Abstract
Brodalumab, a humanized monoclonal antibody that targets the interleukin-17 receptor A, is primarily used to manage moderate-to-severe plaque psoriasis. Although it has demonstrated favorable efficacy and safety in clinical trials, the strict inclusion and exclusion criteria may not fully reflect its safety profile in real-world settings. As its use becomes more widespread in clinical practice, understanding its safety in real-world applications is crucial.
This study employed disproportionality analysis to assess the safety of brodalumab by examining all adverse event reports that identified brodalumab as the primary suspected drug in the FDA Adverse Event Reporting System database since 2017. Techniques such as the Reporting Odds Ratio, Proportional Reporting Ratio, Multi-item Gamma Poisson Shrinker, and Bayesian Confidence Propagation Neural Network were utilized to analyze the adverse events associated with brodalumab. Additionally, the Weibull distribution was used to model the temporal risk of adverse events.
The study identified several adverse reactions already listed on the drug’s label that showed positive signals, including arthralgia, headache, myalgia, suicidal ideation, oropharyngeal pain, injection site mass, and infections. Additionally, we found potential adverse reactions not noted on the drug’s label that exhibited positive signals, including depression, increased blood pressure, peripheral swelling, gait disturbance, inability to walk, stress, myocardial infarction, sepsis, uveitis, nephrolithiasis, and interstitial lung disease. Moreover, this analysis highlighted the critical need for vigilant monitoring of adverse events, especially during the first month following the initiation of treatment.
This study provides initial insights into the real-world safety of brodalumab, confirming known adverse reactions and uncovering additional potential risks. The results deliver vital information that can assist clinicians in making informed decisions when prescribing brodalumab for psoriasis treatment.
Similar content being viewed by others
Introduction
Brodalumab is a novel biologic therapy for psoriasis that blocks IL-17 receptor A, a key receptor involved in IL-17 cytokine binding and the activation of relevant intracellular signaling pathways1,2. Psoriasis is a common, chronic, and immune-mediated skin disease, associated with a number of comorbidities, including metabolic syndrome, cardiovascular disease, and psychiatric disorders3. Psoriasis affects around 2–4% of the population4, and 20% of patients have been estimated to be moderate to severe psoriasis5. The global incidence of psoriasis increased by 26.53% from 1990 to 20196. Psoriatic lesions are infiltrated by a large number of inflammatory cells and interleukin-17 (IL-17) signal pathway plays an important role in the pathogenesis of the disease7. Phase 3 Studies have demonstrated Brodalumab’s effectiveness and safety in the treatment of moderate to severe plaque psoriasis and psoriatic arthritis8,9,10. Meanwhile, brodalumab has been increasingly used in various populations, including adults (aged 18–75)8,11, pregnant and pediatric patients, and those with concomitant chronic infections12. Reports have indicated that the use of brodalumab has led to several challenging adverse events, including subacute cutaneous lupus erythematosus13, ichthyosis14, and autoimmune hepatitis15. Due to the stringent inclusion and exclusion criteria of clinical trials, the safety profile of brodalumab in the general population may not be adequately represented. Moreover, there is currently a lack of real-world safety data on brodalumab. Therefore, post-marketing evaluations utilizing data mining are necessary to explore the potential adverse events associated with brodalumab in real-world settings.
The FDA Adverse Event Reporting System (FAERS) database is a public and valuable reporting database for post-marketing surveillance and early detection of drug safety issues16,17. The database includes the FDA’s collection of all the adverse events information and medication errors. In this study, we aimed to assess the potential adverse events associated with brodalumab by utilizing the FAERS database and several disproportionality analysis techniques. Additionally, we conducted a comprehensive analysis of the time to onset of adverse reactions and explored differences based on age and gender. This study can provide a guide for physicians and health policymakers to monitor adverse reactions and offer recommendations for the rational use of clinical drugs.
