Abstract
The scope of neck lymph node dissection remains controversial for unilateral papillary thyroid carcinoma (UPTC) patients with no clinical evidence of lymph node metastasis (cN0). This study aims to build and validate a model for predicting central lymph node metastasis (CLNM) in UPTC patients through preoperative basic information and intraoperative rapid frozen pathology results. Retrospective analysis covered 1928 patients with PTC from the Wuhan Union Hospital database (2010–2020), randomly split into training and validation sets in a 7:3 ratio. Univariate and multivariate logistic regression analyses were performed to assess the risk factors for ipsilateral CLNM and contralateral CLNM in UPTC patients with cN0. Identified six risk factors for ipsilateral CLNM and seven risk factors for contralateral CLNM in cN0 UPTC patients. Two separate nomograms were constructed to visualize the results. The C-index for predicting ipsilateral and contralateral CLNM nomograms is 0.746 (95% CI 0.723–0.768) and 0.712 (95% CI 0.679–0.744), respectively. The calibration curves presented good agreement between prediction by nomograms and actual observation. The clinical decision curves suggest a net benefit from this model. UPTC patients can use these two nomograms to predict the probability of ipsilateral CLNM and contralateral CLNM separately, enabling risk stratification and aiding in surgical decision-making.
Similar content being viewed by others
Introduction
Papillary thyroid carcinoma (PTC) is the most common thyroid malignant tumor, and the most common metastatic site is cervical lymph nodes. Surgery is the most basic treatment for PTC. For unilateral papillary thyroid carcinoma (UPTC) patients, surgical methods include thyroid lobectomy, total thyroidectomy, and isthmic thyroidectomy1. For patients with no clinical evidence of lymph node metastasis (cN0), lymph node management options primarily include no prophylactic dissection, prophylactic ipsilateral lymph node dissection, or prophylactic bilateral central compartment lymph node dissection. However, there remains significant controversy regarding the extent of surgery and the scope of lymph node dissection2,3.
Prophylactic bilateral lymph node dissection entails greater surgical trauma, with potential serious complications including bilateral recurrent laryngeal nerve injury and permanent parathyroid gland function impairment4,5. According to previous literature, the risk of permanent hypoparathyroidism in the bilateral central compartment lymph node dissection (CND) group (4.6–9.2%) is significantly higher than in the unilateral CND group (1.4–3.2%)6. A retrospective analysis involving 1547 patients undergoing total thyroidectomy found that the incidence of bilateral recurrent laryngeal nerve injury in the bilateral CND group (5.7%) was significantly higher than that in the unilateral CND group (1.4%)7. Which can significantly impact the patients’ quality of life and even their survival8. Balancing the maximum surgical benefit and the risk of surgical complications, predicting the central compartment lymph node metastasis status of UPTC patients, and devising personalized surgical approaches are crucial.
Due to the presence of gas in the central neck region, the sensitivity of ultrasound in diagnosing central lymph node metastasis (CLNM) is approximately 33%, with a specificity of 93%, according to multiple meta-analyses. The sensitivity and specificity of CT are 40% and 89.5%, respectively, while MRI has a sensitivity of about 80% and a specificity of 85% for diagnosing central CLNM. Overall, assessing central neck lymph node metastasis through preoperative imaging is challenging9,10,11. The application of intraoperative rapid frozen pathology in thyroid cancer surgery has become increasingly common. Evaluating intraoperative frozen section pathology to predict thyroid cancer lymph node metastasis and providing appropriate lymph node dissection based on the results holds significant clinical value.
Therefore, this study aims to identify risk factors for ipsilateral and contralateral CLNM in patients with UPTC based on their clinical demographics and intraoperative rapid frozen pathology results. Nomograms were constructed to predict the risk of ipsilateral and contralateral CLNM in these patients. We hope that the predictive model we have developed can assist in making surgical decisions for UPTC patients.
