Abstract
The ongoing challenge of water pollution necessitates innovative approaches to remove organic contaminants from wastewater. In this work, new two-dimensional S-scheme heterojunction photocatalysts Bi2O3/CdS and MoS2/Bi2O3/CdS that are intended for the effective photocatalytic destruction of 4-nitrophenol, a dangerous organic pollutant, are synthesized and characterized. Utilizing a solvothermal method, successfully generated these ternary nanocomposites, which were characterized through various techniques including X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), high resolution transmission electronmicroscopy (HRTEM), Brunauer-Emmett-Telle (BET) and diffuse reflectance spectroscopy (DRS). Our results demonstrated that the Bi2O3/CdS heterojunction achieved an 86% degradation rate of 4-nitrophenol, while the MoS2/Bi2O3/CdS composite exhibited exceptional photocatalytic performance, achieving nearly complete degradation (99%) within 120 min under visible light irradiation. Most importantly the improved photocatalytic activity of MoS2/Bi2O3/CdS heterojunction originated from the release of internal electric field in S-scheme heterojunction. This enhanced activity is attributable to the synergistic effects of the heterojunctions that facilitate more effective charge separation and generation with more OP and RP confirmed the composite synthesis using S-scheme. The S-scheme is further confirmed by XPS, DRS, XPS-VB and photocurrent response. These findings highlight the promising application of these advanced photocatalysts in real-world wastewater treatment processes, offering a sustainable solution to combat water pollution.
Similar content being viewed by others
Introduction
Water pollution is now known as a critical environmental issue, impacting human health and the ecosystem. The release of domestic, agricultural, and industrial effluents into the water bodies has resulted in numerous inorganic and organic pollutants. Here, inorganic pollutants involve heavy metals, sulfates, fertilizers, domestic effluents, etc1,2. While organic pollutants include polycyclic aromatic hydrocarbons, dyes, petroleum, pesticides, phenol, pharmaceutical products, etc3,4. Phenol and compounds derived from it comprise a significant class of pollutants found in water due to certain properties such as stability and water solubility5. Phenols comprised of nitrogen have gained importance not only due to their severe hazardous effects but also due to their poisonous effects on catalysts6,7. Among the vast nitrogen-containing phenols, 4-nitrophenol (4-NP) was selected as a representative of the pollutant group due to its environmental significance8. 4-Nitrophenol is toxic just like the other derivatives of phenol and lies in the list of 114 organic compounds issued by EPA9,10. These nitrophenols have endocrine disruption ability and are also carcinogenic. To remove this phenol the routine wastewater treatment fails to treat because of this researchers are looking at different methods to treat them11. Pollutants from such different sources enter the water system, by creating a complex mixture of contaminants that can affect both human health and the aquatic human system12,13,14. These pollutants are now highly concern because of their potential, persistence, and toxicity for accumulation within aquatic organisms15. Moreover, the removal of such contaminants from wastewater has become a worldwide issue, encouraging extensive research on sustainable and effective treatment methods.
According to WHO polluted water causes numerous health issues like toxic exposure and waterborne diseases16. In various studies, the effect of pesticides has been highlighted especially demonstrating that the exposure to these chemicals further leads to critical health problems17,18. So, water quality assessment to restore the quality of water is very important when alarming levels of contamination in water bodies are revealed. To mitigate pollution the strategies, include attenuation and containment layers to preserve the quality of water19,20. Pesticide contamination is also of great importance in developing countries where regulatory frameworks might not be very strong21. Degradation of synthetic dyes is another approach used to achieve good water quality22. Different methods have been used for the degradation of wastewater, but photocatalytic degradation has been promising in removing such containments from wastewater effectively, especially when using advanced photocatalysts which can harness solar energy for the degradation method23,24,25.
The photocatalysis approach turned out as a potential technique that uses light energy to stimulate chemical reactions to degrade pollutants26,27. This technique has gained a lot of attention due to its ability to harness solar energy, low cost, and efficiency which makes it a sustainable way for water purification28,29. Subsequently, there are many limitations of photocatalysis in practical applications, such as suboptimal stability, insufficient light cultivation, and rapid recombination of charge carriers produced by photolysis30. To eliminate these limitations, researchers have concentrated on introducing advanced photocatalyst materials especially heterojunction systems which can improve the performance of photocatalytic.
Photocatalytic reactions here are thermodynamically favored by S-Scheme heterojunction as it provides a major driving force due to its strong redox abilities31. Photocatalysts may be oxidation photocatalysts or reduction photocatalysts depending upon band structure32. S-scheme builds an internal electric field because of work function differences that further induce the bending band which produces charge recombination by maintaining the high potential of the system for redox reaction. This increased high redox activity is important for the generation of reactive oxygen species which drive the degradation of pollutants33. The S-scheme also has motivated the development of new multi-semiconductor systems like dual as well as triple S-scheme heterojunctions that increase photocatalytic efficiency by giving more optimizing charge transfer pathways and more active sites34. The latter has a high conduction band with major usage in the production of solar cells producing photogenerated electrons and holes which are effective and useless (removed by sacrificial agents) respectively35. So, a heterojunction that is based on Step-Scheme contains an OP and RP having staggered band structures36. CdS37,38 is well known because of its effective visible light absorption as it has a narrow band gap which makes it a suitable candidate for photocatalytic pollutant degradation activity. CdS39 has efficient photocatalytic activity with reasonable stability. Due to their ability to enhance reactivity and facilitate electron transfer, Ba2O₃ and MoS2 have been shown to enhance the degradation efficiency of organic pollutants in photocatalytic systems when incorporated into the system. In pharmaceutical pollutant-specific systems, Ba₂CoMnO5 and LDH/CaCO340 are crucial for enhanced persulfate activation to facilitate enhanced degradation. Moreover, the increasing charge separation efficiency of metal oxides like Bi2O₃41 enhances photocatalytic activity when combined with other catalysts. Therefore, the overall degrading performance is improved by using Bi2O₃ with CdS since it encourages the generation of ROS, which are very important for effective degradation. The fact that the addition of both MoS2 and Bi2O3 to the MoS₂/Ag@WO₃/EG42 results in more stable and efficient photoelectrochemical degradation processes as supported by the investigation results which further strengthen this. Bi2O343 has unique structural properties which increases its photocatalytic activity by facilitating both extending light separation and charge separation. MoS244,45 that has been shown effectively improved photocatalytic performance of these metals. Diclofenac sodium was photodegraded using Co(II/III) oxide and tungsten (VI) oxide p-n heterojunction catalyst46 under visible light with high removal efficiency of 98.7% at pH 10.7. Similarly, Ba2CoMnO5 and LDHCaCO340 were also synthesized and used as catalysts for activation of persulfate for amoxicillin’s degradation. The more efficient performance was achieved with the LDH/CaCO3/PS due to enhanced charge separation and reduced catalytic site leaching and their performances were primarily dependent on designing the semiconductor according to the substrate’s nature. Additionally, it has been claimed that dual Z-scheme heterojunctions are cutting-edge techniques47 for the improvement of charge carrier dynamics and solar light harvesting and, meanwhile, have been applied to many fields from environmental remediation to other fields by current research. Altogether, these studies depict how heterojunction-based systems have developed so far to degrade environmental pollution by using effective photocatalytic techniques.