Results
Descriptive characteristics
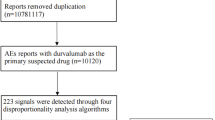
A total of 12,306,668 reported cases were obtained from the FAERS database during the study period (Q1 2017-Q1 2024). After dereplication, this study finally encompassed 1637 adverse event reports associated with brodalumab and 7214 brodalumab-associated adverse events (Fig. 1). The clinical characteristics of brodalumab-related adverse event reports are described in Table 1. These reports included 902 reports (55.1%) from males, 701 reports (42.8%) from females and 34 reports (2.1%) whose gender information was missed. The age group of 18–65 years accounted for 53.8% of the total reports. Healthcare professionals submitted 50.3% of the adverse event reports. Canada, the United States, Japan, Germany, and England were the top five countries with the highest number of reports. In terms of reporting years, the most reported year was 2023 (22.1%), followed by 2020 (17.8%), 2021 (17.3%), 2022 (13.8%), and 2019 (13.7%), respectively. The detailed information regarding adverse event reports associated with brodalumab can be found in Table 1.
Disproportionality analyses
Signal strengths of reports of niraparib at the SOC level are shown in Table 2. Statistically, we found that brodalumab-induced adverse events occurrence targeted 27 organ systems (Fig. 2). The significant SOCs were shown as follows: infections and infestations, skin and subcutaneous tissue disorders, musculoskeletal and connective tissue disorders, surgical and medical procedures, and social circumstances (Table 2). Moreover, we further examined PT signals of the data and the statistically significant signals of the top 100 PTs ranked by frequency was listed in Table 3. All of the top 100 PTs were shown in Supplementary Table 2. The disproportionality analyses confirmed the occurrence of adverse reactions included in the drug’s label, containing arthralgia, headache, myalgia, suicidal ideation, oropharyngeal pain, injection site mass, and infections. In addition, several potential adverse reactions were identified, including depression, blood pressure increased, peripheral swelling, gait disturbance, gait inability, stress, myocardial infarction, sepsis, uveitis, nephrolithiasis, and interstitial lung disease.
Subgroup analyses
Subgroup analyses were subsequently conducted on the top 50 most frequent adverse events. Gender subgroup analysis revealed that require additional attention in male patients include headache, blood pressure increased, and myocardial infarction, while Oropharyngeal pain, Injection site pain, and stress in female patients. Specific signal values for these adverse events can be found in Supplementary Tables 3 and 4.
Age subgroup analysis indicated that depression, stress, suicidal ideation and nasopharyngitis should be paid further attention in the 18–65 age group. In the population over 65 years of age, occurrences of fatigue, headache, and peripheral swelling should be monitored. Signal values for age-related adverse events are provided in Supplementary Tables 5, 6, and 7.
Time to onset and Weibull distribution analysis of adverse events
A total of 498 patients recorded the onset times of adverse events, and the with the specific distribution shown in the Fig. 3. Results indicated that most cases occurred within 1 month (n = 135, 27.1%) after brodalumab administration, and the numbers of cases decreased over time. Additionally, an analysis of the variation of adverse events over time was conducted, as depicted in another Fig. 4. The onset times associated with brodalumab were predicted using the Weibull distribution, showing an early failure model which indicated that the occurrence of adverse events decreases over time. More specific parameters are detailed in the Table 4.
Sensitivity analysis
Brodalumab is commonly used in combination with medications such as clobetasol, prednisone, betamethasone calcipotriene, and vitamin D3. After excluding the four drugs most commonly co-administered with brodalumab in clinical settings, a reanalysis of the top 100 adverse events was conducted. The potential adverse reactions at the PT level were found to be essentially consistent with previous findings, as detailed in Supplementary Table 8, including headache, Covid-19, depression, suicidal ideation, nasopharyngitis, blood pressure increased, cellulitis, influenza, and myocardial infarction.
