Abstract
To explore techniques, advantages and disadvantages of 3D Slicer reconstruction and 3D printing localization technology combined with transcranial neuroendoscopy in ventriculoperitoneal shunt surgery. Retrospective analysis of clinical data of patients with hydrocephalus treated by ventriculoperitoneal shunt surgery using 3D Slicer reconstruction and 3D printing positioning technology combined with transcranial neuroendoscopy in our hospital from October 2021 to March 2023. A total of 33 patients with complete data were collected, including 19 males and 14 females, aged 10–81 years. Pre operative use of 3D Slicer reconstruction and 3D printing localization, and intraoperative use of neuroendoscopy assisted catheterization to complete ventriculoperitoneal shunt surgery. The drainage tube position was confirmed by brain CT and 3D Slicer reconstruction after operation, of which 30 cases were located in the frontal horn or center of the ipsilateral lateral ventricle, and 3 cases were located in the frontal horn or center of the contralateral lateral ventricle. All patients were successfully catheterized and well positioned. According to the unique ventricular system characteristics of each hydrocephalus patient, the 3D Slicer reconstruction technology was used to determine the individualized puncture point and direction, measure the puncture depth, accurately locate the puncture through the 3D printing guide plate, and accurately send the tip of the ventricular catheter into the frontal or central part of the lateral ventricle with the assistance of neuroendoscopic visualization, which improved the success rate of the operation and reduced the risk of tube blockage. At the same time, our team has newly developed a puncture point (“Cai’s point”), which has a puncture path in a non-vascular area and can reduce the risk of puncture bleeding. However, further prospective clinical research is needed to determine its routine location.
Similar content being viewed by others
Introduction
Hydrocephalus is a common disease in neurosurgery characterized by dementia, unstable gait and urinary incontinence, which seriously affects the quality of life of patients and increases the burden on families and society. Ventriculoperitoneal Shunt (VPS) is the main treatment method that can significantly improve patients’ clinical symptoms, improve their quality of life, and restore their social functions. However, due to the fact that the tip of the ventricular catheter is often wrapped by the choroid plexus within the ventricle, or the poor position of the shunting catheter at the ventricular end, infection, and other reasons, the failure rate of conventional VPS surgery is high1. In the United States, the revision surgery after VPS and/or VPS accounts for nearly one third of all neurosurgery operations every year2. It is reported in the literature that the manual lateral ventricle puncture and catheterization based on the anatomical marks of the scalp surface is a “blind puncture” operation, with an inaccuracy rate of nearly 50%3. Current research shows that lateral ventricle catheterization under precise navigation and positioning combined with endoscope can significantly remedy the above defects, reduce the recurrence rate and the incidence of complications1,3.
3D Slicer is a free open-source medical image processing software with low operating system requirements, which can be run on ordinary personal computers. It is reported4,5,6,7 that 3D Silcer has been applied to preoperative planning of various neurosurgery diseases, such as brain tumor, cerebral hemorrhage, cerebral aneurysm, epilepsy. The advantage of 3D Slicer is that it can reconstruct three-dimensional images of intracranial lesions, important structures, sulcus and gyrus, clearly and stereoscopically displaying their relationships, guiding preoperative planning and surgery8.With the development of 3D reconstruction technology, 3D printing has been widely applied in clinical practice9,10. Li et al.11 have applied 3D printing positioning combined with neuroendoscopy technology to the surgical treatment of cerebral hemorrhage and achieved good results. The progress of neuroendoscope technology has changed the visualization and navigation ability of craniocerebral surgery and broadened the field of neurosurgery. Neuroendoscope has the advantages of good lighting, multi angle vision, close observation, etc. It is widely used in various operations of neurosurgery at present12,13,14,15,16.
This retrospective study introduces new methods and technologies for preoperative planning and precise positioning using 3D Slicer reconstruction and 3D printing technology. The combination of intraoperative neuroendoscopy assisted placement of ventricular catheters under direct vision improves surgical efficiency and safety, reduces the incidence of various postoperative complications and the risk of catheter blockage.