The current study demonstrates the synthesis and characterization of 2-D Bi2O3/CdS and MoS2/ Bi2O3/CdS S-Scheme heterojunctions by solvothermal method. The material exhibits enhanced photocatalytic activity for the degradation of 4-nitrophenol from wastewater. In these heterojunctions, synergistic effects are observed which highlight the potential for the development of advanced materials that can discuss the challenge of water pollution. For example, Bi2O3@MoS2 fabrication shows an overpotential 223 mV48 CdS shows 4.57 eV and α-Bi2O3 gives 3.37 eV with a maximum degradation efficiency of 87%49. So, the chosen photocatalysts can get high degradation efficiencies within visible light irradiation which further recommends their practical usage in wastewater treatment approaches. The performance evaluation of these photocatalysts includes testing their capability to degrade 4-nitrophenol under visible light by using a xenon lamp of 1000 W as a source of light. Results demonstrated that the Bi2O3/CdS heterojunction achieved 86% degradation efficiency, while the MoS2/Bi2O3/CdS heterojunction achieved 99% degradation efficiency within 120 minutes’ degradation rate. These results confirm that S-scheme heterojunction systems are used to achieve higher photocatalytic efficiency in contrast to pure catalysts which highlight their ability for real-world wastewater treatment.
Experimental section
Synthesis of Bi2O3
For synthesis of Bi2O3 Cetyl trimethyl ammonium bromide (CTAB ≥ 99.0%, Sigma) (0.05 g) was dissolved in ethanol-water and stirred for 20 min. Then 390 mg of BiNO3.5H2O (BiNPH ≥ 98.0%, Sigma) is added to the above solution to form a transparent solution. After that, 36 mL of Ethylene Glycol (EG ≥ 98.0%, Sigma) was added to the transparent solution upon stirring which was continued for 20 min. The resultant solution was placed for aging for 24 h. After the completion of the aging period, the mixture was stirred for 5 min and then poured into the Teflon steel-lined autoclave (100 mL) fulfilling 3/4th of its volume at 170 °C for 5 h. The product was separated from solvent using centrifugation which was done at 8000 rpm for 6 min then washed thrice with ultrapure pure water as well as with ethanol. Finally, the white-colored product was placed for vacuum drying at 65 °C for some time of 3–4 h. The product was then thermally treated under Argon and airflow at 400 °C at a rate of 2.5 °C/min (ramping time 160 min) for 30 min (reaction time) in a muffle furnace just to create the thermal environment for calcination and to remove unwanted oxidation of product. Upon calcination, the white color of Bi2O3 changed from white to yellow hence, confirming the formation of Bismuth oxide.
Synthesis of CdS
Anhydrous Cadmium Acetate, Cd(CH3COO)2 (0.533 g) (CA ≥ 99.5%, Sigma), was dissolved in 60 mL of ethylene diamine upon stirring for an hour. After that, thiourea (0.448 g) (TU ≥ 99.9%, Sigma) was added subsequently and the mixture solution was further stirred for 30 min. The whole mixture solution was then poured into 100 mL Teflon, which was placed in a heating oven at 105 °C for 8 h. To get rid of any organic and inorganic contaminants, the autoclave is auto-cooled, and the product is centrifuged for eight minutes at 8000 rpm. It is then washed three times with deionized water and commercial-grade ethanol. The product was placed for drying at 60 °C in a vacuum oven overnight and the yellow-colored product was obtained, and the yield of 0.10 g is 98.1%.
Synthesis of MoS2
Molybdenum sulfide was formulated by a facile hydrothermal method. In the synthetic procedure of MoS2, 2.4 g of ammonium molybdate (AM ≥ 99.1%, Sigma) and 0.87 g of elemental sulfur powders (S ≥ 98.4%, Sigma) were mixed and finely ground and added into a 100 mL beaker containing 40 mL of ultrapure water upon stirring. Then 24 mL of hydrazine monohydrate (85%) (HZMH ≥ 99.2%, Sigma), was added dropwise into the solution and continuously stirred for 30 min. The mixture solution was then poured into a 100 ml autoclave, and this autoclave was tightly sealed to build up autogenous pressure and placed in a heating oven at 180 °C for 50 h. The product was produced by cooling the autoclave to ambient temperature, centrifuging it for six minutes at 8000 rpm, and then washing it three times with deionized water and twice with commercial ethanol. The finished product, which was black, was dried for five hours at 60 °C in a vacuum oven.
Synthesis of ternary composite MoS2/Bi2O3/CdS
In a typical synthetic procedure, 22.6 mg of as-prepared bismuth oxide that was obtained after calcination was finely grounded and added to precursors of Cadmium Sulfide. Cadmium acetate, 0.533 g or 2 mmol, was added to 60 mL of ethylene diamine (EDA ≥ 99.9%, Sigma) upon stirring followed by the addition of thiourea, 0.448 g or 2 mmol. Then the as-prepared Bi2O3 with CdS was added to the mixture solution. After this the MoS2 according to binary composite was added with weight% which is 8% (10.7 mg) and the whole mixture solution was sonicated for 30 min to form a uniform dispersion (Fig. 1). After that, the solution was poured into the steel autoclave that is Teflon lined, which is then placed in a heating oven at 105 °C for 8 h. After the reaction’s completion time, the green-colored product was taken out from the autoclave and washed thrice each with ultra-pure water followed by ethanol and then dried in a vacuum oven at 60 °C for 3 h. The dried product obtained was ready for use.
