the Research Institute of Agriculture and Environment providing them. All aspects of the plant studies, including the collection process, adhered to relevant institutional, national, and international guidelines and regulations.
Preparation of plant extract
The extraction process began by boiling 300 ml of deionized water. Next, add 30 g of dried Moringa oleifera leaves to the boiling water. Then, reduce the temperature to 50 °C and maintain it for 1.75 h to optimize extraction efficiency while minimizing the degradation of sensitive compounds. Gentle stirring ensured even distribution of the plant material in the water, which facilitated the release of beneficial compounds into the solution. After the extraction was complete, allow the mixture to cool to room temperature before filtering it. Using filter paper, separate the liquid extract from the solid plant residues. Also measured the pH of the filtered extract and found it to be approximately 5.5, which provided insights into its acidity. Finally, store the filtered plant extract at 4 °C for future use.
Synthesizing MgFe2O4 nanoparticles through environmentally friendly methods
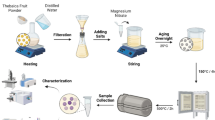
Mix 50 ml of the prepared Moringa extract with 3 g of magnesium nitrate and 3 g of iron (III) nitrate in a clean beaker. Mix the combination thoroughly to make sure the components are fully dissolved. It was evident that the iron-magnesium complex formed as a suspension since there was no precipitation observed. Covering the beaker with aluminum foil prevents contamination and evaporation while allowing the reaction to proceed at room temperature. The 18-h duration suggests that sufficient time is critical for the complexation reaction to reach completion. There is no heat, or additional chemicals should be introduced at this time. After 18 h, move the mixture to an oven and dry at 100 °C for several hours until all liquid evaporates, resulting in a solid powder. The temperature of 100 °C is sufficient for gentle drying without thermal decomposition. Rinse the dried powder several times using distilled water to eliminate any unreacted substances. The final step involves heating the dried powder in air for 2 h at elevated temperatures (500 °C and 700 °C). This approach used in this research was derived from the previous study22.
Characterization of MgFe2O4 nanoparticles
For high-resolution transmission electron microscopy (HRTEM), place a small quantity of MgFe2O4 nanoparticles onto a carbon-coated grid and examine them under a transmission electron microscope (TEM) at an accelerating voltage of 120 kV. Obtain SEM images to evaluate the morphology and size distribution of the MgFe2O4 nanoparticles. To perform elemental analysis, utilize energy-dispersive X-ray spectroscopy (EDS) on the same sample prepared for scanning electron microscopy (SEM) imaging at 5 keV. For the analysis of chemical bonding and surface coatings, employ Fourier-transform infrared spectroscopy (FT-IR) to obtain spectra within the range of 400–4000 cm2. Prepare a powdered sample of the nanoparticles for X-ray diffraction (XRD) and expose it to CuK radiation in a Bruker diffractometer to analyze phase composition and crystallographic structure. The diffuse reflectance (DRS) of MgFe2O4 was conducted on a Cary 5000 UV–Vis-NIR spectrophotometer equipped with an integrating sphere. The photoluminescence (PL) properties were examined with the high sensitivity QE Pro-FL spectrometer that had a signal–noise ratio of > 1000:1 (Ocean optics, FL, USA) with an ultraviolet light of wavelength of 240 nm.
Photocatalytic activity testing of MgFe2O4 nanomaterial
The experiment began with the preparation of a methylene blue (MB) solution. A total of 15 mg of methylene blue was dissolved in 1000 ml of distilled water (15 mg/L). The pH of this MB solution was then adjusted to 5.0. Afterwards, 100 ml of the prepared MB solution was mixed with 15 mg of MgFe2O4 nanomaterial in a separate container to facilitate the interaction of the dye with the nanomaterial. To enhance dye adsorption onto the MgFe2O4, the resulting mixture was stirred for 30 min at room temperature in a dark environment, allowing sufficient time for equilibration before initiating photocatalytic degradation. The mixture was exposed to a 270 W xenon arc lamp, tuned to a wavelength of 460 nm, to provide visible light for the photocatalytic reaction. During the reaction, the UV-Vis absorbance spectra of the solution were collected at 30-min intervals for a total duration of 2 h. The percentage degradation of methylene blue was calculated to measure the efficacy of the photocatalytic process. In proceeding experiments, varying initial concentrations of methylene blue, specifically 5, 10, and 15 ppm, were tested while keeping all other reaction conditions constant. The removal percentage of methylene blue was pursued and recorded concerning contact time, following to the procedures previously outlined. Further investigations explored the influence of varying dosages of MgFe2O4 nanoparticles, set at 5, 10, and 15 mg, while maintaining a constant initial methylene blue concentration and pH of the solution. Next, the effect of pH on the photodegradation rates was examined. The pH of the methylene blue solution was adjusted to 3.0, 5.0, 7.5, and 10 using acid or base solutions before the introduction of the MgFe2O4 photocatalyst.
Electrochemical testing
Preparation of GCE/MgFe2O4 electrode
Combine approximately 3 mg of MgFe2O4 with carbon black and polyvinylidene fluoride (PVDF) in a mortar to create a uniform mixture. Slowly add dimethyl formamide (DMF) until a smooth slurry is achieved. Thoroughly drop coat about 3 µl of this slurry onto the surface of a pre-cleaned glassy carbon electrode (GCE). Place the electrode in an oven at 35 °C for 1 h to facilitate drying. Finally, rinse the electrode with distilled water to eliminate any excess material.
Electrochemical measurements
Electrochemical tests were performed using a CH Instruments auto-lab potentiostat, utilizing both cyclic voltammetry and electrochemical impedance spectroscopy. The electrochemical cell was set up with a glassy carbon electrode as the working electrode, a platinum wire as the counter electrode, and an Ag/AgCl electrode as the reference, using a 3 M NaCl salt bridge solution. A 1 M Na2SO4 electrolyte solution was prepared, and its pH was adjusted to approximately 6.2. Before and after the measurements, the glassy carbon electrode was polished with alumina slurries of 1.0, 0.3, and 0.05 μm using Buehler polishing pads. Cyclic voltammetry was conducted over a potential range of − 0.6 to 0.6 V at a scan rate of 50 mV/s, along with additional measurements at varying scan rates from 20 to 100 mV/s. Electrochemical impedance spectroscopy was conducted with a perturbation amplitude of 10 mV over a frequency range of 100 kHz to 100 mHz, ensuring an oxygen-free environment by purging with high-purity argon gas throughout the testing process.