Methods
Enrollment of patients
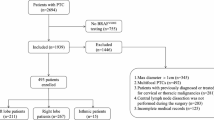
A retrospective review was conducted on the electronic medical records of all adult thyroid cancer patients who underwent total thyroidectomy and bilateral central lymph node dissection at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, from 2010 to 2020. The exclusion criteria are: (1) With previous thyroid surgery, (2) With other malignant tumors, (3) With clinical evidence of CLNM, (4) Non-papillary thyroid carcinoma (Thyroid follicular carcinoma/ medullary carcinoma/ anaplastic carcinoma), (5) Bilateral or isthmic cancers, (6) Without intraoperative frozen pathological examination, (7) Lack of important data. Ultimately, a total of 1928 cases of UPTC patients with cN0 were included in the study (Fig. 1).
Surgical procedures and definitions
The surgeries were performed by experienced surgeons with ample thyroid surgical expertise. All surgical specimens were subjected to microscopic examination and cross-checking by two experienced pathologists.
Anatomical boundaries and composition definition of the central compartment lymph nodes: The upper boundary was the hyoid bone, the lower boundary was the level of the innominate artery, the lateral boundary was the common carotid artery, the anterior boundary was the superficial layer of the deep cervical fascia, and the posterior boundary was the deep layer of the deep cervical fascia. Lymph nodes are divided into left and right lymph nodes based on their position with the midline of the trachea.
Ipsilateral/contralateral CLNM were defined as the presence of lymph node metastasis on the same side or the opposite side of the cancer, including patients with bilateral CLNM.
Data collection and analysis
We gathered data pertaining to demographic traits, thyroid function, intraoperative rapid frozen outcomes, and standard pathological findings of surgical specimens.
All statistical analyses were performed utilizing R software version 4.3.1 (R Foundation for Statistical Computing). Continuous data underwent normality testing, and if they followed a normal distribution, they were presented as the mean and standard deviation (SD). In cases where the data did not conform to a normal distribution, they were reported as the median and interquartile range. For continuous variables that follow a normal distribution, a T-test was used to compare the mean differences between the train and valid sets. If the normal distribution assumption was not met, the Mann-Whitney U test was employed. For categorical variables, the Chi-squared test was used to assess the differences in category distributions between the two sets.
Construction of nomograms
Univariate logistic regression was used to assess the predictive strength of each factor for CLNM. Factors with a p-value < 0.05 in the univariate analysis were then included in the subsequent multivariate logistic regression. Odds ratios (ORs) and their corresponding 95% confidence intervals were calculated based on the multivariate analysis. Finally, using the regression coefficients of each independent risk factor identified in the multivariate analysis, two separate nomograms were developed to predict the risk of ipsilateral and contralateral CLNM in patients with UPTC.
Discrimination and calibration of nomograms
The nomogram’s performance was assessed through discrimination and calibration. Discrimination ability was quantified using the C-index, which ranges from 0.5 to 1.0. A value of 0.5 signifies random chance, while 1.0 indicates perfect stratification of patients into different CLNM groups. Additionally, receiver operating characteristic (ROC) curves and corresponding area under the curve (AUC) values were employed to gauge predictive accuracy. Generally, C-index and AUC values exceeding 0.7 indicate reasonable estimation.
Calibration was evaluated using calibration curves generated from 1,000 bootstrap resamples. These curves illustrated the relationship between predicted probability and the observed outcome frequency. An ideal calibration curve is a straight line with a slope of 1, passing through the origin. The closer the calibration curve aligns with this standard, the better the nomogram’s predictive ability.
Results
Patients characteristics
As shown in Table 1, there were 406 males (21.06%) and 1522 females (78.94%), with a median age of 44 years (range: 36–52 years). Among them, 852 cases (44.19%) exhibited CLNM (Central Lymph Node Metastasis), with 773 cases (40.09%) having ipsilateral CLNM (on the same side) and 317 cases (16.44%) having contralateral CLNM (on the opposite side). Additionally, 238 cases (12.34%) experienced bilateral CLNM simultaneously. We summarized the patient characteristics of the train set (n = 1349) and valid set (n = 579). Because all cases were randomly assigned to two sets, there was no significant difference in the distribution of variables between the two sets (all p > 0.05).