Synthesis of BiVO4 nanosheet
BiVO4 is a visible light active photocatalyst and when integrated into ternary composite it facilitates electron-hole pair separation and serves as a charge separation agent that improves absorption of light. For the synthesis of BiVO4, two solutions were made; Solution-1 contains 2 mmol (0.789 g) of Anhydrous Bismuth Nitrate dissolved in 40 ml of Ethylene Glycol and solution-2 contains 2 mmol (0.233 g) of Ammonium metavanadate dissolved in 40 ml of lukewarm DI water. Both solutions were stirred for 30 min and then solution-2 was added dropwise into solution-1 upon stirring and a prominent color change (transparent to orange) was visualized. The mixture solution was vigorously stirred for 30 min to make a homogenous solution. Then, it was poured into a stainless-steel Teflon autoclave. The autoclave was placed in the heating oven at 100 °C for 12 h. After the completion of the reaction, the green color solution was formed which was centrifuged at 8000 rpm for 8 min. The final product undergoes washing with DI water thrice to eliminate any inorganic impurities and finally washed thrice with commercial ethanol at 8000 rpm for 8 min using the centrifugation machine. For drying, the resultant product was poured into a petri dish and placed in a vacuum oven at 80 °C for 3–4 h.
Synthesis of graphitic carbonitride
Graphitic carbonitride has a layered structure which makes it charge transport efficient and increases the surface area of the material due to which it was required to synthesize. A layered graphitic Carbonitride was synthesized by a one-pot calcination process by using equal masses of both precursors, urea, and melamine. In a simple process, 4.5 g of Urea and 4.5 g of melamine are finely grounded and taken in a 30 ml crucible made of alumina that is covered with its lid so that it gets half-filled. It is then calcined under argon and air atmosphere in a chamber furnace at 550 °C for 4 h at a heating rate of 2.3 °C/min while the ramping time is set to be 239 min. At this high temperature, CO2 and NH3 gases evolve, and intercalation occurs into layers of g-C3N4. Here, urea, being a supplementary material, undergoes complete decomposition at high temperatures. i.e. 550 °C and assist in the formation of layered graphitic carbonitride.
Characterization
The structure and powder X-ray diffraction (XRD) pattern of S-scheme heterojunction MoS2/Bi2O3 and MoS2/Bi2O3/CdS was studied on a PANalytical X’pert MPD Pro X-ray diffractometer. SEM is used to figure out the morphology of nanocomposites. For more analysis of the compositional and optical properties of the material HR-TEM, and EDX were done on FEI Tecnai G2 F30 S-Twin microscopy giving correct results with an Oxford MAX-80 EDX analysis.XPS was equipped with a Kratos Axis-Ultra multifunctional X-ray spectrometer. The BEt surface area was found by N2 adsorption within the automated gas sorption analyzer means with the Micromeritics ASAP 2020 instrument. DRS of prepared sample were measured with Thermo Fisher Scientific evolution UV-VIS absorption spectrometer which is in excellent condition and data collected from HITACHI U4100 spectrometer.
Results and discussion
XRD analysis
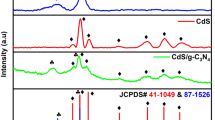
XRD analysis of photocatalysts was performed. The findings (Fig. S1a) show the diffraction pattern of Cadmium Sulfide has a hexagonal phase preferentially that matches well with the reported data in JCPDS card no 41-1049 for CdS (a = 4.1409Å, b = 4.1409 Å, c = 6.7198 Å). There were no characteristic peaks observed for CdO or C, showing that the material is pure CdS nanosheets. It has 3 main peaks according to results (Fig. S1a) centered at 2θ values of 24.45, 26.65, and 28.75 corresponding to the hkl values of (100), (002), and (101) respectively. The peaks at 2θ values of 43.65, 47.8, and 66.5 correspond to (110), (103), and (203) planes respectively. The obtained results confirm the formation of hexagonal Cadmium Sulfide nanosheets prepared via a hydrothermal method. The results (Fig. S1b) show the XRD pattern as synthesized highly crystalline Bismuth oxide. The peaks indexed at 25.56, 27.7, and 32.7 correspond purely to the monoclinic phase of Bi2O3 (210), (201), and (220) crystal planes respectively. The sharpness and intensity of diffraction peaks show that Bi2O3 is crystalline. The distinct diffraction peaks indexed at 2θ values of 41.2, 45.02, 46.16, 46.84,48.4, 51.26, 54.07, 55.42, 57.64 refers to (212), (321), (222), (400), (410), (411), (203), (421), (402) crystal planes respectively match well with the reference pattern in JCPDS card no 27–0050.
Finally, the XRD spectrum of CdS/Bi2O3 and all the characteristic peaks of CdS and Bi2O3 are present in the results (Fig. 2). The successful doping of 10% Bi2O3 nanoparticles over CdS was confirmed due to the presence of common peaks in the nanocomposite but with less intensity. Due to the distortion of a lattice, the loss of crystallinity is observed in the nanocomposite CdS@Bi2O3 thus, broadened peaks have been observed as compared to pure materials. No chemical interaction is found between Bi2O3 and CdS since the diffraction peaks of both the pure materials are present in the composite, and no diffraction pattern for impurities is observed. This is an indication that a cohesive composite material is formed by the combination of Bi2O3 and CdS. Moreover, the secondary peaks are absent which confirms that the nanocomposite is pure.
XRD pattern of MoS2 (JCPDS NO: 37-1492) is shown in Fig. 2. The spectrum shows that most of the intensity integrated with it refers to a broad peak of amorphous substance, while the (002) peak indexed at 14.37 which refers to the crystalline MoS2 is not very intense showing the less crystalline nature of MoS2. This indicates that MoS2 layers are less stacked, and packing is highly disordered. The as-synthesized MoS2 nanospheres are hexagonal in phase and belong to the space group P63/mmc. The crystal planes (100), (006), (110), (107) correspond to the 2θ values of 32.45, 44.15, 57.38, and 62.81 respectively that are well aligned with the JCPDS card no 37-1492. Figure 2 shows the XRD pattern of ternary nano heterojunction, 6% doped CdS/Bi2O3. The diffraction pattern contains the characteristic peaks of hexagonal MoS2 as well as that of binary nanocomposite CdS/Bi2O3 with slight shifting and reduction in the intensity of peaks. This confirms the successful development of the interface between the pure materials. The crystal structure of a material gives information about the optical and electrical features of a material.
XPS analysis
XPS is a characterization technique that gives information about the bonding states and chemical composition42,50. The composite Bi2O3/CdS consists of Bi, S, Cd, and O (Fig. 3 and Fig. S2). The O 1s peaks (Fig. 3a), which give information about the oxygen content of the material, are deconvoluted into a pair of symmetric peaks lying at a binding energy of 529 and 531 eV that are attributed to the lattice oxygen and the chemisorbed oxygen respectively. Bi shows 2 characteristic peaks that determine the oxidation state of Bismuth. Bi 4f7/2 is attributed to the lower energy peak, which is centered at 158 eV, whereas Bi 4f5/2 is attributed to the higher energy peak, which is centered at 163 eV. This verifies that Bi is in the + 3-oxidation state (Fig. 3b). The XPS spectrum of ternary composite shows a doublet of peaks for Mo 3d centered at 225 eV and 231 eV be compatible with Mo 3d5/2 and Mo 3d3/2 indicating the + 4-oxidation state of Mo (Fig. 3c). The peaks emerging at a binding energy of 160.78 eV and 161.7 eV were endorsed to S 2p3/2 and S 2p1/2 respectively suggesting the oxidation state of Sulphur to be -2 in the product.