Results and discussion
High-resolution transmission electron microscopy (HRTEM) is an effective technique for revealing the physical properties of nanostructured materials. It offers significant understandings into various structural attributes, such as lattice parameters, crystallinity, particle size, and morphology. The HRTEM images depicted in Fig. 1A shows the morphological features of both non-annealed and annealed (at 500 °C and 700 °C) MgFe2O4 nanomaterials. The analysis revealed that all samples exhibited nanometer-scale dimensions, characterized by diverse shapes and irregular particle sizes. The non-annealed sample exhibited a spherical morphology with irregular particle sizes averaging around 2 nm. In contrast, the annealed samples demonstrated a notable increase in particle size, ranging from 10 nm to 35 nm, along with a transformation in shape, displaying both cubic and spherical forms, as shown by the particle size distribution in Fig. 1B. This finding is consistent with prior studies suggesting that thermal treatment generally facilitates sintering and particle growth due to enhanced kinetic energy and atomic mobility19,23. Notably, increasing the annealing temperature to 700 °C resulted in substantial particle growth and agglomeration. This effect may be associated with the improved magnetic properties linked to higher temperatures, which foster particle interactions and clustering19. The Selected Area Electron Diffraction (SAED) patterns for the non-annealed samples indicated an amorphous state, while the samples exposed to higher temperatures transitioned into a polycrystalline phase. This transition corroborates earlier research that associates enhanced crystallinity with increased thermal treatment, supporting the successful synthesis of MgFe2O4 with improved structural characteristics26.
Energy dispersive X-ray (EDX) spectroscopy, as shown in Fig. 2A, was employed to assess the elemental composition of the synthesized samples. The EDX spectra confirmed that magnesium (Mg), iron (Fe), and oxygen (O) are the primary elements in the MgFe2O4 nanomaterials, thereby supporting their successful synthesis as shown in Table 1 for elemental composition. The non-annealed sample exhibited additional peaks associated with elements such as potassium (K), phosphorus (P), silicon (Si), sulfur (S), and calcium (Ca), which likely originated from the biological components of the Moringa oleifera extract used in the synthesis. As the annealing temperature increased, the intensity of these peaks faded, indicating a shift towards a more improved MgFe2O4 phase. This observation aligns with prior research that demonstrates how thermal treatment effectively removes organic impurities and enhances material purity23.
The Fourier transform infrared (FTIR) spectra illustrated in Fig. 2B exhibit an absorption band near 550 cm−1, which is linked to the Mg–O–Fe bond, signifying the presence of metal oxides. This observation is consistent with earlier research conducted by Kurian et al.24 and Pradeep et al.25, who detected similar metal-oxygen vibrations in synthesized Magnesioferrite. The finding confirms the successful synthesis of MgFe2O4 nanomaterials through various techniques, including sol-gel and hydrothermal methods24,25. Such comparative analyses emphasize the effectiveness of the environmentally friendly approach utilized in our research, indicating its potential as a suitable alternative to conventional synthesis methods. Additionally, the FTIR spectra indicate multiple absorption bands associated with biological compounds from Moringa oleifera, found within the wavenumber ranges of 2846–3960 cm−1 (O–H stretching), 2340–2400 cm−1 (likely related to CO2), and 1300–1700 cm−1 (C=O stretching). Furthermore, other absorption peaks at 1200–1000 cm−1, 815 cm−1, 624 cm−1, and 488 cm−1 can be attributed to C–H, N–H, and C–O stretching modes. These bands have been recognized in previous studies, such as those referenced in26,27,28,29,30, which underscore the role of Moringa oleifera extracts in the synthesis of metal nanoparticles, highlighting the importance of these organic components in facilitating the biosynthesis process30. The observed absorption peaks are notably distinct around the absorption band of 550 cm−1, indicating the formation of metal-organic complexes and suggesting the successful incorporation of biological compounds in the synthesis of MgFe2O4 nanoparticles24. It is notable that as the temperature increases, there is a corresponding decrease in the intensity of the biological absorption bands within the wavenumber range of 2846–3960 cm−1 (O–H stretching). This change implies that the leading formation of the pure MgFe2O4 phase is facilitated by the annealing process, where higher thermal energy promotes the removal of organic residues while improving the crystallization of the metal oxide structure24. Figure 2C (schematic 1) depicts the proposed mechanism for the synthesis of MgFe2O4 nanomaterial. Upon the dissociation of precursors into metal ions (Mg2+ and Fe3+) in solution, the electrostatic interactions between the negatively charged anions from Moringa oleifera and the positively charged cations lead to the formation of metal-organic complexes. This interaction acts as a template during the synthesis, ultimately resulting in the production of MgFe2O4 nanomaterial following the annealing process. These findings underscore the growing emphasis on sustainable and eco-friendly synthesis methods in nanotechnology, as highlighted by the research of Sahar Zinatloo-Ajabshir and colleagues, who support the use of plant extracts in nanomaterial fabrication to reduce the environmental impact associated with traditional synthesis methods4,5.