Risk factors of ipsilateral and contralateral CLNM
Univariate and multivariate logistic regression analyses were conducted to evaluate ipsilateral CLNM (Table 2). In the univariate analysis, seven factors exhibited statistical significance, including age (p < 0.001), tumor size (p < 0.001), gender (p < 0.001), tumor location (p =0.014), as well as local invasion (Capsular invasion: p < 0.001; ETE: p < 0.001), intraglandular dissemination (p = 0.001), and the presence of nodular goiter (p < 0.001).
Multivariate regression analysis showed that age (OR = 0.95, 95% CI 0.94–0.96, p < 0.001), maximum tumor diameter (OR = 1.06, 95% CI 1.03–1.08, p < 0.001), gender (Male: OR = 2.03, 95% CI 1.52–2.72, p < 0.001), tumor location (Right: OR = 1.34, 95% CI 1.05–1.71, p = 0.019), local invasion (Capsular invasion: OR = 1.85, 95% CI 1.43–2.40, p < 0.001; ETE: OR = 5.53, 95% CI 2.72–11.24, p < 0.001), and intraglandular dissemination (OR = 4.58, 95% CI 1.75–11.97, p = 0.002) associated with ipsilateral CLNM.
For contralateral CLNM, univariate results showed that age (p < 0.001), TSH level (p = 0.017), tumor size (p < 0.001), gender (p <0.001), local invasion (Capsular invasion: p < 0.001; ETE: p < 0.001), multifocality (3 or more: p = 0.017) and intraglandular dissemination (p < 0.001) were statistically significant (Table 2).
Finally obtained by multi-factor regression seven independent factors of age (OR = 0.96, 95%CI: 0.95–0.98, p < 0.001), the TSH level (OR = 1.09, 95%CI: 1.03–1.16, p = 0.006), maximum tumor diameter (OR = 1.03, 95%CI: 1.01–1.06, p = 0.010), gender (Male: OR = 1.82, 95%CI: 1.30–2.56, p < 0.001), local invasion(Capsular invasion: the OR = 1.79, 95%CI: 1.28–2.49, p < 0.001; ETE: OR = 4.10, 95%CI: 2.15–7.84, p < 0.001), multifocality (3 or more: OR = 2.29, 95%CI: 1.03–5.05, p = 0.041) and intraglandular dissemination (OR = 4.56, 95%CI: 1.92–10.82, p < 0.001) were strongly correlated with contralateral CLNM.
Construction of nomograms
We have successfully integrated the aforementioned predictors into our risk prediction model. For patients with ipsilateral CLNM in UPTC, we utilized the six predictors to construct a nomogram. Additionally, for the prediction of contralateral CLNM, we incorporated seven predictors into a separate nomogram. These two nomograms were designed separately based on the independent factors that influence ipsilateral and contralateral CLNM.
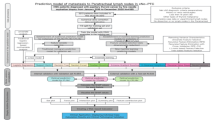
To predict the probability of UPTC patients experiencing ipsilateral and contralateral CLNM, we established two distinct nomograms. The regression coefficients for each variable in the multivariate logistic regression were collected, with the variable having the highest absolute coefficient set to 100 points. The scores for other variables were determined based on the ratio of their coefficients to the maximum coefficient. Each continuous variable was divided into specific intervals, and each categorical variable was divided into categories, with each segment or category assigned a corresponding score (Fig. 2).
The total score for each patient was calculated by summing up the scores corresponding to their selected variables. The predicted probability associated with this total score serves as a valuable tool for predicting the likelihood of CLNM in each individual patient.