Whereas S 2s (Fig. S2) deconvoluted into 2 peaks, a lower energy peak centered at 225 eV and a higher energy peak centered at 231 eV corresponding to S 2s3/2 and S 2s1/2 orbitals of divalent sulfide ions. The XPS spectrum of Cd 3d in Bi2O3/CdS shows 2 high-intensity peaks that correspond to Cadmium atoms present in Cd-S bonds centered at 404 eV and 411 eV that are endorsed to Cd 3d5/2 and Cd 3d3/2 respectively (Fig. 3d). This enriched generation of OP and RP sites confirmed the S-scheme photocatalytic heterojunction composition51. The observed shifts in binding energies of key elements distinguish the S-scheme heterojunction formation of composite from other types like type II or Z-scheme52. The interpretation of these XPS peaks provides valuable insights into the chemical interactions of Bismuth with other elements present in the nanocomposite MoS2/Bi2O3/CdS as well as gives information about the band structure of the composite53. It is useful in understanding the interface properties, surface chemistry, catalysis, and energy conversion54.
The XPS spectrum of the ternary composite shows the O 1s is deconvoluted into 2 peaks at 530.5 eV and 532.4 eV corresponding to divalent and monovalent anionic oxygen. Bi 4f shows 2 peaks, at 159 and 164 eV, credited to Bi 4f7/2 and Bi 4f5/2 with a slight shifting in peak positions as compared to Bi 4f in Bi2O3/CdS indicating the successful formation of ternary heterojunction. S 2p shows 2 peaks centered at 162 eV, and 163 eV attributing to S 2p3/2 and S 2p1/2 correspondingly. The energy band values for each material are different from the others. These energy band values increase and vary from one material to the next material. This shift confirmed that there is tight contact between all three materials supported by the transfer of photogenerated electrons. These photoinduced electrons confirm the S-path because of charge separation and charge transfer when moving from the conduction band to the valance band of the material.
BET analysis
BET is a technique that is used to measure a material’s specific surface area55. Bi2O3/CdS binary nanocomposite is composed of CdS nanoparticles that are uniformly dispersed in a matrix of Bi2O3 as shown in Fig. S3. CdS is involved in the enhancement of optical and photocatalytic properties of the nanocomposite. Specific surface area in the case of composite refers to the total surface area and composites are formed to increase the surface area thus having more active sites for the photocatalytic chemical reactions. The specific surface of composite MoS2/Bi2O3/CdS is 128.65 m2/g which is greater than the pure or individual components. It is because when a composite is formed, binary or ternary, the individual components have varying morphology and surface roughness that fits into the pores and irregularities of other materials making interconnected surfaces. This is called the interlocking effect and is responsible for the increase in the overall surface area of the nanocomposite. Another factor may be the synergistic effect which refers to the increase in the specific surface area of the composite because one component enhances the porosity of the other56. The quantification of specific surface area aids researchers in being aware of the effectiveness and efficiency of Bi2O3/CdS and MoS2/Bi2O3/CdS nanocomposite in applications such as sensors, solar cells, and photocatalysis.
SEM analysis
CdS, Bi2O3, and MoS2 are semi-conducting materials that possess distinct morphologies. Cadmium Sulfide possesses a two-dimensional flower-like structure owing to its high flexibility and thin nanosheets making up the crystal lattice. Such crystal structure of CdS is termed as hexagonal wurtzite resulting in flexible and thin nanosheets along the (111) planes of a crystal. The synthesized Bi2O3 possesses ultra-thin tiny 2-D crystals called nano-flakes making clusters together. The morphological studies of as-prepared samples were performed by SEM. Figure 4 (a) reveals the sheet-like structure of CdS with a smooth and clean surface.
Figure 4 (b) shows the highly crystalline nanoflakes of Bi2O3 formed by hydrothermal method followed by calcination. Figure 4 (c) shows the nanospheres of MoS2. Figure 4 (d) reveals the successful distribution of Bi2O3 nanoflakes over CdS nanosheets thus resulting in the formation of interfacial heterostructure. Fig (e, f) shows the uniform distribution of MoS2 nanospheres on the surface of the CdS/Bi2O3 binary nano heterojunction, and the lattice fringes in particles match well with hexagonal nanocomposite because of CdS thus leading to the effective development of ternary interfacial heterojunction.
EDX analysis
At the nanoscale, the purity and compositional analyses were performed using EDX57. The percentage composition by atomic weight and the compositional elements of Cadmium Sulfide are given as the insets (Fig. S4). Whereas Kα, Lα, and Mα refer to x-rays emitted because of de-excitation from various shells. When an L-shell electron is de-excited to K-shell, the energy released, or the x-ray emitted is called Kα. When an electron jumps from M-shell to L-shell, the x-ray emitted is called Lα. When an electron is de-excited from N-shell to M-shell, the characteristic energy emitted is called Mα. Since Cadmium and Sulphur are present in equal proportion in CdS the EDX spectrum shows approximately the same ratio of both. Bismuth oxide is comprised of two elements Bi and O. The Atomic weight% of respective elements is shown as the insets (Fig. S5). Whereas O Kα refers to the de-excitation of electrons from L-shell to K-shell while Mα and Mβ refer to the electronic de-excitation from N-shell and O-shell respectively. This excess photoexcitation generation confirmed the generation of photocatalytic heterojunction using S-scheme58.
The spectrum shows that the as-synthesized MoS2 is comprised of two constituent elements; Mo and S and their atomic weight% are mentioned (Fig. S6). EDX analysis supports TEM imaging and confirms the presence of all the constituent elements in the binary composite CdS/Bi2O3 and their atomic weight percentages are mentioned in Fig. S7. In this spectrum, the peaks labeled as Cd -L and Cd-M indicate that Cadmium is present in the composite. Whereas the peaks labeled as Bi Mα, Bi Mβ, Bi Lα, and Bi Lβ ensure the presence of Bi in the composite. The peaks S-Kα and O-Kα indicate the presence of Sulphur and Oxygen in the composite. Thus, the EDX spectrum of the binary composite supports TEM results. It can be seen from fig. S8 that all the constituent elements are present in the ternary heterojunction CdS/Bi2O3/MoS2 showing the successful formation of heterojunction.
