Abstract
Yukawa potential and Hulthẻn potential are very useful potential models with applications in different areas of physics. The present study examined theoretic measure and thermodynamic properties of energy levels and wave functions for a combination of these two potentials. The effect of screening parameter on Fisher information and Shannon entropy as well as the effect of temperature on the various thermodynamic properties of the combined potentials and its subsets potentials are well studied. The effects of screening parameter on the thermodynamic properties of the combined potentials are also studied. This study shows equivalence of temperature effect on the combined potential and Hulthẻn potential but a different effect on Yukawa potential. Fisher information and the Shannon entropy for both position and momentum spaces as a function of the screening parameter obey Heisenberg principle, satisfy Cramer-Rao inequality and Bialynick-Birula, Mycielski inequality. The Shannon entropy shows the stability of the system for low screening parameter. It is also shown that the strength of Hulthẻn potential outweighed the strength of Yukawa potential in their combination for thermodynamic properties.
Similar content being viewed by others
Introduction
The exact solutions of both relativistic wave equations and non-relativistic wave equation in quantum mechanics are very useful since wave function and eigenvalues of a system can describe a system completely. The relativistic wave equations (which includes Dirac equation, Klein Gordon equation and Duffin-Kemmer-Petiau equations) are used to analyze spin zero particles, spin half particles and spin one particles. However, non-relativistic wave equation (Schrödinger equation) is used to describe non-relativistic spinless particles. This non-relativistic wave equation has drawn numerous attentions over the years due to its applications in different fields. Authors have examined and reported solutions of the Schrödinger equation with different physical potential terms of interest in various areas of sciences and engineering. Eyube et al.1, for instance, reported potential parameters of improved scarf II potential energy function. The original scarf II potential model was transformed to its improved form and was used to study spectra of some molecules. Sun et al.2, studied exact solutions of an asymmetric double wall potential. These authors examined the effects of the potential parameters on wave function with respect to origin. Barakat3 critically studied anharmonic oscillator potential and found out that his adopted method gives exact results over full range of potential parameters. Das and Arda4, reported exact solution of non-relativistic wave equation for a pseudoharmonic potential for N-dimensions. These authors deduced bound states and special cases of the potential. The variation of energy eigenvalue as a function of dimensions were fully studied. Ejere and Ebomwonyi5, calculated energy band gap of Cu2ZnSnS4 quaternary semiconductor alloy for a Hylleraas potential in the presence of magnetic field. The author reported that the energy band gap of Hylleraas potential depends on the presence of the magnetic field and the confining potential. Dong et al.6, reported exact solutions of the sine hyperbolic type potential and analyzed various energy levels of the potential. Falaye et al.7, studied shifted Tietz-Wei oscillator. They calculated energy spectrum for shifted Tietz-Wei oscillator and applied their result to some diatomic molecules. In another article, Falaye and his group8, studied Schrödinger equation for Tietz-Wei diatomic potential model. They obtained eigensolution techniques and their applications. The study was also applied to Fisher information entropy. Wei et al.9, obtained solutions of Dirac equation for a Coulomb potential in 2 + 1 dimensional systems. The authors examined degeneracies occurring in both spin symmetry and pseudospin symmetry. Taseli10, studied Morse potential in non-relativistic domain and obtained exact solutions for vibrational levels of Morse potential. Sun et al.11, calculated quantum information entropies for an asymmetric trigonometric Rosen–Morse potential and verified BBM relation that proves the correctness of their results. Emeje et al.12, recently obtained thermodynamic properties of standard Coulombic potential. These authors’ examined thermodynamics and its relationship with the potential parameters. They also examined special cases of their potential function. In ref13,14. respectively, temperature effect on thermodynamic behaviour has been reported. Their result shows principle to diffusion-controlled phase transformations in Fe-C-X alloys. Khordad and Ghanbari15, studied thermodynamic properties of potassium