Data and method
General data
This retrospective clinical study collected clinical data from our hospital from October 2021 to March 2023 on patients who underwent ventriculoperitoneal shunt surgery for hydrocephalus using 3D Slicer reconstruction and 3D printing positioning technology combined with transcranial neuroendoscopy. A total of 33 cases with complete data were collected, including 19 males and 14 females, aged 10–81 years old. All patients were diagnosed through brain CT or MRI examination, and 3D Slicer reconstruction and 3D printing were used for localization. Neuroendoscopy assisted catheterization was used during the surgery to complete the ventriculoperitoneal shunt surgery, and the position of the drainage tube was confirmed through brain CT and 3D Slicer reconstruction after the surgery. All patients were successfully catheterized and in good position. The detailed clinical information is showed in Table 1.
3D slicer reconstruction of ventricular model and design of localization guide system
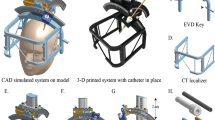
Before surgery, raw data of thin slice CT of the patient’s brain was collected in DICOM format. The above data of the patient was imported into the 3D-Slicer system(https://www.slicer.org), and the ventricular system and scalp were reconstructed. A 3D virtual reality model and a 3D printing positioning guide plate model were created. 3D virtual reality models help us understand the morphology of abnormally enlarged ventricular systems and their positional and structural relationships within the brain. 3D printed positioning guides can assist in determining the optimal location for surface puncture and assist in preoperative positioning. According to our previous clinical experience, the best body surface puncture point designed by us is located between the triangle puncture point and the occipital angle puncture point (we call it “Cai’s point”), through which the puncture can directly reach the lateral ventricle near the frontal horn, and the puncture path is the intracranial avascular area.
Classification of ventricular end catheter position
According to the grading system developed by Benjamin Y et al.17 to evaluate the position of the catheter at the end of the ventricle, we calculated the position of the drainage tube: Level 1, the best position of the catheter tip is located in the frontal horn or the center of the ipsilateral lateral ventricle. Level 2, the tip of the catheter is located in the frontal horn or central part of the contralateral lateral ventricle. Level 3, with the catheter tip located in the non-targeted cerebrospinal fluid gap. Level 4, with the tip of the catheter located within the brain parenchyma.
Ethical approval
All patients or family members have signed informed consent forms for surgery. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of Clinical Research, Our Hospital (WDRY2022-K099). Registration number of the Chinese Clinical Trial Registry is ChiCTR2300074887. This case series has been reported in line with the PROCESS Guideline18.
Typical cases
Case 1
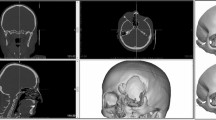
A 53 years old female patient, admitted to the hospital due to postoperative hydrocephalus after cerebral hemorrhage surgery. No history of hypertension, diabetes, etc. At the time of admission, the patient was in a state of tracheal incision 18 days after surgical treatment for cerebral hemorrhage, with blurred consciousness. Figure 1 shows the patient’s preoperative and postoperative brain CT (Fig. 1A-C), preoperative 3D Slicer reconstruction (Fig. 1D-E) and 3D printed guide plate positioning images (Fig. 1F-G), intraoperative neuroendoscopic images (Fig. 1H-I), postoperative 3D Slicer reconstruction virtual reality images (Fig. 1J), and postoperative CTA data (Fig. 1K-L).
(A) Preoperative brain CT. (B,C) Postoperative brain CT with arrow indicating shunt tube. (D) Virtual reality graphics reconstructed using 3D Slicer before surgery. (E) 3D positioning guide plate virtual reality graphics. (F) Preoperative positioning based on 3D printing guide plate. (G) Minimally invasive incision, white triangle represents the “Cai’s point”, black arrow represents the puncture point in the triangle area, and white arrow represents the puncture point in the occipital angle. (H) In the lateral ventricle under neuroendoscope, the black arrow represents the choroid plexus, and the white arrow indicates the septum pellucidum structure. (I) Insertion of ventricular shunt tube under direct vision of neuroendoscopy. (J) Postoperative reconstruction of lateral ventricle and drainage tube with 3D Slicer. (K,L) Postoperative brain CTA.
