Abstract
Stimulants, such as methylphenidate (MPH), are beneficial for attention-deficit/hyperactivity disorder (ADHD), but individual response varies. A deeper understanding of the mechanisms underpinning response is needed. Previous studies suggest that a single MPH dose modulates resting-state functional connectivity (rs-fc). We investigated whether single-dose induced rs-fc changes were associated with post-dose optimization clinical response. Fifty-six adults with ADHD underwent rs-functional magnetic resonance imaging (rs-fMRI) under placebo and a single MPH dose, before starting MPH treatment. Clinical response was measured at two months. We tested if a single MPH dose (vs. placebo) shifted rs-fc; how these shifts were associated with treatment response (categorical approach); and whether these associations were driven by improvement on either ADHD symptom domain. A single MPH dose (vs. placebo) increased rs-fc in three subcortical-cortical and cerebellar-cortical clusters. Enhanced rs-fc between the cerebellar vermis (lobule 6) and the left precentral gyrus was associated with a greater probability of responding to treatment (χ2(7) = 22.740, p = .002) and with an improvement on both inattentive and hyperactive/impulsive symptoms (both p ≤ .001). We provide proof-of-concept that the brain functional response to a single MPH dose, administered before starting routine treatment, is indicative of two-month clinical response in adult ADHD. This may encourage future replication using clinically applicable measures.
Similar content being viewed by others
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental condition characterized by developmentally inappropriate and challenging inattentive and/or hyperactive-impulsive symptoms. ADHD is estimated to affect ~ 5% of children and young people world-wide, mainly males1, and to persist in 40–50% of adults2. The first line pharmacological option is represented by stimulants, such as methylphenidate (MPH). They enhance cognitive and motor functions affected in ADHD by modulating dopamine and norepinephrine transmission in striato-cortical regions3,4,5. They are generally effective in reducing ADHD core symptoms, but clinical response varies among individuals, with lower response rates in adults6,7,8. This is problematic because untreated individuals, and those who do not respond to treatment, are more likely to experience challenges such as academic and occupational failure, substance abuse, and criminal behavior9,10. Moreover, in clinical practice, suitable ADHD medications are frequently selected using a trial-and-error approach and following gradual titration (i.e. dose increments) over weeks11. Hence, individuals with ADHD may need to undergo multiple trials before the most suitable medication is identified. This approach may increase the risk of side effects and costs, and delay beneficial outcomes.
Consequently, there have been increasing efforts to identify neurobiological characteristics associated with treatment response in ADHD12. For instance, prior studies suggested that variants of genes encoding for the dopamine (SLC6A3) and norepinephrine transporters (SLC6A2)13, theta power14,15, and resting-state fronto-striatal functional connectivity16 are associated with varying treatment response. However, these investigations primarily focused on children, and their findings may not necessarily apply to adults17. Further, these previous studies examined how baseline/cross-sectional measures, rather than dynamic/change measures, related to treatment outcome.
Hence, it remains unclear how biological changes (e.g., following a single dose of medication) relate to longer-term treatment response. Specifically, it would be helpful to understand if the brain functional response to a single dose of MPH, administered before starting treatment, can give an indication as to whether and how an individual will respond clinically post-dose optimization. While it is true that neuroimaging currently has limited applicability to clinical practice (largely due to high associated costs), a deeper understanding of the mechanisms underpinning treatment response and the association between single-dose and longer-term response, is warranted to support the development of more individualized treatment approaches.