molecule for generalized Mobius square potential and reported the highest deviation of their predicted result from experimental result as 0.01139 at 800 K for heat capacity, 0.07486 at 4000 K for entropy, 0.10017 at 6000 K for enthalpy and 0.09112 at 5000 K for Gibbs free energy. Their model performed perfectly for these thermal properties of potassium molecule. Onate et al.16, reported molar entropy for iodine molecule, silicon carbide, and carbon phosphide. Their results agreed with experimental data. Their model was tested by calculating average absolute deviation from experimental data that varies from molecule to molecule. Habibinejad et al.17 calculated thermal properties of boron nitride for a q-deformed exponential-type potential. Their model reproduces experimental data with higher deviation compared to other models. Their deviations are recorded as 3.9716% for heat capacity, 2.3567% for Gibbs free energy and 8.5868% for enthalpy. Buchowiecki18 investigated BBr molecule at high temperature in classical approach. In his study, it was discovered that entropy is least sensitive to the approximations in the ro-vibrational coupling while heat capacity is the most sensitive. Eyube et al.19 reported molar Gibbs free energy and molar heat capacity at constant pressure of chlorine molecule, carbon monoxide and bromine molecule for improved generalized Pὂschl-Teller (IGPT) oscillator. Their predicted results were compared with experimental data to verify their calculated result. The model shows a good representation of thermodynamic properties of different molecules. Jia et al.20, calculated thermodynamic properties of nitrogen dimer. Their results perfectly agreed with experimental values at all temperatures. Their model executes complete perfection for thermal calculation of different molecules. Nath21, obtained exact solutions of the time-dependent Schrödinger equation in presence of time- dependent potential. This author carefully calculated analytical forms of the partition function and the corresponding thermodynamic quantities at high temperature using Euler–Maclaurin summation formula over a finite. Nath and Roy22, study energy level and thermodynamic properties such as vibrational mean energy, vibrational entropy, vibrational free energy and vibrational heat capacity for hydrogen molecule and lithium hydride. The effect of temperature parameter on various thermodynamic properties was examined.
It is on record that thermodynamic properties have many useful applications in the human environment. For example, entropy has applications in dissolution, adsorption, phase transition, fluorescence microscopy, material synthesis as well as protein activity23,24,25,26,27,28,29,30,31,32,33, enthalpy on the other hand is very important in adsorption, thermochemical modeling of biomass conversion systems, plasma gasification, and phase transition. Motivated by the usefulness and applications of theoretic measure and thermodynamic properties from the wave functions and energy levels of the Schrödinger equation, Hulthẻn and Yukawa potentials, this study wants to examine some theoretic measure and thermodynamic properties from energy levels of a combination of Yukawa and Hulthẻn potentials. These two potentials are important potentials that are very useful in science. The combination of the Yukawa potential and Hulthẻn potential is given as
where \({\lambda _0}\)and \({\lambda _1}\)are the strengths of Yukawa potential and Hulthẻn potential respectively. A combination of Hulthẻn potential is studied for Klein-Gordon equation by Ahmadov et al.34 where the relativistic effect of the this combination was analysed. These potentials are active potentials in physics. Yukawa potential can be viewed as a screened Coulomb potential, where the exponential term effectively screens the interaction at larger distances. In nuclear physics, Yukawa potential provides a good approximation for the strong nuclear force that binds protons and neutrons together in the nucleus. It is also used in particle physics and condensed matter physics, to describe interactions mediated by massive particles. It is also used as a tensor term to remove energy degeneracies to attain atomic stability. The Hulthẻn potential35 is another potential model used in quantum mechanics to describe interactions between particles. It is often used as an approximation to Yukawa potential and is particularly useful in the study of certain problems in atomic, molecular, and nuclear physics. Their usefulness and applications lead to their choice in this study. The theoretic measure such as the Fisher information and Shannon entropy are used to determine the localization of a particle in a system. They measure the uncertainty of a system through the probability density.