Case 2
A 81 years old female patient, admitted to the hospital due to postoperative hydrocephalus after intracranial aneurysm surgery. The patient has a previous history of intracranial aneurysm rupture and craniotomy clipping surgery. The patient had clear consciousness upon admission, but had poor mental state and delayed reactions. Figure 2 shows the patient’s preoperative brain CT/MRI(Fig. 2A-C), postoperative brain CT(Fig. 2D), preoperative 3D Slicer reconstruction (Fig. 2E-F) and 3D printing guide plate positioning images(Fig. 2G-H), intraoperative neuroendoscopic images(Fig. 2I-K), and postoperative 3D Slicer reconstruction virtual reality images(Fig. 2L).
(A) Preoperative brain CT. (B,C) Preoperative brain MRI. (D) Postoperative brain CT with arrow indicating shunt tube. (E) Virtual reality graphics reconstructed using 3D Slicer before surgery. (F) 3D positioning guide plate virtual reality graphics. (G) Preoperative positioning based on 3D printing guide plate. (H) Minimally invasive incision, white triangle represents the “Cai’s point”, black arrow represents the puncture point in the triangle area, and white arrow represents the puncture point in the occipital angle. (I) Keyhole approach. (J) In the lateral ventricle under neuroendoscope, the black arrow represents the choroid plexus, and the white arrow indicates the septum pellucidum structure. (K) Insertion of ventricular shunt tube under direct vision of neuroendoscopy(White arrow), the black arrow represents the interventricular hole. (L) Postoperative reconstruction of lateral ventricle and drainage tube with 3D Slicer.
Case 3
A 40 years old male patient, admitted to the hospital due to delayed reaction and unstable walking. No history of hypertension, diabetes, etc. When admitted, the patient had a lack of consciousness, poor mental state, accompanied by nausea, no vomiting, delayed reactions, and unstable walking. Figure 3 shows the patient’s preoperative and postoperative brain CT(Fig. 3A-B), preoperative 3D Slicer reconstruction(Fig. 3C-D) and 3D printed guide plate positioning images(Fig. 3E-F), intraoperative neuroendoscopic images(Fig. 3G-H), postoperative 3D Slicer reconstruction virtual reality images(Fig. 3I), and 3D Slicer fusion preoperative brain CTA and postoperative brain CT data reconstruction virtual reality images(Fig. 3J-K).
(A) Preoperative brain CT. (B) Postoperative brain CT with arrow indicating shunt tube. (C) Virtual reality graphics reconstructed using 3D Slicer before surgery. (D) 3D positioning guide plate virtual reality graphics. (E) Preoperative positioning based on 3D printing guide plate. (F) Minimally invasive incision. (G) In the lateral ventricle under neuroendoscope, the black arrow represents the choroid plexus, and the white arrow indicates the perforated septum pellucidum structure. (H) Insertion of ventricular shunt tube under direct vision of neuroendoscopy. (I) Postoperative reconstruction of lateral ventricle and drainage tube with 3D Slicer. (J,K) Use 3D Slicer to fuse postoperative brain CT and preoperative brain CTA data and reconstruct virtual reality images.
Results
In all 33 patients with hydrocephalus treated by ventriculoperitoneal shunt surgery with complete data, 14 cases were secondary hydrocephalus after intracerebral hemorrhage, 8 cases were secondary hydrocephalus after subarachnoid hemorrhage, 5 cases were found hydrocephalus after seeing a doctor with neurological symptoms such as dizziness and unresponsiveness due to unknown reasons, 3 cases were secondary hydrocephalus after brain trauma, and 2 cases were secondary hydrocephalus after brain tumor surgery on the cerebrospinal fluid circulation path, 1 case showed secondary hydrocephalus after intracranial infection. According to Benjamin Y et al.‘s design, 30 cases were classified as Grade 1, located in the frontal horn or central part of the ipsilateral lateral ventricle. 3 cases were classified as Grade 2, located in the frontal horn or central part of the contralateral lateral ventricle.