XRD studies: structural analysis
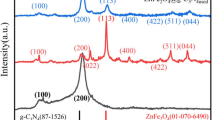
X-ray diffraction (XRD) analysis, illustrated in Fig. 3, was performed to examine the crystalline characteristics of the synthesized MgFe2O4 nanomaterial. The as-prepared sample (non-anneal) displayed an amorphous structure. In contrast, the samples that were calcined (500 and 700 °C) exhibited a distinct crystalline structure, confirming the changes that occur during the annealing process. The XRD patterns of the annealed samples showed a pair of significant diffraction peaks, indicating the development of nanoscale crystalline phases. This shift from an amorphous to a crystalline state is well-established in prior research, supporting the role of thermal annealing in enhancing crystallization12,21. The diffraction peaks observed suggest a face-centered cubic (FCC) structure for the pure MgFe2O4 nanomaterial, which corresponds closely with the standard diffraction patterns for the spinel structure as identified in JCPDS cards (00-045-0946; JCPDS36-0398). At an annealing temperature of 500 °C, the characteristic peaks for the annealed sample included the (200) and (220) planes. When the annealing temperature was increased to 700 °C, additional peaks corresponding to the (220), (222), (311), (111), (400), (422), (511), (440), and (531) crystallographic planes of the spinel phase were observed. This extensive peak distribution further substantiates the high degree of crystallinity and purity of the MgFe2O4 nanomaterial, consistent with previous studies that highlight the absence of crystalline impurities during synthesis21.
The absence of crystalline impurities in the XRD patterns highlights the declaration that our synthesis method effectively yields pure MgFe2O4. Scherrer’s formula (Eq. 1) was used for quantitative analysis, revealed average crystallite sizes of 10 nm and 12 nm for the samples annealed at 500 °C and 700 °C, respectively. These measurements align with findings from other research, which report similar crystallite sizes for MgFe2O4 produced under similar conditions21,31. Additionally, the calculated lattice parameters, obtained from the interplanar spacing relationships (Eq. 2), were found to be 7.3421 Å and 9.4569 Å for the samples treated at 500 °C and 700 °C, respectively. These values agree with those recognized in related studies, further confirming the structural integrity of the synthesized MgFe2O4 nanomaterial and its potential applications in varied fields such as photocatalysis and magnetic technologies21.
where D is the average crystallite size (nm), λ is the x-ray wavelength (15.405 nm) and K is Scherrer’s constant (K¼ 0.89). B is the full width at half maximum observed in radians and θ is the angle of diffraction.
Photoluminescence and diffuse reflectance spectroscopy analysis of MgFe2O4 nanomaterials
Figure 4A illustrates the photoluminescence (PL) spectra of green-synthesized MgFe2O4 nanomaterials, recorded both prior to and following annealing at temperatures of 500 °C and 700 °C. The measurements utilized a LED light source with an excitation wavelength of 240 nm. Distinct broad emission peaks are observed within the range of 400–580 nm, which can be linked to various oxidation states of iron (3d5 → 3d4/4s transitions of Fe+3)32,33,34. This may also indicate the presence of defect-related states or energy transitions involving localized states due to defects. Furthermore, an additional peak at 536 nm is attributed to singly ionized oxygen vacancies or surface defects resulting from trap states or deep-level emissions32,35. The spectrum indicates a broad emission peak with increased intensity in the annealed samples, implying the presence of multiple defect states or a variety of emission sites within the nanostructures. Additionally, the calcined samples exhibit very rough surfaces, which contribute to the scattering of both incident and emitted photons, leading to broader PL spectra, with reduced intensity32,36. The band gap energy values were calculated using Eq. (3) and presented in Table 2.
As particle size increases, Table 2 shows a trend of decreasing optical bandgap, likely due to improved crystallinity and a reduction in defects within the material, often observed at higher annealing temperatures. This enhancement leads to better electronic properties and facilitates increased charge carrier mobility, effectively narrowing the bandgap32,36.
Figure 4B presents the results of diffuse reflectance spectroscopy (DRS) for MgFe2O4, recorded over a wavelength range from 200 to 1000 nm. DRS serves as an effective method for analyzing the optical properties of materials, particularly their ability to reflect light at different wavelengths. Consistent with the typical behavior of oxides, our results indicate significant reflection within the visible spectrum. After undergoing annealing at two different temperatures (500 and 700 °C), the sample demonstrated a reflection of around 80%, suggesting that the material can reflect as much as 80% of light at certain wavelengths. This observation aligns with earlier research, including studies by Tanaka et al. and Tangcharoen, which emphasize that effective light reflection is essential for improving the photocatalytic efficiency of materials, especially in fields such as photocatalysis and solar energy conversion32,33,35.
Photocatalytic activity
The study investigated the photocatalytic activity of MgFe2O4 nanomaterial in degradation of methylene blue (MB), under UV irradiation. To verify that the MB decomposition was mainly due to the photocatalytic properties of MgFe2O4, control experiments were conducted in the absence of the catalyst. The pre-irradiation phase involved allowing the MgFe2O4 catalyst to interact with the MB solution in the absence of light. This stage was designed to reach adsorption equilibrium, ensuring that MB molecules were sufficiently attached to the surface of the catalyst prior to the commencement of the photocatalytic process. The solution was exposed to a xenon arc lamp emitting 270 W of visible light at a wavelength of 664 nm for a duration of 2 h. The absorbance spectrum was recorded across a range of 200 to 1000 nm, revealing two absorption peaks at approximately at 610 and 664 nm, which correspond to methylene blue as shown in Fig. 5A37,38. It is observed that the peak intensity at 664 nm gradually decreases without the appearance of any other new peak. The gradual decrease in MB absorbance over time indicates a successful photocatalytic process, highlighting the ability of MgFe2O4 to transform MB into harmless byproducts39,40. Previous research has shown similar patterns, with different photocatalysts exhibiting their effectiveness in lowering MB concentrations under similar experimental setups37,38,41,42.
The photocatalytic mechanism for the degradation of methylene blue (MB) using magnesium ferrite (MgFe2O4) involves several steps that promote the decomposition of the dye when exposed to visible light. This process can be explained as follows38,43,44,45,46:
Step 1: When MgFe2O4 is irradiated, it absorbs photons with energy equal to or greater than its band gap. This photon absorption excites electrons, promoting them from the valence band to the conduction band, resulting in the formation of electron-hole (e−/h+) pairs (Eq. 4).
Step 2: The high oxidation potential of the holes (h+CB) directly oxidizes organic compounds, such as the dye, leading to the formation of several reactive intermediates (Eq. 5).