The parametric Nikiforov-Uvarov method
The energy levels of the potential (1) will be obtained using the parametric Nikiforov-Uvarov method. Following the work of Tezcan and Sever36, the standard/reference equation for the parametric Nikifov-Uvarov method is given by
According to Tezcan and Sever36, the conditions to obtain the energy equation and its corresponding radial wave function are
The values of the parametric constants in Eqs. (3) and (4) are obtained as follows:
Bound state solutions
In this section, the bound state of the interacting potential will be calculated. To obtain the solution of the radial potential for any quantum system, the radial Schrödinger equation for the non-relativistic energy \({E_{n,\ell }}\) is given by37,38
where \(\hbar\) is the reduced Planck’s constant, \(\mu\) is the reduced mass of the particle, \(\ell\)is the angular quantum number, n is the quantum number and \({R_{n,\ell }}(r)\) is the wave function. The centrifugal term in Eq. (6) can be approximated using the formula39,40
Substituting Eq. (1) and Eq. (7) into Eq. (6) and by defining a transformation of the form \(y={e^{ - \delta r}},\)and inserting it into Eq. (6), we have
Comparing Eq. (8) with Eq. (2), the parameters in Eq. (5) become
Substituting Eq. (10) into Eq. (3) and Eq. (4) respectively, the solution of the radial Schrödinger equation for the potential (1) gives energy levels and wave function respectively as
Theoretic measure
The theoretic quantity that determines the uncertainty in a random variable as it measures the content of an information produced41,42,43,44. To calculate any theoretic quantity, first the normalization constant will be calculated using normalization condition. Thus,
After some simplifications, the normalization constant is obtained as
where
Substituting Eq. (14) into Eq. (12), the complete wave function for quantum state becomes
At the ground state, \(n=0,\)
At the first excited state, \(n=1,\)
At the second excited state, \(n=2,\)
The momentum space wave function is obtained using Fourier transform. The momentum wave function at the ground state is
Fisher information
Fisher information measures the narrowness and the oscillatory nature of the probability distribution. Fisher information for position space and momentum space respectively are given as45,46
.
Shannon entropy
Shannon entropy determines the uncertainty in a random variable as it measures the content of an information produced. The Shannon entropy for position space and momentum space respectively are written as45,46
,
.
The probability density is the square of the wave function generally written in the form \(\:\rho\:\left(r\right)={\left|R\left(y\right)\right|}^{2}\) and \(\:\gamma\:\left(p\right)={\left|R\left(p\right)\right|}^{2}\) for position and momentum spaces.
The pure vibrational partition function and the thermodynamic properties
To calculate a pure vibrational partition from the energy levels, the energy levels in Eq. (11) must be a pure vibrational energy level. For pure vibrational state \(\ell =0,\)then, Eq. (11) becomes
After obtaining the pure vibrational energy levels of the combined potentials, then, the pure vibrational partition function can be calculated. To calculate the pure vibrational partition function, the energy level in Eq. (26) is re-written as
where
The partition function from any energy level is given as47,48,49,50,51
where \(\beta ={({k_\beta }T)^{ - 1}},\)\({k_\beta }\)is the Boltzmann constant, T is the absolute temperature and \({n_{\hbox{max} }}\)is the maximum quantum state obtained from the solution of \({{d{E_n}} \mathord{\left/ {\vphantom {{d{E_n}} {dn.}}} \right. \kern-0pt} {dn.}}\) This maximum quantum state is essentially positive and is given as
This study will adopt the lower-order Poisson summation formula52 that gives an approximate value of a summation to an accurate value to calculate the vibrational partition function. Thus, Eq. (29) becomes15,16,17,52
Substituting Eq. (27) into Eq. (31), the partition function turns to be
where.
Defining \({\lambda _6}={\lambda _2}\left( {\frac{{{\lambda _3} - {{(x+1)}^2}}}{{x+1}}} \right)\) Eq. (32) becomes
where.
Using maple software, Eq. (34) can be evaluated to give the complete pure vibrational partition function as
The factor \({e^{ - 4{\lambda _3}\lambda _{4}^{2}}}\) is infinitesimally small and thus, equivalent to zero. With Eq. (36), the various thermodynamic properties can be calculated as follows.
(i). Enthalpy H. The enthalpy of a system can be calculated using the formula
where R is a gas constant with numerical value of 8.314 J mol− 1 K− 1.