Discussion
Brodalumab, a human monoclonal antibody targeting the IL-17 receptor A, is primarily used to treat moderate to severe plaque psoriasis. It has shown significant efficacy in managing this condition. However, it is associated with various adverse reactions9,18. Comprehensive and systematic monitoring and analysis of these adverse reactions are necessary to further enhance the safety profile of brodalumab in clinical practice. In this pharmacovigilance study, we utilized the FAERS database and conducted four types of disproportionality analyses to evaluate the adverse events associated with brodalumab. This study identified the majority of the adverse reactions contained in the drug’s label, including arthralgia, headache, myalgia, suicidal ideation, oropharyngeal pain, influenza, injection site mass, and infections. Moreover, several potential adverse reactions not list on the drug label were also confirmed, such as depression, blood pressure increased, peripheral swelling, gait disturbance, gait inability, stress, myocardial infarction, sepsis, uveitis, nephrolithiasis, and interstitial lung disease.
Clinical trials have identified that patients treated with brodalumab have reported higher incidences of infections, which are listed as adverse reactions on the drug’s label19. In this study, we identified several infection-related adverse events including nasopharyngitis, cellulitis, influenza, sepsis, and oral candidiasis. Phase 3 clinical trials reported nasopharyngitis and upper respiratory tract infection as common adverse events, with a slightly higher rate of infections in the brodalumab 210 mg group compared to the 140 mg group and placebo8,20,21. The majority of reported infection-related adverse events included nasopharyngitis, pharyngitis, bronchitis, urinary tract infection, influenza, cellulitis, and sinusitis20,21,22. Brodalumab has also been demonstrated to cause mild to moderate candida infections and the exposure-adjusted event rate of suspected Candida infection was 3.5 events per 100 patient‐years (E/100 PY)8,23. In another phase 3 clinical trial, only one patient was diagnosed with candidiasis during the entire study period of 64 weeks24. Clinically, this suggests that while candida infections are relatively rare, physicians should remain aware of their potential in patients undergoing brodalumab treatment. In addition, patients treated with brodalumab were at risk of exposure of COVID-19 25,26, further emphasizing the need for close monitoring. While serious infections were infrequently reported, isolated cases of serious cryptococcal meningitis, serious coccidioidomycosis infection, serious pneumonia, and urosepsis documented in clinical trials20,22,27. The impairment of IL-17 immunity, due to brodalumab binding to IL-17 A, may account for various infections in treated patients28. These findings suggested that clinicians should remain vigilant for signs of infection, particularly in patients with a history of recurrent infections or other related risk factors, and discontinue brodalumab if a serious infection develops during treatment.
In this study, psychiatric disorders including suicidal ideation and depression were identified as adverse events associated with brodalumab. Suicidal ideation and behavior (SIB) have been reported in patients receiving brodalumab, among patients treated with brodalumab, predominantly in those with a history of depression and/or SIB, as noted on the drug label27. A review of clinical trials found that 3 of 4464 patients receiving brodalumab were confirmed to have committed suicide, potentially as a result of worsening psoriasis following discontinuation of the medication29. Psychiatric adverse events such as depression and anxiety were also reported as potential risks in several clinical trials29,30. However, several studies have reported that the incidence of psychiatric adverse events and SIB events was not significantly higher in brodalumab group compared to placebo8,23. Nonetheless, given the severity of these potential side effects and our findings, it is crucial to conduct thorough mental health assessments before and during treatment and prescribers should carefully weigh the potential risks of brodalumab treatment, especially in patients with a history of depression or SIB.
Headache has been reported to be a common adverse event occurred in phase 3 clinical trials and is also listed on the drug’s label9,23. For instance, in the study conducted by Lebwohl et al., approximately 6–7% of patients receiving brodalumab reported headaches, compared to 3% in the placebo group8. Most reported headaches are mild to moderate in severity. While the underlying mechanism of brodalumab-induced headaches remains unclear, monitoring for this side effect is crucial during clinical application, particularly given its potential impact on a patient’s quality of life. Healthcare providers should be vigilant in assessing the frequency and severity of headaches in patients undergoing brodalumab therapy. Adjustments in treatment or supportive care, such as recommending over-the-counter analgesics or other interventions, may be necessary to improve patient comfort and adherence to therapy.