Step 3: Hydroxyl radicals (·OH), which are highly reactive species, can be produced through the degradation of water molecules (Eq. 3) or by the reaction between hydroxyl ions (OH–) and holes (Eq. 4). The excited electrons (e−) and holes (h+) then migrate to the surface of MgFe2O4, where they interact with adsorbed oxygen and water, resulting in the generation of reactive oxygen species (ROS) such as hydroxyl radicals (Eqs. 6 and 7).
Superoxide anions (O2·−) are formed by the reduction of molecular oxygen, which occurs due to the presence of conduction band electrons at the surface of the photocatalyst (Eq. 8).
Reactive oxygen species can subsequently interact with methylene blue, facilitating its oxidation or degradation. This mechanism generally leads to the disintegration of the MB molecule into smaller, less pigmented fragments or may result in its total mineralization into oxygen and water (Eqs. 9 and 10). Additionally, the electrons in the conduction band play a role in generating hydroxyl radicals, which are recognized as the key agents responsible for the degradation of organic compounds38,43,44,45,46
The percentage degradation was calculated using the equation provided in Eq. (11) below42,43,44,45,46.
where Co represents the initial MB dye concentration and C denotes the final MB dye concentration.
The degradation percentage results illustrated in Fig. 5A indicate that the MgFe2O4 catalyst achieved around 81% degradation of methylene orange (MO) after 120 min of exposure to visible light. While the study reports a significant 81% degradation of methylene blue (MB), it is important to note that this value is somewhat lower than the rates documented by Fatima Allawi et al.47 and Ahmed48 in existing literature, where various photocatalysts have demonstrated degradation rates surpassing 90%. In Table 3 displays the comparison of degradation efficiency of MgFe2O4 in methylene blue. It is worth mentioning that MgFe2O4 stands out due to its cost-effectiveness, environmental safety, and ease of synthesis, which are essential considerations in the advancement of sustainable photocatalytic materials.
Effect of MgFe2O4 NPs dose
The study of the effect of MgFe2O4 nanomaterial dose from aqueous solutions highlights the critical role that the dosage of nanomaterials plays in the adsorption process. The experiments were conducted under control of parameters such as initial MO concentration, solution pH, and temperature, with a focus on varying the dosage of MgFe2O4 nanomaterial within the range of 5, 10, 15 mg, as depicted in Fig. 5B. It was observed that increasing the catalyst dose led to a marked enhancement in the removal efficiency of MO. This increase can be attributed to the availability of more active sites for adsorption as the dosage increases. Each nanoparticle has a predictable number of active sites that can interact with the dye molecules; therefore, a higher dose provides more sites for the dye molecules to bind, which facilitates greater adsorption49,50. At a dosage of 15 mg, the removal was high as 81%, illustrating the effectiveness of this specific dose in the adsorption process.
Effect of concentration of methylene blue (MO)
The experiments involved varying concentrations of MO while maintaining other reaction parameters constant. The results, illustrated in Fig. 5C, reveal a significant association between the concentration of MO and the percentage of removal over time at the specified concentrations of 5, 10, and 15 ppm. It is evident that an increase in MO concentration corresponds to an increase in removal efficiency. At higher concentrations, the difference in concentration between the bulk solution and the surface of the adsorbent becomes increasingly pronounced, which enhances the movement of MO molecules toward the active sites on the adsorbent. Additionally, the observed increase in removal efficiency can be attributed to the stronger interactions between the MO molecules and the surface of MgFe2O4 nanoparticles at higher concentrations. The increased ionic strength from concentrated MO solutions can result in enhanced electrostatic interactions and more favorable adsorption conditions. Previous research, as noted in49,50 supports this thought, indicating that higher concentrations of ionic pollutants can trigger nucleation events on the adsorbent’s surface, thereby promoting more effective adsorption.
Kinetics of photodegradation of methylene blue (MB)
The dye degradation reaction kinetics by the MgFe2O4 catalyst during the photocatalytic reaction were explained according to the pseudo-first-order kinetic model.
where Ct and C0 are the initial concentration of the dye and the concentration at the photocatalytic degradation time t (min), respectively. t is the time interval after each exposure of the suspension to the visible light, and Ka is the apparent rate constant (min−1)48,49,50.
Figure 5D demonstrates the relationship between the plot of − ln (Ct/Co) and irradiation time. This analysis aims to establish the reaction order of the photodegradation process. The linearity observed in the fitted curves indicates that the degradation of methylene blue (MB) follows pseudo-first-order kinetics (R2 > 98) when using a complete dose of MgFe2O4 nanomaterial. Additionally, as shown in Fig. 5D, an increase in the dose of MgFe2O4 nanomaterial results in a decrease in the apparent first-order rate constants, suggesting a non-elementary characteristic of the removal reactions. The kinetic rates for the photodegradation efficiency of MB with doses of 5, 10, and 15 mg of MgFe2O4 are 0.11776, 0.05978, and 0.01533 min−1, respectively. This dependence of reaction rate constants on the dose of MgFe2O4 NPs aligns well with the existing literature48,49,50.
Influence of pH on the photodegradation efficiency of methyl blue using MgFe2O4 as a photocatalyst
This study investigates how varying pH levels influence the photodegradation of methyl blue (MB) when utilizing MgFe2O4 nanomaterial as a photocatalyst. The experimental setup involved modifying the pH of the MB solution to four distinct values: 3.0, 5.0, 7.5, and 10.0, achieved through the addition of hydrochloric acid (HCl) and sodium hydroxide (NaOH). The findings reveal a notable pattern in the removal efficiency of methyl blue. As the pH increased from 3.0 to 5.0, the removal efficiency improved significantly, peaking at 81% at pH 5.0. However, this efficiency declined at pH levels of 7.5 and 10.0, as illustrated in Fig. 6A. The fluctuations in removal rates can be linked to various chemical interactions that depend on pH50. Under acidic conditions at pH 3, the higher concentration of protons (H+) facilitates the adsorption of anionic dyes such as methyl blue onto the MgFe2O4 photocatalyst’s surface50. The increased protonation of the dye molecules enhances their vulnerability to photodegradation, resulting in greater degradation efficiency as more dye molecules occupy the active sites on the photocatalyst. In contrast, in alkaline conditions, the ionization of methyl blue reduces its adsorption to the MgFe2O4 surface, leading to the observed reduction in removal efficiency at elevated pH levels50.