Discrimination and calibration of nomograms
The AUC of the ROC curves for the nomogram to predict ipsilateral CLNM in the train set, valid set, and all cases were 0.746 (95% CI 0.719–0.773), 0.744 (95% CI 0.703–0.784), and 0.746 (95% CI 0.724–0.768), respectively. The concordance index (c-index) was 0.746 (95% CI 0.719–0.773), 0.750 (95% CI 0.740–0.790), and 0.746 (95% CI 0.723–0.768), respectively.
The AUC of the ROC curves for the nomogram to predict contralateral CLNM in the train set, valid set, and all cases were 0.709 (95% CI 0.670–0.747), 0.716 (95% CI 0.656–0.776), and 0.713 (95% CI 0.681–0.745), respectively (Fig. 3). The c-index was 0.708 (95% CI 0.669–0.746), 0.733 (95% CI 0.673–0.793), and 0.712 (95% CI 0.679–0.744), respectively.
ROC curves of the nomograms. (a–c) The ROC curves of the nomogram were used to predict ipsilateral CLNM in the train set, valid set and all cases. (d–f) The ROC curves of the nomogram were used to predict CLNM in the train set, valid set and all cases. ROC the receiver-operating characteristic curve, AUC area under the curve.
We performed 1000 bootstrap resampling and calibrated the established models, and all calibration curves exhibited a good linear fit (Fig. 4).
Calibration curves of the nomograms. (a–c) The calibration curves of the nomogram were used to predict ipsilateral CLNM in the train set, valid set and all cases. (d–f) The calibration curves of the nomogram were used to predict contralateral CLNM in the train set, valid set and all cases. The x-axis indicates the predicted CLNM probability, and the y-axis indicates the actual CLNM probability. The diagonal 45° line (gray line) indicates that the prediction agreed with actuality.
Evaluation of clinical usability of nomograms
We quantified the net benefit at different predicted probabilities using clinical decision curves (DCA) (Fig. 5). In the overall sample of UPTC patients, when the probability of ipsilateral CLNM in patients is between 40 and 99%, clinical decisions guided by the predictive model are superior to treating all patients or treating none. Additionally, when the probability of contralateral CLNM ranges from 16 to 73%, our constructed nomogram yields a net benefit.
The clinical decision curves of the nomograms. (a–c) The DCA curves of the nomogram were used to predict ipsilateral CLNM in the train set, valid set and all cases. (d–f) The DCA curves of the nomogram were used to predict contralateral CLNM in the train set, valid set and all cases. The horizontal axis of the image is the probability threshold range calculated by the prediction model, and the vertical axis is the clinical net benefit. The red lines represent the net benefit curve of treatment for patients within each prediction threshold of the clinical prediction model. The gray lines represent the clinical net revenue when treatment is administered to all patients, while the black lines represent the revenue when no treatment is given to any patient. When the red line is above both the gray and black lines, it can be considered that using the predictive model can yield clinical net revenue.
Risk stratification
We stratified patients’ risk of ipsilateral and contralateral lymph node metastasis into high-risk, moderate-risk, and low-risk groups based on the total scores from the two nomograms (Table 3).
Discussion
In this study, we identified six risk factors for ipsilateral CLNM, including age, gender, tumor size, tumor location, local invasion, and intraglandular dissemination. Additionally, we identified seven risk factors for contralateral CLNM, which include age, gender, tumor size, TSH level, multifocality of the tumor, local invasion, and intraglandular dissemination. We established and validated predictive models for ipsilateral and contralateral CLNM in UPTC patients. Based on the nomogram scores, we proposed risk stratification for lymph node metastasis and according to different risk scores. Patients can assess the risks of ipsilateral and contralateral CLNM individually through the two nomograms we constructed, enabling the development of personalized treatment strategies.