Elemental mapping & TEM
The distribution of elements in space can be visualized via Elemental Mapping. The elemental mapping of CdS/Bi2O3 nanocomposite shown in Figure S9 demonstrates the uniform distribution of elements Cadmium, Bismuth, Sulfur, and Oxygen in the nanosheets which indicates that all the component elements are homogenously dispersed and well-mixed. Similarly, the mapping of MoS2/Bi2O3/CdS shows that all the component elements; Cd, Bi, S, O, and Mo are uniformly distributed in the ternary composite (Fig. 5). When the active components are homogenously distributed, it results in charge separation and energy transfer more efficiently and leads to optimal photocatalytic performance. At the nanoscale, the morphology and crystal structure of Bi2O3/CdS and MoS2/Bi2O3/CdS can be visualized by TEM and HRTEM. TEM image of Bi2O3/CdS revealed that the composite has 2-D ultra-thin, highly flexible, almost transparent nanosheets. This is attributed to the dominating ratio of CdS nanosheets in the binary heterojunction. The thinness and high flexibility of these 2-D nanosheets along the crystallographic planes revealed that the cadmium Sulfide nanosheets are decorated with Bi2O3 nanoflakes, forming the binary nano-heterojunction.
DRS analysis
The electronic properties of semiconductors can be determined by DRS by using the band gap of semiconductor materials. The Tauc plot Fig. 6 shows the direct bandgap of CdS by plotting the energy of photon on the x-axis and (αhʊ)2 on the y-axis to be 2.5 eV. The percentage of reflected light is converted into Absorbance by Kubelka-Munk transformation. The bandgaps obtained by DRS are 3.2, 2.12, 2.46, and 2.35 eV for Bi2O3, MoS2 (Fig. S10), Bi2O3/CdS, and MoS2/Bi2O3/CdS respectively. These bandgap values indicate that as the heterojunction or composite forms, the bandgap decreases owing to more absorption of the visible light by the composite especially at 3.2 eV because of more oxygen vacancies and defects in the crystal thus, leading to a better catalyst for the photocatalytic applications using S-scheme.
In thermogravimetric analysis, CdS shows a weight loss of approximately 10% up to 300 °C that might be attributed to the loss of moisture in the material. Then a further weight loss of 5% is observed up to 700 °C due to the loss of solvent residues. This weight loss according to thermionic studies59 suggests that CdS retains its functional properties and is high-crystalline and therefore compared to others. For Bi2O3, 8% weight loss occurs up to 400 °C which may be attributed to the loss of moisture and any solvent residues but after that, the material is stable till 800 °C. For binary composite Bi2O3/CdS, a 12% weight loss is observed till 400 °C due to the loss of water molecules present then a further weight loss of 10% is observed till 800 °C owing to the loss of solvent molecules. For MoS2, initially, a weight loss of 3% is observed till 100 °C then a sudden increase in weight loss occurs up to 350 °C that is attributed to loss of moisture, solvent, or any impurities. The ternary composite shows an initial weight loss of 7% till 300 °C then a further weight loss of 4% is observed up to 500 °C, and again a very sharp increase in weight loss is observed till 800 °C. Therefore, TGA is useful in determining the thermal stability of materials.
Photodegradation of 4-nitrophenol
The degradation studies of Bi2O3, CdS, MoS2, Bi2O3/CdS, MoS2/Bi2O3/CdS were performed and the photocatalytic removal of 4-nitrophenol was done by using a 100ppm solution of 4-nitrophenol. Initially, a 1000 ppm stock solution was prepared from which aliquots were taken to prepare 100 ppm of the pollutant. The amount of catalyst that was added was 30 mg. Upon addition of 30 mg of catalyst in 100ppm solution of 4-nitrophenol, it was stirred and then sonicated for 30 min to a uniform dispersion. After that, the solution was placed in the dark for 30 min to establish adsorption-desorption equilibrium, and an aliquot was taken in the dark. Then, the solution was placed under constant stirring which was then exposed to the xenon lamp source. At regular intervals of 10 min, the aliquots were subsequently taken and then analyzed by UV-Vis spectrophotometer for pollutant removal or degradation. So, the extent and rate of degradation were assessed using UV-Vis spectrophotometry on these aliquots. We used control measurements carried out in the dark at the start of the process to assess the catalyst’s activity. The variations in degradation rates during the process highlighted the various photocatalytic efficiencies of the different catalyst combinations. The data obtained indicates the optimum catalyst combination finally for degrading 4-nitrophenol, showing how each catalyst affects its degradation in light.
The UV-Vis absorption spectrum of Bi2O3, CdS, MoS2, Bi2O3/CdS, MoS2/Bi2O3/CdS shows that pure 4-nitrophenol shows the phenolate ion peak in the dark that is of high intensity (Fig. 7). The intensity of the phenolate ion peak was observed and it shows a continuous decrease with an increase in the time interval, indicating that the 4-nitrophenol is undergoing the degradation process in the presence of a light source. For CdS, due to its photostability, it shows only the 50% degradation of 4-nitrophenol pollutant whereas the Bi2O3 photocatalyst shows the removal of 58% due to an increased rate of electron-hole recombination. The best photocatalytic activity found for Bi2O3/CdS was 86% in 120 min of light exposure which indicates the sufficient removal of pollutants as compared to that of pure photocatalysts (Fig. 8). To further improve the efficiency of binary heterojunction, a ternary heterojunction MoS2/Bi2O3/CdS was synthesized that showed excellent degradation efficiency of 99% in 120 min of light irradiation and this ternary also show most minimum weight loss of 4% upto 400 °C which means material also maintain thermal stability. On average, the remarkable efficiency presented by the MoS2/Bi2O3/CdS composite indicates how material composition may be optimized to achieve high photocatalytic performance gains. Improved charge mobility that reduces recombination rates supports more active sites for interacting pollutants during light exposure. More importantly, such catalysts’ recyclability and stability over a long time would need to be checked for several cycles without losing much activity.
Photomineralisation and photocurrent studies
Photomineralisation is a chemical process that involves the decomposition of organic pollutants into H2O and CO2 by using a photocatalyst and a light source60,61. This study entails the mineralization of 4-nitrophenol by using the as-synthesized photocatalysts CdS, MoS2, Bi2O3, Bi2O3/CdS, and MoS2/Bi2O3/CdS. It was found that the composites showed better activity in photocatalysis compared to pure materials. This is due to the effective transfer and isolation of charge carriers between the components. The ROS, for instance, radicals such as superoxide radical (O2·−) & hydroxyl radical (HO·) are produced as primary oxidants during the reaction and serve for the mineralization of 4-nitrophenol. Upon degradation, the pollutant is not completely removed, and it may be transformed into toxic byproducts. In this study, the potential of MoS2/Bi2O3/CdS ternary composite as a promising photocatalyst for the efficient and effective removal of 4-nitrophenol, an organic pollutant, from wastewater has been highlighted.