(ii). Gibbs free energy G. The Gibbs free energy is calculated using the formula
(iii). Entropy S. Entropy is calculated using the formula
(iv). Heat capacity at constant pressure \({C_p}\) Heat capacity at constant pressure is calculated using the formula
Discussion
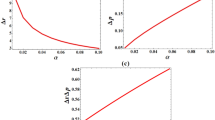
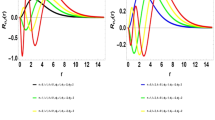
Table 1 presents energy eigenvalues of the interacting potential as a function of screening parameter for the ground state and first excited state with \(\ell =0\) and \(\ell =1.\) For \(\ell =0,\) the energies are completely bounded both at ground and at first excited state. For \(\ell =1,\)the energies are positive as the screening parameter goes above 0.2. Table 2 shows Fisher information for position space and momentum space as a function of the screening parameter at ground state and first excited state. As the screening parameter increases, Fisher information for position space and momentum space varies inversely with each other. At every value of the screening parameter, Fisher information for position space at the ground state are lesser than their counter path at first excited state but Fisher information for momentum space at ground state are higher than their counter path at first excited state. There is a diffused density distribution in momentum space at ground state and at excited states. This correspond to a localized density distribution in position space at both the ground state and excited state. Thus, there is more uncertainty in momentum space as the screening parameter increases. The results obey Heisenberg principle and satisfied Cramer-Rao inequality for one dimensional case with minimum bound of 4.56264314 at ground state and 4.98409273 at excited. Table 3 shows Shannon entropy for both position space and momentum space as a function of the screening parameter at ground state and first excited state. In both cases, the Shannon entropy in momentum space are bounded at lower screening parameter with entropy squeezing. For negative entropy, a moving particle becomes condensed so that it does not move at all. The system becomes more stable at this moment. It is therefore noted that with increasing \(S(\gamma ),\)\(S(\rho )\) decreases but only to the extent that their sum stays above the stipulated lower bound of the value (1 + ln) to obey and satisfied the Bialynick-Birula, Mycielski inequality for one dimensional case. Table 4 presents enthalpy and Gibbs free energy respectively as a function of temperature for a combination of Yukawa and Hulthẻn potential (YHP), Yukawa potential (YP) and Hulthẻn potential (HP). A rise in temperature leads to a rise in enthalpy for the three potentials but, the enthalpy for YHP and that of HP are closer both at lower temperatures and higher temperatures while enthalpy for YP at different temperatures are very far apart from those of YHP and HP. At the lowest temperature, YHP and HP system respectively has enthalpy less than 1JMol− 1K−1 but, YP system has higher enthalpy at all temperatures. This could probably be due to the screening parameter that multiplied the strength of the potential. However, it is observed that the enthalpy for the combined potential is less than the enthalpy of the individual subset potentials at both lower and higher temperatures. The change in enthalpy at higher temperatures are higher than the change in enthalpy at lower temperatures for all the potentials. The temperature and Gibbs free energy varies inversely with each other. It is also shown here that Gibbs free energy of YHP and HP are closer compared to the Gibbs free energy of YP. Again, the Gibbs free energy of YP are higher than the Gibbs free energy of YHP and HP at both lower and higher temperatures. Similarly, the Gibbs free energy of the combined potential is less than the Gibbs free energy of individual subset potentials. The Gibbs free energy at all temperatures for all potentials are less than 1JMol− 1K−1. Table 5 shows entropy and heat capacity respectively for YHP, YP and HP as a function of temperature. An increase in temperature from 200 K to 1200 K decreases the entropy of YHP but increases as the temperature rises above 1200 K. For HP, entropy decreases as temperature rises from 200 K to 1000 K but, increases as temperature rises above 1000 K. However, for YP, entropy increases as temperature rises for all temperature range studied. This behaviour is contrary to the behaviour exhibited by YHP and HP respectively. Except at 200 K, the entropy of YP is higher than the entropy of YHP and HP respectively for the temperature range studied. However, temperature and heat capacity vary inversely with each other for YHP and HP respectively but, for YP, the temperature and the heat capacity vary directly with each other. The entropy for YHP and HP respectively, are very high at every temperature while the entropy for YP remains between 15JMol− 1K−1 and 15.3JMol− 1K−1 for the temperature range studied. At every 200 K change in temperature, there is a minute rise in the entropy of the YP while a significant decrease in entropy is observed for the YHP and HP respectively. Table 6 presents the various thermodynamic properties as a function of the screening parameter at low temperature for YHP. The various thermodynamic properties except the heat capacity increases as the screening parameter goes up. However, between the value of 0.15 and 0.25, the entropy deceases but increases as the screening parameter increases from 0.25.