Determining the point of zero charge (PZC) for MgFe2O4 nanomaterial is essential for comprehending its surface properties across different pH levels, especially in contexts such as adsorption and wastewater treatment48,49,50. In this study, 15 mg of the nanocomposite was suspended in 50 mL of a 0.01 M NaCl solution to assess the PZC of MgFe2O4 nanoparticles. The pH was adjusted to values of 3.0, 5.0, 7.5, and 10 using hydrochloric acid (HCl) and sodium hydroxide (NaOH). To facilitate effective interaction between the nanomaterial and the solution, the samples were agitated at 200 rpm for a duration of 48 h. The point of zero charge (PZC) is an essential factor for understanding the surface characteristics of materials, particularly regarding adsorption mechanisms and their interactions with ions in solution48,49. The data illustrated in Fig. 6B indicates that there is no notable difference between the initial and final pH values, suggesting that at a pH of 7.2, the surface charge of the MgFe2O4 nanocomposite is neutral. When the pH is below 7.2, the nanocomposite exhibits a positive surface charge, which can enhance the adsorption of negatively charged species (anions) from the solution. While, at pH levels above 7.2, the nanocomposite displays a negative charge, promoting the adsorption of positively charged species (cations)48,49.
The stability and reusability of the photocatalyst were assessed through the photodegradation of methyl orange (MO) using 10 mg of MgFe2O4 nanomaterial under visible light irradiation, as illustrated in Fig. 6C. Initially, the MgFe2O4 photocatalyst was employed to degrade MO under visible light. Following the completion of the first test cycle, the catalyst was centrifuged, was rinsed with water and ethanol, and then dried to prepare it for recycling. This procedure is crucial for eliminating any residual dye that may adhere to the catalyst’s surface, as such residues could impair its performance in later cycles47,50. The reused 10 mg of MgFe2O4 photocatalyst was then subjected to the same experimental conditions for five additional cycles, as shown in the Fig. 6C. By the end of the fifth cycle, the photodegradation efficiency of the MgFe2O4 catalyst exhibited a slight decrease. This reduction in efficiency may be due to several factors, including alterations in the catalyst’s morphology, leaching of active components, or blockage of active sites by organic molecules. Numerous studies have reported similar gradual decreases in photocatalytic efficiency after multiple reuse cycles47,50.
Electrochemical characterization of MgFe2O4 nanomaterial.
The electrochemical performance of MgFe2O4 annealed at 700 °C and activated with carbon black, MgFe2O4 annealed at 700 °C, and MgFe2O4 non-annealed was investigated using cyclic voltammetry (CV), linear sweep voltammetry (LSV), and electrochemical impedance spectroscopy (EIS). Cyclic voltammetry is the most extensively used technique for acquiring qualitative information about electrochemical reactions17. It enables the rapid identification of redox potentials distinctive of the electroactive species under investigation, providing considerable information about the thermodynamics of a redox process, the kinetics of heterogeneous electron-transfer reactions, and the analysis of coupled electrochemical reactions or adsorption processes17,18,19,20.
CV and linear sweep curves in Fig. 7A and B no electrochemical process on the surface area of a bare glassy carbon electrode, since there are no peaks revealed. Therefore, the electrochemical process was clearly observed after the modification of the glassy electrode with 3 different MgFe2O4 nanomaterials. The voltammogram curves revealed broad peaks caused by the redox reaction, which is responsible for the pseudo capacitance behaviour. The shape curves primarily indicate the facile reduction and oxidation processes of the loaded glassy carbon materials, which are reversible in nature. The high current response exhibited by the voltametric curves of the nanomaterials clearly shows that they effectively promote electron transfer between the electrolyte and the electrode surface. The redox couple observed confirmed the reduction and oxidation processes of the nanomaterial taking place between the alkaline electrolyte and surface area of the glassy carbon electrode17,18. The voltametric curves also show a high-rate capability of interaction, which is aided by the electrode material’s structure17,18. The area under the cyclic voltammogram curve for the annealed sample and activated with black carbon increased significantly, resulting in improved electrochemical performance, which may be attributed to the high conductivity of carbon black mesopores in Mg2+ and Fe2+ active sites. In addition, carbon black in nanomaterials could improve the speed of electrolyte ions in the electroactive sites, so it is useful to elevate the reaction area at the interface between electrode and electrolyte18,19,20. The cyclic voltametric curves of the materials are shown in Fig. 8A–C at various scan rates ranging from 20 to 100 mV/s and at voltages ranging from − 0.6 to 0.6 V. The peak current density increases with scan rate, showing the excellent rate capability of the electrode and suggesting a fast diffusion-controlled electrolyte ion transport kinetic at the interface. The redox peaks can be clearly observed within the potential region for all scan rates, which indicates the fast and excellent electrochemical reversible redox reaction and can be attributed to the Faradaic redox reaction in the alkaline electrolyte. The CV curves remain in a quasi-rectangular shape, indicating high electronic conductivity and great reversibility for the MgFe2O4 based electrode material.
The EIS technique was employed to evaluate the electrolyte resistance (Rs), or The EIS technique was employed to evaluate the electrolyte resistance (Rs), or equivalent series resistance, and the charge-transfer resistance (Rct) over the interface between the electrode and electrolyte. The technique provides information such as the resistance of the electrolyte between a working electrode (WE) and a reference electrode, the interfacial charge transfer resistance on WE due to adsorbed species, charge transfer resistance due to Faradaic processes, diffusion processes (the Warburg factor), and the adsorption of the reactant or product on the interface. The impedance consists of two different plots, i.e., the Nyquist and Bode plots. The first plot is imaginary vs. real impedance at different frequencies, and the second describes absolute impedance vs. logarithmic frequency. Both interpret impedance as a function of a frequency18,19,20,51,52. The charge transfer kinetics of MgFe2O4 at 700 °C + C, MgFe2O4 at 700 °C and MgFe2O4 at R.T. electrodes material in 1 mol/L Na2SO4 electrolyte were analysed through the EIS spectra, shown in Fig. 9A. It displays two parts: (i) the semicircle in the low-frequency region due to the combination of charge-transfer resistance and the double layer capacitance, that is related to the charge transfer resistance (Rct), and (ii) other diffusion components resultant a straight line in the high-frequency region originated from Warburg impedance. The equivalent circuit as an inserted image in Fig. 9A contains the electrolyte resistance Rs, Warburg diffusion layer, double- layer capacitance Cdl which is in parallel with charge transfer resistance Rct. These parameters play significant roles in understanding the electrochemical processes in solution.