Based on the predictive model, we have proposed a risk stratification model for lymph node metastasis in UPTC, categorizing patients into three risk groups. Accordingly, we have provided different treatment recommendations for each risk group. For patients in the low-risk group, prophylactic lymph node dissection should be avoided to reduce surgical complications. For patients in the moderate-risk group, prophylactic lymph node dissection may be considered. For patients in the high-risk group, prophylactic lymph node dissection is recommended to reduce the chance of recurrence.
Regarding the relationship between age and the risk of lymph node metastasis in PTC patients, previous studies have already confirmed it. Liu et al., in a study of 966 cases of PTC, demonstrated that being younger than 45 years old is an independent risk factor for CLNM12. A study conducted in 2021 suggests that being younger than 55 years old may result in a higher rate of lymph node metastasis13. In general, being younger in age may increase the risk of central compartment lymph node metastasis. While PTC predominantly affects females, male patients seem to exhibit a higher malignancy level, which is closely associated with tumor progression and lymph node metastasis14,15,16.
A larger tumor size is universally recognized as an independent risk factor for the occurrence of CLNM in PTC17,18,19. The relationship between tumor location and thyroid lymph node metastasis has been established in previous research. In isolated primary tumor patients, having the tumor located in the lower part of the thyroid is significantly associated with a higher risk of CLNM20. However, some studies have demonstrated that tumors located in the upper part of the thyroid are more prone to cervical lateral lymph node metastasis21,22.
TSH is more commonly known for its role in postoperative suppressive therapy in differentiated thyroid carcinoma (DTC), as elevated TSH levels may stimulate the growth of residual thyroid tissue or cancer cells. Therefore, clinical guidelines often recommend TSH suppression therapy for DTC patients at intermediate to high risk of recurrence23. However, its relationship with CLNM has also been gradually confirmed. Gao Y et al. suggest that a serum TSH level greater than or equal to 5.162 mIU/L is an independent predictor of CLNM24, Xiang Y and Shahrokh believe that elevated TSH levels are associated with lymph node metastasis25,26. In our study, a high TSH level was identified as an independent risk factor for contralateral CLNM in UPTC, but it did not appear to have a significant impact on ipsilateral CLNM.
Some studies suggest that multifocality of the tumor is associated with lymph node metastasis in PTC27. In this study, tumor multifocality is associated with contralateral CLNM in UPTC patients. When compared to a single lesion, having two cancer foci did not show significant statistical significance regarding contralateral CLNM. However, having ≥ 3 lesions emerged as an independent risk factor for contralateral CLNM.
The extent of local invasion affects the lymph node metastasis in PTC, especially when the tumor invades the thyroid capsule or even extends beyond the thyroid into surrounding tissues such as the strap muscles, trachea, esophagus, and so on. In such cases, cancer cells may spread through lymphatic vessels within the thyroid capsule and the surrounding tissues28, significantly increasing the probability of CLNM29,30,31. The presence of intraglandular dissemination of the tumor is a well-recognized risk factor affecting the prognosis of PTC32. It is also an independent risk factor for PTC lymph node metastasis33. Y Lei et al., through follow-up studies, found that patients who had intraglandular dissemination had a significantly increased risk of developing neck lymph node metastasis after PTC surgery34.
To the best of our knowledge, this may be the first nomogram model for predicting lymph node metastasis in UPTC. Although the nomogram demonstrated good performance in discrimination and calibration, it has several limitations. Firstly, this study is a retrospective analysis with inherent biases. Secondly, the registration information regarding tumor location was not complete, which may have introduced some errors into the results. Lastly, being a single-center study, it was challenging to obtain external data for validation.
Conclusion
In summary, this study demonstrates that the risk factors for ipsilateral lymph node metastasis in UPTC include younger age, larger tumor diameter, male gender, tumor located on the right lobe, local invasion, and intraglandular dissemination. The risk factors for contralateral lymph node metastasis in UPTC include younger age, larger tumor diameter, high TSH levels, male gender, having three or more unilateral lobe lesions, local invasion, and intraglandular dissemination. The two nomograms constructed provide individualized assessment and reliable predictions for ipsilateral and contralateral CLNM in UPTC patients, offering guidance for personalized surgery. Further prospective research is needed to confirm and refine this model.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Tufano, R. P. & Kandil, E. Considerations for personalized surgery in patients with papillary thyroid cancer. Thyroid 20, 771 (2010).