Conclusion
The energy and thermal properties of a combined Yukawa and Hulthẻn potential are fully studied in the absence of the rotational quantum number. The Fisher information and Shannon entropy obeyed and satisfied their condition of reality. The Shannon entropy exhibit squeezing effect and stability at low screening parameter for the momentum space. The thermodynamic properties of the two subset potentials exhibit different features. However, the thermodynamic properties of the Hulthẻn potential and the thermodynamic properties of the mother potential exhibit similar beheviours. For most thermodynamic properties, the result of the mother potential is also less than the result of each of the sunset potentials. This study shows that even though YP and HP look alike, their results are never closer. In our next study, we will examine the vibrational mean energy and vibrational free energy using both Possion summation formula and the use of classical limit at high temperature.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Eyube, E. S., Notani, P. P. & Izam, M. M. Potential parameters and eigen spectra of improved scarf II potential energy function for diatomic molecules. Mol. Phys. e1979265 (2021).
Sun, G-H., Dong, Q. & Bezerra, V. B. Dong. Exact solutions of an asymmetric double well potential. J. Math. Chem. 60, 605–612 (2022).
Barakat, T. The asymptotic iteration method for the eigenenergies of the anharmonic oscillator potential . Phys. Lett. A. 344, 411–417 (2005).
Das, T. & Arda, A. Exact analytical solution of the -dimensional radial schrödinger equation with pseudoharmonic potential via Laplace transform approach. Advan High. Energy Phys. 2015 Article ID 137038 (2015).
Ejere, A. I. I. & Ebomwonyi, O. Hylleraas potential quantum well in Cu2ZnSnS4 quaternary semiconductor alloy in the presence of magnetic field. Mater. Res. Express. 6, 096411 (2019).
Dong, Q. et al. Exact solutions of the sine hyperbolic type potential. J. Math. Chem. 57, 1924–1931 (2019).
Falaye, B. J., Ikhdair, S-M. & Hamzavi, M. Shifted Tietz–Wei oscillator for simulating the atomic interaction in diatomic molecules. J. Theor. Appl. Phys. 9, 151–158 (2015).
Falaye1, B. J., Oyewumi, K. J., Ikhdair, S. M. & Hamzavi, M. Eigensolution techniques, their applications and Fisherʼs information entropy of the Tietz–Wei diatomic molecular model. Phys. Scr. 89, 115204 (2014).
Dong, S-H. & Ma, Z-Q. Exact solutions to the Dirac equation with a Coulomb potential in 2 + 1 dimensions. Phys. Lett. A. 312, 78–83 (2003).
Taseli, H. Exact solutions for vibrational levels of the Morse potential. J. Phys. A: Math. Gen. 31, 779–788 (1998).
Sun, G-H., Dong, S-H. & Saad, N. Quantum information entropies for an asymmetric Trigonometric Rosen–Morse potential. Ann. Phys. 525, 934–943 (2013).
Emeje, K. O., Onate, C. A., Okon, I. B., Omugbe, E. & Eyube, E. S. Olanrewaju and E. Aghemenloh. Eigensolution and Thermodynamic properties of Standard Coulombic potential. J. Low Temp. Phys. https://doi.org/10.1007/s10909-024-03074-5 (2024).
Jiang, Y., Liu, L., Yan, J. & Wu, Z. Room-to-low temperature thermo-mechanical behavior and corresponding constitutive model of liquid oxygen compatible epoxy composites. Compos. Sci. Technol. 245, 110357 (2024).