The photoluminescence (PL) and photocurrent in Fig. 9 data demonstrate the dynamics of electron-hole recombination in different photocatalysts. Figure 9 (a) reveals that the intensity of PL is highest for CdS followed by Bi2O3/CdS but lowest for MoS2/Bi2O3/CdS composite. This indicates that the composite MoS2/Bi2O3/CdS minimizes electron-hole recombination, enhancing photocatalytic performance, and increasing charge separation. Our PL results show that the S-scheme heterojunction is present by comparing the PL spectra of CdS, Bi₂O₀/CdS, and MoS₂/Bi₂O₃/CdS. This shows that the electrons and holes are moving in opposite directions. Figure 9 (b) supports these results where the photocurrent response for MoS2/Bi2O3/CdS composite is more stable and higher than that of Bi2O3/Cd and CdS. An effective charge transfer with less electron-hole recombination is implied by a high and stable photocurrent. Results obtained confirmed that MoS2/Bi2O3/CdS composite achieves excellent electron-hole separation, making composite an efficient photocatalyst for 4-nitrophenol mineralization because of negligible recombination losses. We can see that the electrons and holes move in opposite directions by comparing the TPS curves of the MoS₂/Bi₂O₀/CdS heterojunction with those of CdS, Bi₂O₀, and Bi₂O₃/CdS. This proves that the S-scheme heterojunction exists.
The reusability of the catalyst MoS2/Bi2O3/CdS was performed for 4 cycles. All showed remarkable stability up to approximately 98% (Fig. S11), indicating that the heterojunction catalyst is efficient enough for charge separation and can be reused. For the first cycle, the catalyst gave 99% photodegradation efficiency. In the 2nd run, the catalyst gave 98.5% degradation efficiency. In the 3rd cycle, the catalyst gave 98.8% degradation efficiency while in the 4th cycle, the photodegradation efficiency of the catalyst reached 98%. The overall efficiency remained almost constant showing that the catalyst was stable up to 4 cycles.
Figure 10 of XPS-VB spectra represents a more favorable band structure of composite material for charge-efficient separation as compared to individual material and confirmed the usage of S-scheme. This reduced electron-hole recombination is more probably responsible for the improvement in the performance of photocatalysts. In Fig. 10 the valence band spectra (VB) of CdS are at 1.64 eV while the VB spectra of Bi2O3 are located at 2.98 eV. The VB spectra of Bi2O3/CdS exhibit at 1.62 eV which is very close to CdS and suggests that the material is influenced by CdS. The valance band edge for MoS2/Bi2O3/CdS positioned at 1.53 eV which is less close to the VB edge of CdS as compared to Bi2O3/CdS. The shift observed for the VB edge towards CdS in both material composites confirms the effective charge transfer of photogenerated holes from material Bi2O3 to CdS. So, S-scheme composite material is beneficial because it reduces electron-hole separation and promotes charge separation which is an important limiting factor within the photocatalytic efficiency.
The charge transfers and charge separation process in MoS2/Bi2O3/CdS composite under the irradiation of light using S-scheme is schematically depicted in Fig. 11. Due to advantageous band alignment, the photogenerated electrons of CdS in the conduction band (CB) are excited and then transfer to the conduction band of Bi2O3. At the same time, the holes in Bi2O3’s valance band transferred to the valance band of CdS. The addition of MoS2 also facilitates charge separation by creating pathways for the transfer of electrons. The effective separation of charge driven by the electric field created at interfaces among different components in S-scheme stops the charge carrier’s recombination and increases the photocatalytic activity of the composite.
Conclusion
The synthesized MoS2/Bi2O3/CdS heterojunction nanocomposites exhibit remarkable photocatalytic abilities for the degradation of 4-nitrophenol in wastewater. The integration of these advanced materials resulted in an unprecedented degradation efficiency of 99% within 120 min. Our characterization studies confirm the structural integrity and optical properties conducive to enhanced photocatalytic activity, driven by effective charge separation mechanisms facilitated by the S-scheme architecture. Furthermore, the S-scheme between Bi2O3, and CdS along with a highly conductive MoS2 electron sink work together to stimulate charge transfer and separation, resulting in increased photocatalytic activity as mentioned in XPS, DRS, and photocurrent response. The demonstrated capability of these composites not only addresses critical environmental health concerns but also paves the way for their application in broader contexts, such as supercapacitors and electrochemical water splitting. Future investigations will explore various pollutants to further establish their versatility and efficacy in water purification strategies. This research underscores the potential of S-scheme heterojunction photocatalysts as vital tools in the quest for sustainable environmental remediation technologies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bahadur, A., Iqbal, S., Alsaab, H. O., Awwad, N. S. & Ibrahium, H. A. Designing a novel visible-light-driven heterostructure Ni–ZnO/SgC 3 N 4 photocatalyst for coloured pollutant degradation. RSC Adv. 11(58), 36518–36527 (2021).
Bao, T. et al. Highly efficient nitrogen fixation over S-scheme heterojunction photocatalysts with enhanced active hydrogen supply. Natl. Sci. Rev. 11(5), nwae093 (2024).
Bahadur, A., Saeed, A., Shoaib, M., Iqbal, S. & Anwer, S. J. Modulating the burst drug release effect of waterborne polyurethane matrix by modifying with polymethylmethacrylate. J. Appl. Polym. Sci. 136(13), 47253 (2019).
Shan, Z. et al. Hollow dodecahedra graphene oxide-cuprous oxide nanocomposites with effective photocatalytic and bactericidal activity. Front. Chem. 9, 755836 (2021).
Bahadur, A. et al. Regulating the anticancer drug release rate by controlling the composition of waterborne polyurethane. Reactive Funct. Polym. 131, 134–141 (2018).
Liu, W. et al. Treatment of CrVI-containing mg(OH)2 nanowaste. Angew. Chem. Int. Ed. 47(30), 5619–5622 (2008).
Liu, W. et al. Effective extraction of cr(VI) from hazardous gypsum sludge via controlling the phase transformation and chromium species. Environ. Sci. Technol. 52(22), 13336–13342 (2018).