Our study also identified blood pressure increased as a potential adverse reaction. Considering that hypertension and cardiac diseases are common comorbidities in patients with psoriasis31,32, it is essential to monitor the blood pressure of those treated with brodalumab. Elevated blood pressure can exacerbate underlying cardiovascular conditions, potentially leading to more serious complications. Regular monitoring allows healthcare providers to detect any changes early and intervene appropriately, ensuring that patients receive the most effective care while minimizing the risk of adverse cardiovascular outcomes.
Myocardial infarction was identified as a potential adverse event in this study. Recent studies have focused on biologics-induced major adverse cardiovascular events (MACEs), such as myocardial infarction, cerebrovascular accident, and cardiovascular death. In a pooled analysis, the exposure-adjusted rate of MACE was 0.9 E/ 100PY in the brodalumab 140 mg group in period 1, compared to 0.5 E/100 PY in the placebo group. In period 2, the rates were 0.7 E/100 PY and 1.0 E/100 PY for the brodalumab 210 mg and 140 mg groups, respectively. All patients who experienced MACE had a history of cardiovascular disorders and/or cardiovascular risk factors such as hypertension20. However, in several clinical trials, MACEs were not observed in patients treated with brodalumab9,22,27. Therefore, the association between brodalumab and MACE needs further investigated. Considering the strong link between psoriasis and cardiovascular diseases, along with findings from this study that identified myocardial infarction as a potential adverse event associated with brodalumab, it is imperative that patients with a history of cardiovascular disorders or risk factors undergo careful assessment before starting treatment with brodalumab. Regular monitoring throughout treatment is also recommended to detect any potential cardiovascular issues early.
Moreover, nephrolithiasis was identified as a potential adverse event associated with brodalumab in our study. The typical symptoms of nephrolithiasis include sudden-onset, crampy flank pain, hematuria, nausea, and vomiting. About 50% of patients experience nausea and vomiting due to the shared splanchnic innervation between the renal capsule and the intestines. The presence of fever and chills during the onset is uncommon and may indicate an infected stone or a concurrent urinary tract infection33,34,35. Therefore, regular imaging and initial laboratory tests, including assessments for hematuria and creatinine clearance, are essential to effectively evaluate and manage nephrolithiasis36.
In addition, this study revealed that oropharyngeal pain and interstitial lung disease were the potential adverse events of brodulumab. The causes of oropharyngeal pain included infections37, tumors38,39, oral mucositis40, and neuralgia41. Clinicians should positively explore the causes and provide appropriate treatment when patients experienced oropharyngeal pain. Drug-induced interstitial lung disease have been reported to be caused by antineoplastic drugs42, rituximab43, statins44 and etc. However, interstitial lung disease induced by brodalumab has not been previously reported, which underscores the importance of heightened clinical vigilance when prescribing brodalumab, especially in patients with respiratory symptoms, and warrants further research to better understand this potential complication. Early identification and intervention are essential for managing interstitial lung disease effectively and minimizing long-term pulmonary damage.
Headache, blood pressure increased and cardiovascular adverse events including such as myocardial infarction warrant particular attention in male patients, potentially due to associations with sex hormones. In females, the occurrence of oropharyngeal pain, injection site pain, and stress should be monitored regularly. For patients over the age of 65, fatigue, headache, and peripheral swelling should be carefully concerned during the period of medication use. Additionally, our findings indicated that the most cases occurred within the first month of brodalumab administration, with a subsequent decline over time. This highlights the need for vigilant monitoring of adverse events during the initial month of treatment. Moreover, sensitivity analysis was conducted to identify persistent potential adverse reactions associated with brodalumab monotherapy, including headache, depression, suicidal ideation, nasopharyngitis, and blood pressure increased. Such impactful adverse events can influence treatment adherence, adversely affecting therapeutic efficacy.