Discussion
VPS is the most commonly used method in neurosurgery for the treatment of hydrocephalus, but the failure rate of this operation is high, the recurrence rate in one year is as high as 40%, and the recurrence rate in two years is about 50%19. Mechanical obstacles are the most common cause of shunt failure, of which about 67.7% are ventricular duct obstruction20. Neuroendoscopy can obtain a direct view of the area inside the ventricle and around the catheter, which can help us identify the different causes of mechanical obstruction of the catheter. Literature reports that the lateral foramen of the ventricular catheter can be blocked by choroid plexus, ependyma, brain tissue, granulation tissue, newly formed blood vessels19. There is no choroid plexus tissue in the frontal horn of the lateral ventricle. Inserting a ventricular catheter into the frontal horn of the lateral ventricle can reduce the risk of choroid plexus obstruction. But when the volume of the ventricle significantly shrinks after shunt surgery, the tip of the catheter may insert into the brain tissue, leading to blockage of the lateral foramen of the catheter. Without endoscope assisted direct vision catheterization, we could not grasp the length of the drainage tube in the brain ventricle. If excessive emphasis was placed on putting the catheter into the lateral ventricle frontal horn, resulting in the catheter insertion being too long, it is very likely that the tip of the catheter will be inserted into the lateral ventricle frontal horn brain parenchyma. Even if there is no insertion at that time, once the drainage effect is good, the catheter tip may still enter the brain parenchyma after the ventricular system shrinks, ultimately leading to shunt failure. Therefore, routine VPS is considered to be the neurosurgery operation with the highest failure rate.
The failure rate of traditional ventricular catheter insertion methods is high because they largely rely on personal experience and familiarity with neuroanatomical features, without fully considering the anatomical variations of individual patients. At the same time, because “blind” placement may require multiple attempts to place the catheter in the so-called “optimal position,” repeated catheter placement often causes new damage to normal brain tissue. An increase in the number of catheter placements is positively correlated with an increased risk of complications, and multiple placements increase the probability of bleeding or infection3. To address the above issues, the precise navigation combined with neuroendoscopic assisted catheter placement method will be very beneficial. Research21 has shown that using image guidance during the placement of ventricular catheters can greatly reduce the number of successful insertions required and improve the accuracy of catheterization. However, due to the high cost, large volume, complex operation, and long preparation time of currently available navigation devices, conventional navigation devices are usually not used to assist in the placement of ventricular catheters.
In recent years, multimodal fusion imaging technology such as 3D Slicer, as a new imaging tool, has been widely used in the field of neurosurgery4. Its characteristics are free and open source, simple operation, low requirements for computer configuration, and ordinary personal computers can run, while functional expansion and continuous optimization and upgrading can be carried out. In order to achieve a low-cost and convenient preoperative navigation method, our research team cleverly adopted the concept of “virtual reality”, which involves the 3D Slicer reconstruction of a semi-transparent ventricular system and scalp 3D model cleverly overlaid on the patient’s head through the dual exposure function of the Sina/MosoCam mobile app, forming a composite view, and slowly adjusting the phone until the most suitable position is found22. In this way, neurosurgeons can “visualize” the relationship between the ventricular system and the scalp through the mobile phone lens. Moneer KF et al.23 showed that the accuracy of this navigation system developed using 3D Slicer is almost identical to that of traditional navigation systems, with an error of only 2 mm, but the cost is almost free. If operated properly, this new technology can replace traditional navigation methods with advantages such as less preparation time and lower equipment requirements.