Anwer, S. et al. Synthesis of edge-site selectively deposited au nanocrystals on TiO2 nanosheets: an efficient heterogeneous catalyst with enhanced visible-light photoactivity. Electrochim. Acta. 283, 1095–1104 (2018).
Li, N. et al. Dual S-scheme MoS(2)/ZnIn(2)S(4)/graphene quantum dots ternary heterojunctions for highly efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 676, 496–505 (2024).
Li, N. et al. Unraveling charge transfer dynamics in AgBr/Bi(4)Ti(3)O(12)/Bi(2)Sn(2)O(7) ternary S-scheme heterojunction photocatalyst. J. Colloid Interface Sci. 669, 175–189 (2024).
Su, P. et al. Boosted photoinduced charge separation in FeNi2S4@ Cd0. 9Zn0. 1S step-scheme heterojunction for photothermal assisted photocatalytic water splitting into hydrogen. J. Colloid Interface Sci. 680, 529–540 (2025).
Irfan, R. M. et al. Co 3 C as a promising cocatalyst for superior photocatalytic H 2 production based on swift electron transfer processes. J. Mater. Chem. C. 9(9), 3145–3154 (2021).
Bahadur, A. et al. Biocompatible waterborne polyurethane-urea elastomer as intelligent anticancer drug release matrix: a sustained drug release study. Reactive Funct. Polym. 119, 57–63 (2017).
Li, Y. et al. D-A conjugated polymer/CdS S-scheme heterojunction with enhanced interfacial charge transfer for efficient photocatalytic hydrogen generation. Small 20(31), e2312104 (2024).
Liang, H. et al. Efficient photocatalytic hydrogen peroxide production over S-scheme in(2)S(3)/molten salt modified C(3)N(5) heterojunction. J. Colloid Interface Sci. 669, 506–517 (2024).
Shoaib, M. et al. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers. J. Drug Deliv. Sci. Technol. 39, 277–282 (2017).
Nawaz, R. et al. Designing an innovative 2D/2D step scheme α-Fe2O3/BiOBr/MoS2 ternary integrated heterojunction with unparalleled visible-light-induced remarkable photocatalytic H2 evolution. Int. J. Hydrog. Energy. 99, 112–122 (2025).
Luo, H. et al. Rational design of Bi(2)sn(2)O(7)/Bi(5)O(7)I S-scheme heterojunction for visible photocatalytic oxidation of emerging pollutants. J. Colloid Interface Sci. 659, p569–581 (2024).
Luo, H. Y. et al. Construction of a BiVO(4)/V(S)-MoS(2) S-scheme heterojunction for efficient photocatalytic nitrogen fixation. Nanoscale Adv. 6(6), 1781–1789 (2024).
Rahman, M. S. U. et al. Magnetic mesoporous bioactive glass for synergetic use in bone regeneration, hyperthermia treatment, and controlled drug delivery. RSC Adv. 10(36), 21413–21419 (2020).
Ma, H. et al. S-Scheme heterojunction of cs(2)SnBr(6)/C(3)N(4) with interfacial electron exchange toward efficient photocatalytic NO abatement. J. Colloid Interface Sci. 671, 486–495 (2024).
Shoaib, M. et al. Magnesium doped mesoporous bioactive glass nanoparticles: a promising material for apatite formation and mitomycin c delivery to the MG-63 cancer cells. J. Alloys Compd. 866, 159013 (2021).
Ghasemi, S. et al. Recent progress on Z-and S-scheme photocatalysis: mechanistic understanding toward green applications. Curr. Opin. Chem. Eng. 47, 101059 (2025).
Pandey, A. et al. Construction of a stable S-scheme NiSnO(3)/g-C(3)N(4) heterojunction on activated carbon fibre for the degradation of glyphosate in water under flow condition. Chemosphere 347, 140709 (2024).
Cui, J. et al. Engineering of Bi2O3-BiOI 2D/2D S-scheme heterojunction for efficient photocatalytic organic hazards removal. Separ. Purif. Technol. 354, 129136 (2025).
Qi, K. et al. TiO(2)-based photocatalysts from type-II to S-scheme heterojunction and their applications. J. Colloid Interface Sci. 675, 150–191 (2024).
Qian, A. et al. Photocatalytic hydrogen production from pure water using a IEF-11/g-C(3)N(4) S-scheme heterojunction. ChemSusChem. 17(6), e202301538 (2024).
Rana, G. et al. Recent advances in photocatalytic removal of antiviral drugs by Z-scheme and S-scheme heterojunction. Environ. Sci. Pollut Res. Int. 31(28), 40851–40872 (2024).
Chaharlangi, N., Molaei, P. & Yousefi, R. One-step fabrication of S-scheme ZnO/g-C3N4 composites for enhanced environmental photocatalysis. J. Alloys Compds. 1010, 177289 (2025).
Sarngan, P. P. et al. Unveiling efficient S-scheme charge carrier transfer in hierarchical BiOBr/TiO(2) heterojunction photocatalysts. Nanoscale 16(40), 19006–19020 (2024).
Shen, Q. et al. A MOF@MOF S-scheme heterojunction with lewis acid-base sites synergistically boosts cocatalyst-free CO(2) cycloaddition. ChemSusChem. e202401362 (2024).
Shen, Y. et al. g-ZnO/Si(9)C(15): a S-scheme heterojunction with high carrier mobility for photo-electro catalysis of water splitting. Phys. Chem. Chem. Phys. 26(6), 5569–5578 (2024).
Malefane, M. E. et al. s-scheme3D/3D Bi0/BiOBr/P doped g‐C3 N4 with oxygen vacancies (Ov) for photodegradation of pharmaceuticals: in‐situ H2O2 production and plasmon induced stability. ChemSusChem. e202401471 (2024).
Malefane, M. E. et al. Triple S-scheme BiOBr@ LaNiO3/CuBi2O4/Bi2WO6 heterojunction with plasmonic bi-induced stability: deviation from quadruple S-scheme and mechanistic investigation. Adv. Compos. Hybrid. Mater. 7(5), 181 (2024).
Wang, C. et al. A novel dual S-scheme Co(9)S(8)/MnCdS/Co(3)O(4) heterojunction for photocatalytic hydrogen evolution under visible light irradiation. Nanoscale 16(36), 17009–17023 (2024).
Wang, K. et al. Enhanced photocatalytic activity of magnetically recyclable spherical Fe(3)O(4)/Cu(2)o S-scheme heterojunction. Environ. Technol. 45(20), 3986–4002 (2024).
Wang, S. et al. Photothermal-enhanced S-scheme heterojunction of hollow core-shell FeNi(2)S(4)@ZnIn(2)S(4) toward photocatalytic hydrogen evolution. Small 20(30), e2311504 (2024).