Through the analysis of adverse events using the FAERS database, this study provides a comprehensive evaluation of the real-world safety of brodalumab, offering valuable guidance for clinical decision-making. Clinicians should remain vigilant for infections, particularly in patients with a history of recurrent infections, and consider discontinuing treatment if serious infections develop. Meanwhile, given the potential psychiatric side effects, such as depression and suicidal ideation, mental health assessments are essential both before and during treatment. Regular monitoring of blood pressure and cardiovascular health is also recommended, especially for patients with pre-existing risk factors. Additionally, this study further emphasizes the importance of individualized monitoring, as certain adverse events may differ by sex or age. For example, cardiovascular events are more common in males, while oropharyngeal pain is more frequently observed in females, and elderly patients may experience increased fatigue and peripheral swelling. In summary, these findings provide valuable insights into the safety profile of brodalumab, assisting clinicians in making more informed prescribing decisions for their patients.
However, this study has several limitations worth noting. Firstly, as a spontaneous reporting system, the FAERS database is susceptible to underreporting and reporting bias, which could affect the understanding of adverse events associated with brodalumab. Underreporting typically occurs with minor or well-known adverse reactions, as healthcare providers or patients might consider these reactions well-documented, not requiring further reporting, or irrelevant to drug use. Additionally, for new drugs or those that have recently garnered public attention, adverse event reporting might surge due to increased scrutiny, a phenomenon known as “publicity bias”. This could lead to an excessive focus on the safety of certain drugs while neglecting potentially equally important safety issues. Moreover, the relatively small sample size included in this study may not offer a comprehensive overview of identified potential adverse reactions, necessitating further data accumulation. Next, the FAERS database often lacks detailed information on drug exposure and other factors, limiting exploration of their effects on adverse events. Furthermore, although this study identified brodalumab as the primary suspect drug, it did not restrict its use to specific indications, potentially affecting conclusion specificity. In addition, this study was limited to analyzing adverse drug reactions/events and did not comprehensively cover the use of biologicals within controlled environments accompanied by risk management plans or their overall risk-benefit evaluation. This limitation may restrict a full understanding of the overall safety profile of brodalumab. Future research should integrate risk management strategies with patient monitoring in real-world settings to provide a more comprehensive drug safety assessment. Lastly, while disproportionality analysis was effective in identifying positive signals for adverse events, it does not establish a causal relationship between brodalumab and these adverse events. Further prospective studies are essential to validate these findings and enhance the understanding of brodalumab’s safety profile.
In conclusion, this study used disproportionality analysis to investigate adverse events associated with brodalumab, confirming several risks listed on the drug’s label and identifying potential issues such as depression, increased blood pressure, and myocardial infarction. Patients with a history of mental health issues should receive extra psychological support. Patients should be informed about potential side effects and encouraged to report symptoms promptly, while clinicians should conduct thorough pre-treatment evaluations and maintain regular monitoring throughout the therapy.
Methods
Data source and processing
Our study utilized the FAERS database to perform the pharmacovigilance study of brodalumab in the post-marketing setting. The FAERS database gathers spontaneous adverse event reports from healthcare professionals, pharmaceutical manufacturers, and patients from various regions. Seven types of information make up the FAERS database: demographics (DEMO), drug information (DRUG), adverse events (REAC), outcomes (OUTC), report sources (RPSR), therapy start and end dates (THER), and indications (INDI). The adverse event reports related to brodalumab from Q1 2017 to Q1 2024 were searched and downloaded from the FAERS database. The flow chart of this study is depicted in Fig. 1, which illustrates the process of data extraction, processing, and analysis procedures.
Subsequently, we conducted deduplication and standardization of the adverse events. Following the criteria recommended by FDA, we removed the duplicates according to CASEID, FDA_DT, and PRIMARYID45. Next, the terminology for adverse events was standardized using the Medical Dictionary for Regulatory Activities (MedDRA 26.1), which classifies events at both the system organ class (SOC) and preferred term (PT) levels.
Time-to-onset and weibull distribution analysis
Time-to-onset (TTO) of adverse events is defined as the duration from the initiation date of brodalumab use (START_DT in the THER file) to the occurrence date of the adverse event (EVENT_DT in the DEMO file). The median (interquartile range, IQR) was utilized to assess the TTO of brodalumab. The Weibull distribution is capable of predicting the variability of adverse event risk over time, which is characterized by scale α and shape β parameters.