Our early clinical practice22 has shown that using the dual exposure function of the Sina/MosoCam mobile app for projection positioning requires manual matching of body surface markers such as the patient’s eyes, nose, or auricle, which requires extremely high stability of the operator’s hand. Although difficulties can be overcome through mobile phone brackets, there are still certain shortcomings. Therefore, on the basis of 3D Slicer reconstruction, we have developed a new 3D printing guide positioning system, which directly matches the patient’s body surface markers such as eyes, ears, and nose through 3D printing technology, which can effectively solve the above problems. In the later research of our team, we used 3D Slicer to reconstruct the 3D graphics of the ventricular system and head face body surface signs, and then designed a suitable puncture point (We call it Cai’s point). The principle of selecting the puncture point is that through the puncture point perpendicular to the surface of the brain tissue, the puncture point can reach the lateral ventricle nearest and the tip of the ventricular catheter can reach the frontal horn or center of the ipsilateral lateral ventricle through fine adjustment. Then, based on the above design, we cut out a personalized 3D guide plate model that includes both eyes, nasal roots, auricles, and puncture points, and import the data into a 3D printer to print out for preoperative positioning. After mastering the entire design process proficiently, it takes an average of about 20 min, while the simplified personalized positioning guide printing takes an average of about 2 h. As VPS is a flat diagnosis surgery, we have enough time to design, plan, and print the guide. At the same time, by using postoperative brain CTA reconstruction images (Fig. 1K-L) or 3D Slicer to fuse postoperative brain CT with preoperative brain CTA data (Fig. 3J-K), the reconstructed images can clearly display that the drainage tube puncture channel area is a non-vascular area within the brain tissue, which will greatly reduce the risk of puncture bleeding.
Conventional VPS ventricular catheterization usually has three types: lateral ventricle frontal angle, occipital angle and triangle area. The triangle area has been abandoned because of the large area of choroid plexus, which is most likely to lead to duct obstruction. Generally, lateral ventricle frontal angle or occipital angle without choroid plexus is selected. The frontal horn puncture of the lateral ventricle is the easiest, but the subcutaneous tunnel of the shunt tube is long and needs to bypass the upper part of the external auditory canal. The operation is complex and requires an additional scalp incision. At the same time, the frontal puncture is easy to induce epilepsy. The occipital angle puncture of lateral ventricle theoretically has a low incidence of epilepsy, but the occipital angle volume is small, and the patient needs to be placed in a special lateral position when puncture, which increases the difficulty of puncture. In our clinical practice, our specific puncture point (Cai’s point) is located between the lateral ventricle triangle and the occipital angle puncture point. No special position is required during the puncture. Through the direct visualization function of neuroendoscope, the operator can identify the internal structure of the ventricle, such as the pulse plexus, ependyma, and interventricular foramen, and can also make minor adjustments to the catheter. To deliver the catheter to areas without choroid plexus structure such as the frontal horn or central part of the ipsilateral lateral ventricle. Our three special cases demonstrate a normal thickness transparent septum(Fig. 1H), an extremely thin transparent septum(Fig. 2J), and a perforated transparent septum structure(Fig. 3G)under neuroendoscopy. The interventricular foramen structure (Fig. 2K) can also be seen using a 30 ° working lens.
Neuroendoscopic assisted treatment of hydrocephalus seems to be an alternative minimally invasive treatment method, which can assist in performing third ventricular floor fistula surgery or VPS. Research24 shows that the combination of neuroendoscope and VPS is the preferred treatment method for patients with complex multilocular hydrocephalus. It can reduce the amount of surgical bleeding, shorten the operation time, reduce the incidence rate of complications and shorten the hospital stay24. Meanwhile, placement of ventricular catheters under neuroendoscopic guidance can also reduce the risk of incorrect placement of ventricular catheters25. Neuroendoscopic assisted VPS can safely remove the membrane attached to the ventricular wall caused by infection during surgery, and can also use suction and other invasive instruments to remove deposits in the ventricle, reducing the risk of shunt blockage26. Wei et al.19 first described three surgical revision strategies for VPS mechanical obstruction assisted by neuroendoscopy, which can reduce surgical time, incidence of ventricular hemorrhage, and risk of infection. All 35 revision patients they reported showed no signs of shunt obstruction or recurrence of hydrocephalus during a 5-year follow-up period. And in one case, active bleeding occurred during the surgery. With the assistance of neuroendoscopy, the bleeding point was quickly found and successfully stopped. Then, the ventricle was repeatedly flushed with artificial cerebrospinal fluid to ensure that there was no residual blood in the ventricle, leading to catheter blockage in the later stage. This is also one of the advantages of using neuroendoscopic assisted VPS.