Wang, T. et al. Situ construction of hollow coral-like porous S-doped g-C(3)N(4)/ZnIn(2)S(4) S-scheme heterojunction for efficient photocatalytic hydrogen evolution. Adv. Sci. (Weinh). 11(33), e2403771 (2024).
Zang, Y. et al. S-scheme heterojunction of CoMoO4/PCN with lower overpotential for photocatalytic water oxidation. Sep. Purif. Technol. 354, 129146 (2025).
Malefane, M. E. et al. Boosted persulfate activation using Ba2CoMnO5 and LDH/CaCO3 for amoxicillin degradation: a comparative study. Adv. Sustain. Syst. 2400434.
Mafa, P. J. et al. Experimental and theoretical confirmation of CeFeCu trimetal oxide/Bi2O3 S-scheme heterojunction for boosted photocatalytic degradation of sulfamethoxazole and toxicity evaluation. J. Clean. Prod. 429, 139519 (2023).
Mafa, P. J. et al. Visible light responsive MoS2/Ag@ WO3/EG photoanode with highly stable Z-scheme induced circular electron motion pioneered by exfoliated graphite for bisphenol a photoelectrodegradation. Chem. Eng. J. 464, 142462 (2023).
Wang, X. et al. Fabrication of 0D/1D S-scheme CoO-CuBi(2)O(4) heterojunction for efficient photocatalytic degradation of tetracycline by activating peroxydisulfate and product risk assessment. J. Colloid Interface Sci. 661, 943–956 (2024).
Xi, Y. et al. Nanoarchitectonics of S-scheme heterojunction photocatalysts: a nanohouse design improves photocatalytic nitrate reduction to ammonia performance. Angew Chem. Int. Ed. Engl. 63(38), e202409163 (2024).
Xie, K. L. et al. Rationally designed S-scheme CeO(2)/g-C(3)N(4) heterojunction for promoting visible light driven CO(2) photoreduction into syngas. ChemSusChem. e202400969. (2024).
Malefane, M., Feleni, U. & Kuvarega, A. Cobalt (II/III) oxide and tungsten (VI) oxide pn heterojunction photocatalyst for photodegradation of diclofenac sodium under visible light. J. Environ. Chem. Eng. 8(2), 103560 (2020).
Malefane, M. E. et al. Understanding the principles and applications of dual Z-scheme heterojunctions: how far can we go? J. Phys. Chem. Lett. 14(4), 1029–1045 (2023).
Liu, Y. et al. Bi2O3@ MoS2 heterojunction for enhanced photoelectrocatalytic hydrogen evolution. J. Electroanal. Chem. 957, 118127 (2024).
Lin, S. T. et al. High-performance α-Bi2O3/CdS heterojunction photocatalyst: innovative design, electrochemical performance and DFT calculation. J. Nano Res. 71, 13–28 (2022).
Mukwevho, N. et al. Photo-Fenton like reaction for the degradation of methyl orange using magnetically retrievable NiFe2O4/CoMoS4 heterojunction photocatalyst. J. Water Process. Eng. 65, 105882 (2024).
Malefane, M. E., Managa, M., Nkambule, T. T. I. & Kuvarega, A. T. Attuned band structure in triple S-scheme heterojunctions for naproxen degradation under visible light. Chem. Eng. J. 497, 155094 (2024).
Malefane, M. E. et al. Modulation of Z-scheme photocatalysts for pharmaceuticals remediation and pathogen inactivation: design devotion, concept examination, and developments. Chem. Eng. J. 452, 138894 (2023).
Yan, P. et al. Construction of a in(2)O(3)/ultrathin g-C(3)N(4) S-scheme heterojunction for sensitive photoelectrochemical aptasensing of diazinon. J. Colloid Interface Sci. 679(Pt A), 653–661 (2024).
Yin, H. Y. et al. Multifunctional In-MOF and its S-Scheme Heterojunction toward Pollutant Decontamination via fluorescence detection, physical adsorption, and Photocatalytic REDOX. Inorg. Chem. 63(4), 1816–1827 (2024).
You, Y. et al. Rational Design of S-Scheme heterojunction toward efficient photocatalytic cellulose reforming for H(2) and formic acid in pure water. Adv. Mater. 36(4), e2307962 (2024).
Yuan, L. et al. Metal-organic framework-based S-scheme heterojunction photocatalysts. Nanoscale 16(11), 5487–5503 (2024).
Yuan, M. et al. Fabrication of interface engineered S-scheme heterojunction nanocatalyst for ultrasound-triggered sustainable cancer therapy. Adv. Sci. (Weinh). 11(15), e2308546 (2024).
Malefane, M. E. et al. Induced S-scheme CoMn-LDH/C-MgO for advanced oxidation of amoxicillin under visible light. Chem. Eng. J. 480, 148250 (2024).
Iqbal, S., 2020. Spatial charge separation and transfer in L-cysteine capped NiCoP/CdS nano-heterojunction activated with intimate covalent bonding for high-quantum-yield photocatalytic hydrogen evolution. Applied Catalysis B: Environmental, 274, p.119097.
Yue, F. et al. S-scheme heterojunction Cu-porphyrin/TiO(2) nanosheets with highly efficient photocatalytic reduction of CO(2) in ambient air. J. Colloid Interface Sci. 665, 1079–1090 (2024).
Zhang, D. et al. Synthesize magnetic ZnFe(2)O(4)@C/Cd(0.9)zn(0.1)S catalysts with S-scheme heterojunction to achieve extraordinary hydrogen production efficiency. J. Colloid Interface Sci. 657, 672–683 (2024).
Acknowledgements
This work was financially supported by the Guangdong Province Key Construction Discipline Research Ability Improvement Project (2021ZDJS081, 2022ZDJS052, 2022ZDJS053 and 2022ZDJS054). Key Research Platforms and Projects for Ordinary Universities of Guangdong Provincial Department of Education (2022ZDZX3029). Researchers Supporting Project number (RSP2025R242), King Saud University, Riyadh, Saudi Arabia. This research was supported by the International Collaborative Research Program (ICRP2023008), Internal Faculty/Staff Research Support Programs (IRSPC2024007) at Wenzhou Kean University.
Author information
Authors and Affiliations
Contributions
The manuscript was written with contributions from all authors. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Z., Roosh, M., Lu, M. et al. Empowering wastewater treatment with step scheme heterojunction ternary nanocomposites for photocatalytic degradation of nitrophenol. Sci Rep 15, 3299 (2025). https://doi.org/10.1038/s41598-025-86975-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86975-z