Statistical analysis
In this study, we employed four commonly used disproportionality analysis methods to assess positive signals in adverse events associated with brodalumab: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS). Detailed formulas for these analyses are provided in Supplementary Table 1. All statistical analyses were performed using R software (Version 4.2.2).
Data availability
The database used in this study can be accessed at: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
References
Krstic, J. et al. An overview of Interleukin-17A and Interleukin-17 receptor a structure, Interaction and Signaling. Protein Pept. Lett. 22, 570–578. https://doi.org/10.2174/0929866522666150520145554 (2015).
Russell, C. B. et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J. Immunol. 192, 3828–3836. https://doi.org/10.4049/jimmunol.1301737 (2014).
Armstrong, A. W., Read, C. & Pathophysiology Clinical presentation, and treatment of Psoriasis A Review. Jama-J Am. Med. Assoc. 323, 1945–1960. https://doi.org/10.1001/jama.2020.4006 (2020).
Chandran, V. & Raychaudhuri, S. P. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J. Autoimmun. 34, J314–321. https://doi.org/10.1016/j.jaut.2009.12.001 (2010).
American Academy of Dermatology et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Sect. 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J. Am. Acad. Dermatol. 65, 137–174. https://doi.org/10.1016/j.jaad.2010.11.055 (2011).
Mou, Y. et al. Global trends in the incidence of psoriasis from 1990 to 2019. Eur. J. Dermatol. 32, 207–213. https://doi.org/10.1684/ejd.2022.4245 (2022).
Hawkes, J. E., Chan, T. C. & Krueger, J. G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immun. 140, 645–653. https://doi.org/10.1016/j.jaci.2017.07.004 (2017).
Lebwohl, M. et al. Phase 3 studies comparing brodalumab with Ustekinumab in Psoriasis. N Engl. J. Med. 373, 1318–1328. https://doi.org/10.1056/NEJMoa1503824 (2015).
Farahnik, B. et al. Brodalumab for the treatment of psoriasis: a review of phase III trials. Dermatol. Ther. (Heidelb). 6, 111–124. https://doi.org/10.1007/s13555-016-0121-x (2016).
Bauer, E., Lucier, J. & Furst, D. E. Brodalumab -an IL-17RA monoclonal antibody for psoriasis and psoriatic arthritis. Expert Opin. Biol. Ther. 15, 883–893. https://doi.org/10.1517/14712598.2015.1045410 (2015).
Papp, K. et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br. J. Dermatol. 183, 1037–1048. https://doi.org/10.1111/bjd.19132 (2020).
Kaushik, S. B., Lebwohl, M. G. & Psoriasis Which therapy for which patient: focus on special populations and chronic infections. J. Am. Acad. Dermatol. 80, 43–53. https://doi.org/10.1016/j.jaad.2018.06.056 (2019).
Ang, E., Hadjieconomou, S. & Kalavala, M. Brodalumab-induced subacute cutaneous lupus erythematosus. Clin. Exp. Dermatol. 46, 926–927. https://doi.org/10.1111/ced.14583 (2021).
Mantovani, L. et al. A possible case of brodalumab-induced ichthyosis. J. Dtsch. Dermatol. Ges. 21, 288–290. https://doi.org/10.1111/ddg.14976 (2023).
Serizawa, N., Hoashi, T., Saeki, H. & Kanda, N. A case of Autoimmune Hepatitis during Brodalumab Treatment for Psoriasis. J. Nippon Med. Sch. 87, 359–361. https://doi.org/10.1272/jnms.JNMS.2020_87-607 (2020).
Vogel, U. et al. Investigating overlap in signals from EVDAS, FAERS, and VigiBase((R)). Drug Saf. 43, 351–362. https://doi.org/10.1007/s40264-019-00899-y (2020).