Resistances in the equivalent circuit diagram within electrochemical cell describe the movement of moving charges that occur between the electrode and the electrolyte surface or vice versa and non-Faradic charges that are modelled as constant phase elements. The Warburg impedance describes the effects of mass transport or diffusion of ions to and from the electrodes during electrochemical reactions. It is characterized by the Warburg element, which can be represented by a straight line in the Nyquist plot at an angle of 45 degrees51,52. The double-layer capacitance represents the capacitance at the interface between the electrolyte and the electrode surface. The capacitance is essential in determining how quickly the electrode can respond to changes in voltage or current. Charge transfer resistance (Rct) represents the resistance to the electrochemical reaction occurring at the electrode surface. It quantifies the difficulty of charge transfer between the electrode and the electrolyte during oxidation or reduction reactions. A high Rct indicates that the charge transfer reactions are slow, which can limit the overall rate of the electrochemical process. In contrast, a low Rct indicates that the charge transfer is efficient, corresponding to good performance of the electrochemical system. These EIS results reveal the good electronic conductivity as well as electrochemical stability of GCE/MgFe2O4 nanomaterial18,19,20,51,52.
The Bode plot presented in Fig. 9B illustrates the relationship between impedance and phase angle as functions of frequency. It reveals maximum phase angles of 65°, 75°, and 80° for the non-annealed sample, the sample annealed at 700 °C, and the sample annealed at 700 °C with activated carbon, respectively. These phase angles are notably close to 90°, suggesting a high degree of metallic conductivity51,52. The non-annealed sample, with a phase angle of 65°, may exhibit an increased number of defects or grain boundaries, which can impact the movement of free electrons. However, these imperfections do not significantly impede conductivity. In contrast, the annealed samples display enhanced phase angles of 75° and 80°, indicating that the annealing process typically improves the crystalline structure of the material. This enhancement reduces defects and grain boundaries, thereby facilitating better metallic conductivity51,52. Moreover, the increase in phase angle may suggest additional capacitance effects owing to energy storage capabilities resulting from structural modifications during the annealing process. The sample incorporating activated carbon, which shows a phase angle of 80°, likely experiences further complexity in its electrical characteristics. This increase in phase angle can be attributed to the porous nature of activated carbon, which may enhance the charge storage capacity within the material. Overall, these observations indicate that both annealing, and the inclusion of activated carbon have significant effects on the electrical properties of the materials, leading to improved conductivity and energy storage potential18,19,20. Based on the excellent electrochemical results, we validate that MgFe2O4 nanomaterials are highly suitable electrode materials for electrochemical applications due to their excellent findings in voltametric response, electrochemical kinetics, high electroactivity, and good conductivity18,19,20,51,52.
Conclusion
In this research, Magnesioferrite (MgFe2O4) nanomaterials were successfully synthesized using green chemistry methods, specifically through the utilization of Moringa oleifera extract and varying calcination temperatures of room temperature, 500 °C, and 700 °C. Various characterization techniques, including High-Resolution Transmission Electron Microscopy (HRTEM) and Energy Dispersive Spectroscopy (EDS), were employed to analyze the morphology, particle size distribution, elemental composition, and electrochemical properties of the nanomaterials. The average crystallite sizes after annealing measured 10 nm and 35 nm for the samples calcined at 500 °C and 700 °C, respectively. Lattice parameters were recorded at 7.3421 Å and 9.4569 Å, confirming the structural integrity of the materials. Electrochemical evaluations demonstrated that MgFe2O4 exhibits excellent conductivity and capacitance, highlighting its potential for energy storage applications. The MgFe2O4 nanomaterial showed remarkable photocatalytic performance in degrading methylene blue, achieving an 81% degradation rate within 120 min under light conditions. This study detailed the photocatalytic mechanism, emphasizing the formation of reactive species that facilitate the breakdown of organic pollutants. The nanomaterial also exhibited good stability and reusability over multiple degradation cycles, underscoring its practical utility in wastewater treatment. The methylene blue degradation process followed first-order kinetics, with a high-rate constant indicating a rapid photodegradation process and effective interaction between the dye and the photocatalyst. Future studies should aim to optimize synthesis parameters and operational conditions to further enhance photocatalytic performance. It is crucial to evaluate the effectiveness of MgFe2O4 in breaking down various hazardous dyes and pollutants to assess its versatility as a photocatalyst. Investigating hybrid systems that integrate MgFe2O4 with other nanomaterials may enhance photocatalytic activity and broaden its application range. Additionally, conducting field trials in actual wastewater treatment settings will help evaluate the practical applicability and scalability of MgFe2O4 nanomaterials in industrial contexts.
Data availability
All data generated or analyzed during this study are included within the manuscript.
References
Dubal, D. P., Gomez-Romero, P., Sankapal, B. R. & Holze, R. Nickel cobaltite as an emerging material for supercapacitors: An overview. Nano Energy 11, 377–399 (2015).
Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 91, 37–50 (2000).
Uke, S. J., Akhare, V. P., Bambole, D. R., Bodade, A. B. & Chaudhari, G. N. Recent advancements in cobalt oxides, manganese oxides and their composite as an electrode material for supercapacitor: A review. Front. Mater. 4, 21 (2017).
Zinatloo-Ajabshir, S., Morassaei, M. S. & Salavati-Niasari, M. Eco-friendly synthesis of Nd2Sn2O7-based nanostructure materials using grape juice as green fuel as photocatalyst for the degradation of erythrosine. Compos. Part B Eng. 167, 643–653 (2019).