Some scholars may be concerned that neuroendoscopic assisted surgery may increase the risk of VPS infection, which is one of the common causes of surgical failure. The reported infection rates for routine VPS surgery vary greatly, ranging from 0.17–27.8%27. Indeed, considering that the use of neuroendoscope assisted VPS will increase the overall surgical time, and the use of a key surgical instrument will also increase the likelihood of infection, it can be inferred that the risk of surgical related infections will significantly increase. Alexandre et al.27 reviewed many literatures and found that the use of neuroendoscope itself is not a risk factor for surgical infection. The infection risk of neuroendoscope surgery is similar to other conventional neurosurgery surgery, and the use of neuroendoscope assisted VPS will not increase the risk of surgical infection.
Conclusions
According to the unique ventricular system characteristics of each hydrocephalus patient, the 3D Slicer reconstruction technology was used to determine the individualized puncture point and direction, measure the puncture depth, accurately locate the puncture through the 3D printing guide plate, and with the help of neuroendoscope, accurately send the tip of the ventricular catheter into the frontal horn or center of the lateral ventricle, which improved the success rate of shunt surgery and reduced the risk of tube blockage. At the same time, our team has newly developed a puncture point (“Cai’s point”), which is located in a bloodless area within the brain tissue, which can significantly reduce the risk of puncture bleeding. However, further prospective clinical research is needed to determine its specific routine location. The progress of neuroendoscope technology makes most neurosurgery surgery can be operated directly without the aid of microscope. These tools help to expand the field of neurosurgery surgery, and are worth promoting in neurosurgery surgery.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Song, Z. et al. The utility of combined neuroendoscopic- and laparoscopic-assisted ventriculo- peritoneal shunt as a treatment for patients with communicating hydrocephalus. Technol. Health Care. 29(S1), 3–10 (2021).
Hochstetler, A., Raskin, J. & Blazer-Yost, B. L. Hydrocephalus: historical analysis and considerations for treatment. Eur. J. Med. Res. 27(1), 168 (2022).
Li, Y. et al. A wearable mixed-reality holographic computer for guiding external ventricular drain insertion at the bedside. J. Neurosurg., 1–8. (2018).
Hou, X. et al. 3D slicer and Sina appilication for surgical planning of giant invasive spinal schwannoma with scoliosis: a case report and literature review. Neurochirurgie 66(5), 396–399 (2020).
Liao, R. et al. 3D-slicer software-assisted neuroendoscopic surgery in the treatment of hypertensive cerebral hemorrhage. Comput. Math. Methods Med. 2022, 7156598 (2022).
Wang, H. W. et al. A supplemental technique for preoperative evaluation of giant intracranial aneurysm. J. Neurol. Surg. Cent. Eur. Neurosurg. 82(5), 424–429 (2021).
Liu, Q. et al. FreeSurfer and 3D slicer-assisted SEEG implantation for drug-resistant epilepsy. Front. Neurorobot. 16, 848746 (2022).
Ma, J. et al. Preoperative visualization of cranial nerves in skull base tumor surgery using diffusion tensor imaging technology. Turk. Neurosurg. 26(6), 805–812 (2016).
Lan, Q. et al. Application of 3D-printed craniocerebral model in simulated surgery for complex intracranial lesions. World Neurosurg. 134, e761–e770 (2020).
Bonda, D. J. et al. The recent revolution in the design and manufacture of cranial implants: modern advancements and future directions. Neurosurgery 77(5), 814–824 (2015). discussion 24.
Li, Y. et al. Clinical value of 3D-printed navigation technology combined with neuroendoscopy for intracerebral hemorrhage. Transl Stroke Res. 12(6), 1035–1044 (2021).
Cai, Q. et al. Hemorrhagic stroke treated by transcranial neuroendoscopic approach. Sci. Rep. 11(1), 11890 (2021).