Kuang, W. et al. Application of the thermodynamic extremal principle to diffusion-controlled phase transformations in Fe-C-X alloys: modeling and applications. Acta Mater. 159, 16 (2018).
Khordad, R. & Ghanbari, A. Theoretical prediction of thermal properties of K2 diatomic molecule using generalized mobius square potential. Int. J. Thermophys. 42, 115 (2021).
Onate, C. A. et al. Obaje and T.O. Etchie. Theoretical prediction of molar entropy of modified shifted Morse potential for gaseous molecules. Chem. Phys. 582, 112294 (2024).
Habibinejad, M., Khordad, R. & Ghanbari, A. Specific heat at constant pressure, enthalpy and Gibbs free energy of boron nitride (BN) using q-deformed exponential-type potential. Phys. B. 613, 412940 (2021).
Buchowiecki, M. Ro-vibrational coupling in high temperature thermochemistry of the BBr molecule. Chem. Phys. Lett. https://doi.org/10.1016/j.cplett.2017.12.051
Eyube, E. S., Onate, C. A., Omugbe, E. & Nwabueze, C. M. Theoretical prediction of Gibbs free energy and specific heat capacity of gaseous molecules. Chem. Phys. 560, 111572 (2022).
Jia, C-S. et al. Prediction of entropy and Gibbs free energy for nitrogen. Chem. Eng. Sci. 202, 70–78 (2019).
Nath, D. Study of uncertainty, average energy, and thermodynamic quantities of the time dependent quantum system. J. Math. Chem. 60, 1819–1839 (2022).
Nath, D. & Roy, A. K. Ro-vibrational energy and thermodynamic properties of molecules subjected to Deng–Fan potential through an improved approximation. Int. J. Quant. Chem. 121, e26616 (2021).
Otten, D. E., Shaffer, P. R., Geissler, P. L. & Saykally, R. J. Elucidating the mechanism of selective ion adsorption to the liquid water surface. Proc. Natl. Acad. Sci. USA 109, 701–705 (2013).
Zherebtsov, V. I. & Peganova, M. M. Water solubility versus temperature in jet aviation fuel. Fuel 102, 831–834 (2012).
Boycheva, S., Zgureva, D. & Vassilev, V. Kinetic and thermodynamic studies on the thermal behaviour of fly ash from lignite coals. Fuel 108, 639–646 (2013) (2013).
Yahiatne, I., Hennig, S. & Huser, T. Optical fluctuation microscopy based on calculating local entropy values. Chem. Phys. Lett. 587, 1–6 (2013).
Stadie, N. P., Murialdo, M., Ahn, C. C. & Fultz, B. Anomalous isosteric enthalpy of adsorption of methane on zeolite-templated carbon supporting information. J. Am. Chem. Soc. 135, 990–993 (2013) (2013).
Song, W., Martsinovich, N., Hecklabd, W. M. & Lackinger, M. Thermodynamics of halogen bonded monolayer self-assembly at the liquid-solid interface. Chem. Commun. 50, 13465–13468 (2014).
Moses, S. A. et al. Creation of a low-entropy quantum gas of polar molecules in an optical lattice. Science 350, 659–662 (2015).
Ammendola, P., Raganati, F. & Chirone, R. CO2 adsorption on a fine activated carbon in a sound assisted fluidized bed: thermodynamics and kinetics. Chem. Eng. J. 322, 302–313 (2017).
Wang, H. et al. A physical-constrained decomposition method of Infrared Thermography: Pseudo restored Heat Flux Approach based on ensemble bayesian variance Tensor Fraction. IEEE Trans. Ind. Informat. 20 (3), 3413–3424 (2024).
Chen, Y., Sun, S., Zhang, T., Zhou, X. & Li, S. Effects of post-weld heat treatment on the microstructure and mechanical properties of laser-welded NiTi/304SS joint with ni filler. Mat. Sci. Eng. A. 771, 138545 (2020).
Zhang, Z., Bu, Y., Wu, H., Wu, L. & Cui, L. Parametric study of the effects of clump weights on the performance of a novel wind-wave hybrid system. Renew. Energy. 219 (Part 1), 119464 (2023).