Zinatloo Ajabsbir, S., Zeidabadi, M. A., Amiri, M. & Sharifianjazi, F. Innovative sono-synthesis of cerium dioxide nanomaterials using mentha extract with efficient activity for cancer therapy application. Results Eng. 23, 102720 (2024).
Zinatloo-Ajabshir, S. & Salavati-Niasari, M. Preparation of magnetically retrievable CoFe2O4@SiO2@Dy2Ce2O7 nanocomposites as novel photocatalyst for highly efficient degradation of organic contaminants. Compos. Part B 174, 106930 (2019).
Zinatloo-Ajabshir, S., Morassaci, M. S., Amiri, O., Salavati-Niasari, M. & Foong, L. K. Nd2Sn2O7 nanostructures: Green synthesis and characterization using date palm extract, a potential electrochemical hydrogen storage material. Ceram. Int. 46, 17186–17196 (2020).
Hamzeh, S., Mahmoudi-Moghaddam, H., Zinatloo-Ajabshir, S., Amiri, M. & Azari, A. Simple fabrication of mesoporous praseodymium cerate via an eco-friendly route for development of carbendazim electrochemical sensor. J. Electrochem. Soc. 171, 037508 (2024).
Zinatloo-Ajabshir, S., Esfahani, M. H., Marjerrison, C. A., Greedan, J. & Behzad, M. Enhanced electrochemical hydrogen storage performance of lanthanum zirconium oxide ceramic microstructures synthesized by a simple approach. Ceram. Int. 49, 37415–37422 (2023).
Kim, H. G. et al. Fabrication of CaFe2O4/MgFe2O4 bulk heterojunction for enhanced visible light photocatalysis. Chem. Commun. 39, 5889–5891 (2009).
Liu, Y. L. et al. Simple synthesis of MgFe2O4 nanoparticles as gas sensing materials. Sens. Actuators B 107, 600–604 (2005).
Chen, D., Zhang, Y. Z. & Tu, C. J. Preparation of high saturation magnetic MgFe2O4 nanoparticles by microwave-assisted ball milling. Mater. Lett. 82, 10–12 (2012).
Gupta, R. K. & Yakuphanoglu, F. Epitaxial growth of MgFe2O4 (111) thin films on sapphire (0001) substrate. Mater. Lett. 65, 3058–3060 (2011).
Kannan, K., Radhika, D., Gnanasangeetha, D., Sivarama Krishna, L. & Gurushankar, K. Show more Y3+ and Sm3+ co-doped mixed metal oxide nanocomposite: Structural, electrochemical, photocatalytic, and antibacterial properties. Appl. Surf. Sci. Adv. 4, 100085 (2021).
Chinnaiah, K. et al. Ag nanoparticles synthesized by Datura metel L. Leaf extract and their charge density distribution, electrochemical and biological performance. Chem. Phys. Lett. 807, 140083 (2022).
Sarojini, P., Leeladevi, K., Kavitha, T. & Gurushankar, K. Design of V2O5 blocks decorated with garlic peel biochar nanoparticles: A sustainable catalyst for the degradation of methyl orange and its antioxidant activity. Materials 16, 17 (2023).
Zhu, M., Meng, D., Wang, C. & Diao, G. Facile fabrication of hierarchically porous CuFe2O4 nanospheres with enhanced capacitance property. ACS Appl. Mater. Interfaces 5, 6030–6037 (2013).
Ichiyanagi, Y. et al. Magnetic properties of Mg-ferrite nanoparticles. J. Magn. Mater. 310, 2378–2380 (2007).
Meng, W. Q., Li, F., Evans, D. G. & Duan, X. Preparation of magnetic material containing MgFe2O4 spinel ferrite from a Mg–Fe(III) layered double hydroxide intercalated by hexacyanoferrate(III) ions. Mater. Chem. Phys. 86(86), 1–4 (2004).
Chinnaiah, K., Kannan, K., Chen, Y. S. & Gurushankar, K. Exploring Ag/Mn3O4 composite nanorods as an attractive battery-type electrode material for supercapacitors. J. Phys. Chem. Solids 196, 112310 (2025).
Taghavi Fardood, S. et al. Biosynthesis of MgFe2O4 magnetic nanoparticles and their application in photodegradation of malachite green dye and kinetic study. Nanochem. Res. 4, 86–93 (2019).
Matinise, N. et al. Green synthesis of novel zinc iron oxide (ZnFe2O4) nanocomposite via Moringa oleifera natural extract for electrochemical applications. Appl. Surf. Sci. 446, 66–73 (2018).
Carter, C. B. & Williams, D. B. Transmission Electron Microscopy 2nd edn. (Springer, 2009).
Kurian, J. & Mathew, M. J. Structural, optical and magnetic studies of CuFe2O4, MgFe2O4 and ZnFe2O4 nanoparticles prepared by hydrothermal/solvothermal method. J. Magn. Magn. Mater. 451, 121–130 (2018).
Pradeep, A. & Chandrasekaran, G. FTIR study of Ni, Cu and Zn substituted nano-particles of MgFe2O4. Mater. Lett. 60, 371–374 (2006).
Labde, B. K., Sable, M. C. & Shamkuwar, N. R. Structural and infra-red studies of Ni1+xPbxFe2−2xO4 system. Mater. Lett. 57, 1651–1655 (2003).
Mojumdar, S. C., Melnıḱ, M. & Jóna, E. Thermal and IR properties of Mg(II) complexes with heterocyclic ligands. Thermochim. Acta 352–353, 127–132 (2000).
Shen, Yu., Zhao, Q., Li, X., Hou, Y. & Chen, G. Surface photovoltage property of magnesium ferrite/hematite heterostructured hollow nanospheres prepared with one-pot strategy. Colloids Surf. A Physicochem. Eng. Asp. 403, 35–40 (2012).
Maensiri, S., Sangmanee, M. & Wiengmoon, A. Magnesium ferrite (MgFe2O4) nanostructures fabricated by electrospinning. Nanoscale Res. Lett. 4, 221–228 (2008).