Cai, Q. et al. Extradural anterior clinoidectomy and aneurysm clipping using transcranial neuroendoscopic approach: a case report. Med. (Baltim). 98(17), e15288 (2019).
Cai, Q. et al. Surgical treatment of a posterior inferior cerebellar artery aneurysm via transcranial neuroendoscopic approach: a case report. Med. (Baltim). 98(17), e15304 (2019).
Cai, Q. et al. Cerebral arteriovenous malformation treatment by full transcranial neuroendoscopic approaches. Neuropsychiatr Dis. Treat. 16, 1899–1905 (2020).
Cai, Q. et al. Microvascular decompression using a fully transcranial neuroendoscopic approach. Br. J. Neurosurg., 1–4. (2021).
Yim, B. et al. Optimizing ventriculoperitoneal shunt placement in the treatment of idiopathic intracranial hypertension: an analysis of neuroendoscopy, frameless stereotaxy, and intraoperative CT. Neurosurg. Focus. 40(3), E12 (2016).
Agha, R. A. et al. The PROCESS 2020 Guideline: updating Consensus Preferred Reporting of CasESeries in surgery (PROCESS) guidelines. Int. J. Surg. 84, 231–235 (2020).
Wei, Q. et al. Value of the application of neuroendoscope in the treatment of ventriculoperitoneal shunt blockage. World Neurosurg. 116, e469–e475 (2018).
Singh, I. et al. Comparison of total versus partial revision of primary ventriculoperitoneal shunt failures. Surg. Neurol. Int. 4, 100 (2013).
AlAzri, A. et al. Placement accuracy of external ventricular drain when comparing freehand insertion to neuronavigation guidance in severe traumatic brain injury. Acta Neurochir. (Wien). 159(8), 1399–1411 (2017).
Zhou, L. et al. Clinical application of 3D slicer combined with Sina/MosoCam multimodal system in preoperative planning of brain lesions surgery. Sci. Rep. 12(1), 19258 (2022).
Faraj, M. K., Kailan, S. L. & Al-Neami, A. Q. H. A new simple, cost-effective Navigation System (EASY Navigator) for neurosurgical interventions. World Neurosurg. 164, 143–147 (2022).
Martinez-Berganza, M. T. et al. Biventricular hydrocephalus due to idiopatic occlussion of foramina of Monro. Neurologist 17(3), 154–156 (2011).
Deopujari, C. E. et al. Neuroendoscopy for post-infective hydrocephalus in children. Childs Nerv. Syst. 34(10), 1905–1914 (2018).
Qin, G. et al. Neuroendoscopic lavage for ventriculitis: case report and literature review. Neurochirurgie 66(2), 127–132 (2020).
Giannetti, A. V., Pimenta, F. G. & Clemente, W. T. Does the simultaneous use of a neuroendoscope influence the incidence of ventriculoperitoneal shunt infection? World Neurosurg. 98, 171–175 (2017).
Funding
This work was supported by National Natural Science Foundation of China (82271518; 81971158; 81671306); The Interdisciplinary Innovative Talents Foundation from Renmin Hospital of Wuhan University (JCRCFZ-2022-030); Guiding projects of traditional Chinese medicine in 2023 ~ 2024 by Hubei provincial administration of traditional Chinese medicine (ZY2023F038).
Author information
Authors and Affiliations
Contributions
Silei Zhang and Qiang Cai studied concept and design, critical revision of manuscript for intellectual content, acquisition of data.Long Zhou, Pan Lei and Ping Song collected and analysised data, and wrote the main manuscripts.Zhiyang Li, Huikai Zhang, Hangyu Wei, Lun Gao, Qiuwei Hua, Hui Ye and Qianxue Chen collected and interpretated of data.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All patients or family members have signed informed consent forms for surgery. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of Clinical Research, Our Hospital (WDRY2022-K099).
Patient or family member consent statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, L., Lei, P., Song, P. et al. Clinical application of 3D slicer reconstruction and 3D printing localization combined with neuroendoscopy technology in VPS surgery. Sci Rep 15, 2609 (2025). https://doi.org/10.1038/s41598-025-86731-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86731-3