Feng, Z. et al. Real-world safety of PCSK9 inhibitors: a pharmacovigilance study based on spontaneous reports in FAERS. Front. Pharmacol. 13, 894685. https://doi.org/10.3389/fphar.2022.894685 (2022).
Iznardo, H. & Puig, L. The safety of brodalumab for the treatment of psoriasis. Expert Opin. Drug Saf. 19, 365–372. https://doi.org/10.1080/14740338.2020.1730326 (2020).
Blair, H. A. & Brodalumab A review in moderate to severe plaque psoriasis. Drugs 78, 495–504. https://doi.org/10.1007/s40265-018-0888-4 (2018).
Reich, K. et al. Safety of Brodalumab in Plaque Psoriasis: Integrated Pooled Data from five clinical trials. Acta Derm Venereol. 102, adv00683. https://doi.org/10.2340/actadv.v102.1993 (2022).
Kim, T. H. et al. Brodalumab, an anti-interleukin-17 receptor A monoclonal antibody, in axial spondyloarthritis: 68-week results from a phase 3 study. Rheumatol. (Oxford). 62, 1851–1859. https://doi.org/10.1093/rheumatology/keac522 (2023).
Okubo, Y. et al. Efficacy and safety of Brodalumab, an anti-interleukin-17 receptor a monoclonal antibody, for Palmoplantar Pustulosis: 16-Week results of a Randomized Clinical Trial. Am. J. Clin. Dermatol. https://doi.org/10.1007/s40257-024-00876-x (2024).
Papp, K. A. et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol. 175, 273–286. https://doi.org/10.1111/bjd.14493 (2016).
Seo, S. J., Shin, B. S., Lee, J. H. & Jeong, H. Efficacy and safety of brodalumab in the Korean population for the treatment of moderate to severe plaque psoriasis: a randomized, phase III, double-blind, placebo-controlled study. J. Dermatol. 48, 807–817. https://doi.org/10.1111/1346-8138.15733 (2021).
Lebwohl, M. et al. Brodalumab: 4-Year US Pharmacovigilance Report. J. Drugs Dermatol. 22, 419–422. https://doi.org/10.36849/JDD.7344 (2023).
Lebwohl, M. G. et al. Brodalumab: 5-Year US Pharmacovigilance Report. Dermatol. Ther. (Heidelb). 14, 1349–1357. https://doi.org/10.1007/s13555-024-01162-8 (2024).
Mease, P. J., Helliwell, P. S., Hjuler, K. F., Raymond, K. & McInnes, I. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann. Rheum. Dis. 80, 185–193. https://doi.org/10.1136/annrheumdis-2019-216835 (2021).
Puel, A. et al. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr. Opin. Allergy Clin. Immunol. 12, 616–622. https://doi.org/10.1097/ACI.0b013e328358cc0b (2012).
Lebwohl, M. G. et al. Psychiatric adverse events during treatment with brodalumab: Analysis of psoriasis clinical trials. J Am Acad Dermatol 78, 81–89 e85, (2018). https://doi.org/10.1016/j.jaad.2017.08.024
Papp, K. et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol 71, 1183–1190 e1183, (2014). https://doi.org/10.1016/j.jaad.2014.08.039
Orlando, G. et al. Psoriasis and Cardiovascular diseases: an Immune-mediated Cross Talk? Front. Immunol. 13, 868277. https://doi.org/10.3389/fimmu.2022.868277 (2022).
Hu, M. Y., Yang, Q. & Zheng, J. The association of psoriasis and hypertension: focusing on anti-inflammatory therapies and immunological mechanisms. Clin. Exp. Dermatol. 45, 836–840. https://doi.org/10.1111/ced.14327 (2020).
Gottlieb, M., Long, B. & Koyfman, A. The evaluation and management of urolithiasis in the ED: a review of the literature. Am. J. Emerg. Med. 36, 699–706. https://doi.org/10.1016/j.ajem.2018.01.003 (2018).
Teichman, J. M. Clinical practice. Acute renal colic from ureteral calculus. N Engl. J. Med. 350, 684–693. https://doi.org/10.1056/NEJMcp030813 (2004).