Luster, M. et al. European perspective on 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: Proceedings of an interactive international symposium. Thyroid 29 7 (2019).
Alsubaie, K. M. et al. Prophylactic central neck dissection for clinically node-negative papillary thyroid carcinoma. Laryngoscope 132 1320 (2022).
Thomas, A. M., Fahim, D. K. & Gemechu, J. M. Anatomical variations of the recurrent laryngeal nerve and Implications for injury prevention during surgical procedures of the neck. Diagnostics 10 (2020).
Xiang, D. et al. Endoscopic thyroidectomy along with bilateral central neck dissection (ETBC) increases the risk of transient hypoparathyroidism for patients with thyroid carcinoma. Endocrine 53, 747 (2016).
Li, Y. & Lao, L. Comparison of prophylactic ipsilateral and bilateral central lymph node dissection in papillary thyroid carcinoma: a meta-analysis. Braz. J. Otorhinolar. 89, 101318 (2023).
Salem, F. A., Bergenfelz, A., Nordenstrom, E. & Almquist, M. Central lymph node dissection and permanent hypoparathyroidism after total thyroidectomy for papillary thyroid cancer: population-based study. Br. J. Surg. 108, 684 (2021).
Seo, S. T. et al. Transient and permanent hypocalcemia after total thyroidectomy: early predictive factors and long-term follow-up results. Surgery 158, 1492 (2015).
Zhao, H. & Li, H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: diagnosis of central and lateral compartment nodal metastases. Eur. J. Radiol. 112, 14 (2019).
Cho, S. J. et al. Diagnostic performance of MRI to detect metastatic cervical lymph nodes in patients with thyroid cancer: a systematic review and meta-analysis. Clin. Radiol. 75, 561 (2020).
Xing, Z. et al. Thyroid cancer neck lymph nodes metastasis: Meta-analysis of US and CT diagnosis. Eur. J. Radiol. 129, 109103 (2020).
Liu, C. et al. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer 19, 622 (2019).
Liu, W., Wang, S. & Xia, X. Risk factor analysis for central lymph node metastasis in papillary thyroid microcarcinoma. Int. J. Gen. Med. 14, 9923 (2021).
Yu, X. et al. Independent risk factors predicting central lymph node metastasis in papillary thyroid microcarcinoma. Horm. Metab. Res. 49, 201 (2017).
Liu, Y., Lv, H., Zhang, S., Shi, B. & Sun, Y. The impact of coexistent Hashimoto’s thyroiditis on central compartment lymph node metastasis in papillary thyroid carcinoma. Front. Endocrinol. 12, 772071 (2021).
Sun, J., Jiang, Q., Wang, X., Liu, W. & Wang, X. Nomogram for preoperative estimation of cervical lymph node metastasis risk in papillary thyroid microcarcinoma. Front. Endocrinol. 12, 613974 (2021).
Gao, X., Luo, W., He, L., Cheng, J. & Yang, L. Predictors and a prediction model for central cervical lymph node metastasis in papillary thyroid carcinoma (cN0). Front. Endocrinol. 12, 789310 (2021).
Guang, Y. et al. Clinical study of ultrasonographic risk factors for central lymph node metastasis of papillary thyroid carcinoma. Front. Endocrinol. 12, 791970 (2021).
Gui, C. Y., Qiu, S. L., Peng, Z. H. & Wang, M. Clinical and pathologic predictors of central lymph node metastasis in papillary thyroid microcarcinoma: a retrospective cohort study. J. Endocrinol. Invest. 41, 403 (2018).