Ahmadov, A. I., Aslanova, S. M., Orujova, M. S., Badalov, S. V. & Dong, S-H. Approximate bound state solutions of the Klein-Gordon equation with the linear combination of Hulthén and Yukawa potentials. Phys. Lett. A. 383, 3010–3017 (2019).
Bayrak, O. & Boztosun, I. Bound state solutions of the Hulthén potential by using the asymptotic iteration method. Phys. Scr. 76, 92–96 (2007).
Tezcan, C. & Sever, R. A General Approach for the exact solution of the Schrödinger equation. Int. J. Theor. Phys. 48, 337–350 (2009).
Qiang, W. C. & Dong, S-H. Proper quantization rule. EPL 89, 10003 (2010).
S-H. Dong. The SU (2) realization for the Morse potential and its coherent states. Can. J. Phys. 80, 129 (2002).
Greene, R. L. & Aldrich, C. Variational wave functions for screened Coulomb potential. Phys. Rev. A. 14, 2363 (1976).
Wei, G-F., Dong, S-H. & Bezerra, V. B. The relativistic bound and scattering states of the Eckart potential with a proper new approximate scheme for the centrifugal term. Int. J. Mod. Phys. A. 24, 161–172 (2009).
Orlowski, A. Information entropy and squeezing of quantum fluctuations. Phys. Rev. A. 56, 2545 (1997).
Dehesa, J. S., Toranzo, I. V. & Puertas-Centeno, D. Entropic measures of Rydberg-like harmonic states. Int. J. Quant. Chem. 117, 48 (2017).
Najafizade, S. A., Hassanabadi, H. & Zarrinkamar, S. Nonrelativistic Shannon information entropy for Kratzer potential. Chin. Phys. B. 25, 040301 (2016).
Dehesa, J. S., Assche, W. W. & Yáñez, R. J. Information entropy of classical orthogonal polynomials and their application to the harmonic oscillator and coulomb potentials. Meth Appl. Analy. 4, 91 (1997).
Yahya, W. A., Oyewumi, K. J. & Sen, K. D. Position and momentum information-theoretic measures of the Pseudoharmonic potential. Int. J. Quant. Chem. 115, 1543–1552 (2015).
Romera, E. Dehesa. The Fisher–Shannon information plane, an electron correlation tool. J. Chem. Phys. 120, 8906–8912 (2004).
Dong, S-H. & Cruz-Irisson, M. Energy spectrum for a modified Rosen-Morse potential solved by proper quantization rule and its thermodynamic properties. J. Math. Chem. 50, 881–892 (2012) (2012).
Khordad, R. & Sedehi, H. R. R. Thermodynamic properties of a double ring-shape quantum dot at low and high temperature. J. Low Temp. Phys. 190, 200–212 (2018).
Khordad, R. & andA Ghanbari. Theoretical prediction of thermodynamic functions of TiC: Morse ring-shape potential. J. Low Temp. Phys. 199, 1198–1210 (2020).
Khordad, R. & Ghanbari, A. Analytical calculations of thermodynamic functions of lithium dimer using modified Tietz and Badawi-Bessis-Bessis potentials. Comput. Theor. Chem. 1155, 1–8 (2019) (2019).
Liu, G-H., Ding, Q-C., Wang, C-W. & Jia, C-S. Unified non-fitting explicit formulation of thermodynamic properties for five compounds. J. Mol. Str. 1294, 136543 (2023).
Strekalov, M. L. An accurate closed-form expression for the partition function of Morse oscillators. Chem. Phys. Lett. 439, 209–212 (2007).
Author information
Authors and Affiliations
Contributions
Conceptualization: C.A. Onate Methodology: O.O. Ajani and J.A. Akinpelu Formal Analysis: C.A. Onate and B.B. Deji-jinadu Software: O. Ebomwonyi Original draft: C.A. Onate.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Onate, C.A., Deji-jinadu, B.B., Ajani, O.O. et al. Information theory and thermodynamic properties for a combined potential model. Sci Rep 15, 2626 (2025). https://doi.org/10.1038/s41598-025-86335-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86335-x