Xiao, S. H., Jiang, W. F., Li, L. Y. & Li, X. J. Low-temperature auto-combustion synthesis and magnetic properties of cobalt ferrite nanopowder. Mater. Chem. Phys. 106, 82–87 (2007).
Batoo, K. M., Kumar, S., Lee, C. G. & Alimuddin,. Influence of Al doping on electrical properties of Ni–Cd nano ferrites. Curr. Appl. Phys. 9(4), 826–832 (2009).
Tangcharoen, T. Enhanced room temperature ferromagnetism and versatile optical properties in MgFe2O4 spinel ferrite prepared under different calcination temperatures. Results Mater. 23, 100596 (2024).
Tanaka, K., Nakashima, S., Fujita, K. & Hirao, K. High magnetization and the Faraday effect for ferrimagnetic zinc ferrite thin film. J. Phys. Condens. Matter 15, L469–L474 (2003).
Zhang, X. X., Schoenes, J., Reim, W. & Wachter, P. Evidence for 3dn to 3dn-14s transitions in magnetite and in lithium and magnesium ferrites. J. Phys. C Solid State Phys. 16, 6055–6072 (1983).
Kaur, N. & Kaur, M. Envisioning the composition effect on structural, magnetic, thermal and optical properties of mesoporous MgFe2O4-GO nanocomposites. Ceram. Int. 44, 4158–4168 (2018).
Sadat, M. E. et al. Photoluminescence and photothermal effect of Fe3O4 nanoparticles for medical imaging and therapy. Appl. Phys. Lett. 105, 091903 (2014).
Yao, J. & Wang, C. Decolorization of methylene blue with TiO2 sol via UV irradiation photocatalytic degradation. Int. J. Photoenergy 2010, 643182 (2010).
Shahid, M. et al. Photocatalytic degradation of methylene blue on magnetically separable MgFe2O4 under visible light irradiation. Mater. Chem. Phys. 139, 566–571 (2013).
Phuruangrat, A. et al. Synthesis of Ag/Bi2MoO6 nanocomposites using NaBH4 as reducing agent for enhanced visible-light-driven photocatalysis of rhodamine B. J. Inorg. Organomet. Polym. Mater. 30, 322–329 (2020).
Phuruangrat, A. et al. Synthesis and characterization Ag nanoparticles supported on Bi2WO6 nanoplates for enhanced visible-light-driven photocatalytic degradation of rhodamine B. J. Inorg. Organomet. Polym. Mater. 30, 1033–1040 (2019).
Cabrera, A. F., Rodríguez Torres, C. E., Marchetti, S. G. & Stewart, S. J. Degradation of methylene blue dye under dark and visible light conditions in presence of hybrid composites of nanostructured MgFe2O4 ferrites and oxygenated organic compounds. J. Environ. Chem. Eng. 8(5), 104274 (2020).
Danyliuk, N., Tatarchuk, T., Kannan, K. & Shyichuk, A. Optimization of TiO2-P25 photocatalyst dose and H2O2 concentration for advanced photooxidation using smartphone-based colorimetry. Water Sci. Technol. 84, 469–483 (2021).
Zinatloo-Ajabshir, S., Mortazavi-Derazkola, S. & Salavati-Niasari, M. Nd2O3 nanostructures: Simple synthesis, characterization and its photocatalytic degradation of methylene blue. J. Mol. Liq. 234, 430–436 (2017).
Mortazavi-Derazkola, S., Zinatloo-Ajabshir, S. & Salavati-Niasari, M. New facile preparation of Ho2O3 nanostructured material with improved photocatalytic performance. J. Mater. Sci. Mater. Electron. 28, 1914–1924 (2017).
Zinatloo-Ajabshir, S., Zinatloo-Ajabshir, Z., Salavati-Niasari, M., Bagheri, S. & AbdHamid, S. B. Facile preparation of Nd2Zr2O7–ZrO2 nanocomposites as an effective photocatalyst via a new route. J. Energy Chem. 26, 315–323 (2017).
Nachimuthu, S. et al. Lawsonia inermis mediated synthesis of ZnO/Fe2O3 nanorods for photocatalysis—Biological treatment for the enhanced effluent treatment, antibacterial and antioxidant activities. Chem. Phys. Lett. 804, 139907 (2022).
Allawi, F., Musa Juda, A. & Radhi, S. W. Photocatalytic degradation of methylene blue over MgO/α-Fe2O3 nano composite prepared by a hydrothermal method. AIP Conf. Proc. 2290, 030020 (2020).
El-Khawaga, M. A., Farrag, A. A., Elsayed, M. A., El-Sayyad, G. S. & El-Batal, A. I. Promising antimicrobial and azo dye removal activities of citric acid-functionalized magnesium ferrite nanoparticles. J. Clust. Sci. 33, 197–213 (2022).
Elsayed, M. A. Ultrasonic removal of pyridine from wastewater: Optimization of the operating conditions. Appl. Water Sci. 5, 221–227 (2015).
Kaur, M., Ubhi, M. K., Grewal, J. K. & Singh, D. Insight into the structural, optical, adsorptive, and photocatalytic properties of MgFe2O4-bentonite nanocomposites. J. Phys. Chem. Solids 154, 110060 (2021).
Kannan, K. et al. Investigation of the electrochemical behavior of CuO–NiO–Co3O4 nanocomposites for enhanced supercapacitor. Materials 17, 3976 (2024).
Chinnaiah, K. et al. Exploring the potential of Withania somnifera-mediated Ag/Mn3O4 nanocomposites as electrode material for high-performance supercapattery device. J. Taiwan Inst. Chem. Eng. 157, 105441 (2024).
Acknowledgements
This research was generously supported by the UNESCO-UNISA Africa Chair in Nanosciences and Nanotechnology, to whom we are all grateful.
Funding
Funding was provided by UNISA_UNESCO (Grant No. 22ACN22024).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matinise, N., Fall, A. Electrochemical and physical properties of magnesioferrite nanomaterial and photocatalytic degradation of methylene blue. Sci Rep 15, 2164 (2025). https://doi.org/10.1038/s41598-025-85510-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85510-4