Zhong, X. et al. Prophylactic central lymph node dissection performed selectively with cN0 papillary thyroid carcinoma according to a risk-scoring model. Gland Surg. 11, 378 (2022).
Back, K., Kim, J. S., Kim, J. H. & Choe, J. H. Superior located papillary thyroid microcarcinoma is a risk factor for lateral lymph node metastasis. Ann. Surg. Oncol. 26, 3992 (2019).
Feng, J. W. et al. Nomograms for the prediction of lateral lymph node metastasis in papillary thyroid carcinoma: stratification by size. Front. Oncol. 12, 944414 (2022).
Papaleontiou, M. et al. Thyrotropin suppression for papillary thyroid cancer: A physician survey study. Thyroid 31 1383 (2021).
Gao, Y., Qu, N., Zhang, L., Chen, J. Y. & Ji, Q. H. Preoperative ultrasonography and serum thyroid-stimulating hormone on predicting central lymph node metastasis in thyroid nodules as or suspicious for papillary thyroid microcarcinoma. Tumour Biol. 37, 7453 (2016).
Xiang, Y. et al. Serum TSH levels are associated with postoperative recurrence and lymph node metastasis of papillary thyroid carcinoma. Am. J. Transl. Res. 13, 6108 (2021).
Shahrokh, M., Alsultan, M. & Kabalan, Y. The relationship between papillary thyroid carcinoma and preoperative TSH level: a cross-sectional study from Syria. Medicine 102, e34283 (2023).
Wang, D. et al. Preoperative and pathological predictive factors of central lymph node metastasis in papillary thyroid microcarcinoma. Auris Nasus Larynx 49, 690 (2022).
Kim, S. K. et al. Predictive factors for lymph node metastasis in papillary thyroid microcarcinoma. Ann Surg. Oncol. 23, 2866 (2016).
Vasileiadis, I. et al. Papillary thyroid microcarcinoma: clinicopathological characteristics and implications for treatment in 276 patients. Eur. J. Clin. Invest. 42, 657 (2012).
Yan, B., Hou, Y., Chen, D., He, J. & Jiang, Y. Risk factors for contralateral central lymph node metastasis in unilateral cN0 papillary thyroid carcinoma: a meta-analysis. Int. J. Surg. 59, 90 (2018).
Jiang, L. H. et al. Predictive risk-scoring model for central lymph node metastasis and predictors of recurrence in papillary thyroid carcinoma. Sci.. Rep. UK 10, 710 (2020).
Dzepina, D., Zurak, K., Petric, V. & Cupic, H. Pathological characteristics and clinical perspectives of papillary thyroid cancer: study of 714 patients. Eur. Arch. Oto Rhino L. 271, 141 (2014).
Qian, B., Guo, S., Zhou, J., Qu, X. & Zhang, S. Intraglandular dissemination is a risk factor for lymph node metastasis in papillary thyroid carcinoma: a propensity score matching analysis. Gland Surg. 10, 3169 (2021).
Lei, Y. et al. Conventional papillary thyroid carcinoma with intraglandular lymphatic dissemination shows more aggressive features. Jpn. J. Clin. Oncol. 52, 1311 (2022).
Author information
Authors and Affiliations
Contributions
The study was jointly designed by XC.Q. and SN.R. The collection of raw data is completed by JL.Z. Data analysis and visualization were performed by LQ.H. and B.Q. The first draft of this manuscript was written by LQ.H., ST.G. and L.M. commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study follows the 2013 revised Helsinki Declaration and has been approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Approval No. 0304-01). This is an observational study that does not involve collecting personal information of patients, therefore the ethics committee of our institution eliminates the need for obtaining informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, L., Qian, B., Zhu, J. et al. Construction and validation of nomograms to predict central lymph node metastasis in clinical node-negative unilateral papillary thyroid carcinoma. Sci Rep 15, 2662 (2025). https://doi.org/10.1038/s41598-025-86201-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86201-w