Pfau, A. & Knauf, F. Update on Nephrolithiasis: Core Curriculum 2016. Am. J. Kidney Dis. 68, 973–985. https://doi.org/10.1053/j.ajkd.2016.05.016 (2016).
Mayans, L. & Nephrolithiasis Prim. Care 46, 203–212, doi:https://doi.org/10.1016/j.pop.2019.02.001 (2019).
Baracco, G. J. Infections caused by Group C and G Streptococcus (Streptococcus dysgalactiae subsp. Equisimilis and others): epidemiological and clinical aspects. Microbiol. Spectr. 7 https://doi.org/10.1128/microbiolspec.GPP3-0016-2018 (2019).
Belcastro, A., Smith, B. D., Heidel, R. E. & Hechler, B. L. Incidence of pain complaints in oropharyngeal squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 132, 626–632. https://doi.org/10.1016/j.oooo.2021.03.005 (2021).
Vilarim, R. C. B. et al. Characteristics and prevalence of orofacial pain as an initial symptom of oral and oropharyngeal cancer and its impact on the patient’s functionality and quality of life. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 134, 457–464. https://doi.org/10.1016/j.oooo.2022.07.001 (2022).
Raber-Durlacher, J. E., Elad, S. & Barasch, A. Oral mucositis. Oral Oncol. 46, 452–456. https://doi.org/10.1016/j.oraloncology.2010.03.012 (2010).
Do, K. et al. Effectiveness of Radiofrequency Ablation for Treatment of Glossopharyngeal Neuralgia: a systematic review of the current literature. Pain Physician. 27, 97–110 (2024).
Conte, P. et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. 7, 100404. https://doi.org/10.1016/j.esmoop.2022.100404 (2022).
Goswami, R. P. et al. Rituximab in the treatment of systemic sclerosis-related interstitial lung disease: a systematic review and meta-analysis. Rheumatol. (Oxford). 60, 557–567. https://doi.org/10.1093/rheumatology/keaa550 (2021).
Fernandez, A. B., Karas, R. H., Alsheikh-Ali, A. A. & Thompson, P. D. Statins and interstitial lung disease: a systematic review of the literature and of food and drug administration adverse event reports. Chest 134, 824–830. https://doi.org/10.1378/chest.08-0943 (2008).
Zou, F. et al. Adverse drug events associated with linezolid administration: a real-world pharmacovigilance study from 2004 to 2023 using the FAERS database. Front. Pharmacol. 15, 1338902. https://doi.org/10.3389/fphar.2024.1338902 (2024).
Acknowledgements
The authors express their gratitude to the Dermatology Department at the First Affiliated Hospital of Xi’an Jiaotong University. Kaidi Zhao expresses heartfelt thanks to all the staff and leadership of the Department of Dermatology at the Second Affiliated Hospital of Xi’an Jiaotong University for their invaluable support.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82273541), Funds of Shaanxi Province (2021ZDLSF03-01), and institutional foundation of the first affiliated hospital of Xi’an Jiaotong University (No. QYJC06).
Author information
Authors and Affiliations
Contributions
K.H., K.Z., D.Z., and Y.Z. designed the project. K.H. and K.Z. sourced data from the database. K.Z., T.Y., and M.L. visualized the data and prepared figure and tables. J.L. and X.L. prepared the supplementary tables. W.D., M.L., and B.C. revised the data. D.Z. supervised this study. Y.Z. provided the funding for this study. K.H. and T.Y. wrote the original draft. K.Z., D.Z., and Y.Z. reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, K., Zhao, K., Yin, T. et al. A real-world Pharmacovigilance study of brodalumab based on the FDA adverse event reporting system. Sci Rep 15, 2346 (2025). https://doi.org/10.1038/s41598-025-86976-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86976-y
Keywords
This article is cited by
-
A pharmacovigilance study of vortioxetine based on data from the FDA adverse event reporting system
Scientific Reports (2025)