As recently reviewed in Parlatini et al.18, previous functional magnetic resonance imaging (fMRI) studies have reported that individuals with ADHD have brain functional alterations in fronto-striatal, parieto-temporal and cerebellar areas, and suggested that stimulants may shift or reduce some of these regional alterations19,20,21. Accordingly, several studies have investigated if stimulants induce functional changes at a brain network level. This includes research in young people, e.g., a study of 23 boys with ADHD, which reported that an acute dose of MPH ‘normalized’ (i.e., abolished) between-group differences in regional homogeneity (a measure of local functional connectivity) in fronto-cerebellar-parietal regions, and that the drug-induced shift in the parietal cortex was associated with symptom reduction at 8 weeks in the 7 participants that completed follow-up22. Similarly, a study in 16 adolescents with ADHD demonstrated that a single dose of MPH ‘normalized’ rs-fc in multiple functional networks23. Moreover, it includes a study in 50 children and 49 adults with ADHD which showed that MPH challenge decreased subcortical (striatal and thalamic) rs-fc in children; and increased it in adults, suggesting that the effects of MPH on rs-fc are age-dependent24. Further, a study in 24 adults with ADHD reported that a single dose of MPH altered (both increased and decreased) subcortical and cortical rs-fc in a region/connection-dependent manner25. A recent clinical trial also demonstrated that, in 20 adults with ADHD, MPH altered connectivity within, but not between, several networks, including the default mode network (DMN)26. Finally, a study in 22 adults with ADHD showed that MPH not only ‘normalised’ rs-fc in some networks; but also re-organised it in others27. Together, these studies represent important first steps in discerning the neurobiological basis of treatment response in people with ADHD. However, it remains unclear how change in regional/network functional connectivity elicited by an acute MPH challenge in adults with ADHD, and in which networks precisely, relates to subsequent, longer-term, treatment response23,28,29,30.
To address this, we tested if a single, acute dose of MPH (compared to placebo, PLC) ‘shifted’ functional connectivity in whole brain resting state networks; and if this change was associated with post-titration treatment response in adults with ADHD. Given prior reports that MPH optimizes activation and functional connectivity within fronto-parieto-striato-cereballar regions during attention and inhibition tasks in individuals with ADHD19,31,32, we hypothesized that MPH induces significant changes within these networks and that these changes are associated with longer-term clinical treatment response.
Methods
Participants
The initial sample included 60 male adults with ADHD aged 18–45 years, who were recruited at the Adult ADHD Clinic, Maudsley Hospital (London, UK)33. Details on sample selection have been previously described, including power calculation34. We included only males because ADHD is more commonly diagnosed in males35, and there is preliminary evidence of sex differences in brain connectivity36,37,38 and response to pharmacological treatment39. Potential limitations of this approach are presented in the discussion. The diagnosis of ADHD was confirmed by a clinician according to the DSM-5 criteria40. We only included participants with no current co-occurring conditions and an intelligence quotient (IQ) above 70. IQ was measured using the Wechsler Abbreviated Scale of Intelligence (WASI)41; and handedness was assesssed through a modified version of the Edinburgh Handedness Inventory (EHI)42. Recruitment focused on the inclusion of medication-naïve adults, but we also included a small minority of individuals who were previously medicated and had stopped treatment at least one year before the start of the study (see Results). Finally, we included 17 neurotypicals that did not differ from the ADHD participants in sex, age, and IQ in secondary analyses to support the interpretation of the results.
Research protocol and experimental procedure
Data used in this study were collected as part of a larger trial (NCT03709940), which employed a single-blind placebo-controlled cross-over design, followed by a longitudinal open-label phase. Details of the protocol have been described in33, which reports the results of our diffusion imaging data analysis. In brief, the study was conducted over three sessions, two at baseline (TIME 1 and TIME 2) and one after two-month MPH treatment (TIME 3, i.e., follow-up). During the baseline sessions, each participant with ADHD underwent rs-fMRI scanning twice, once under PLC (ascorbic acid 50 mgs) and once under a clinically effective dose of short-acting MPH (20 mgs). Half of participants received PLC first, and the other half received MPH first; this cross-over approach aimed to balance potential expectation and practice effects. Participants were blinded to the content of the capsules (single-blind design). The MPH dose used (20 mgs) was slightly higher than that recommended as a starting dose (15 mgs/day) by the NICE guidelines (www.nice.org.uk), as that dose was previously shown to affect brain activation during fMRI tasks in adults43. Neuropsychological tests (reported in44) started one hour after drug administration and the scans three hours after. Considering that the maximum plasma concentration (Cmax) after 20 mgs of MPH is attained on average 90 min after administration, with a one-two hour range43, the set timing allowed participants to perform tests and functional MRI acquisitions under an optimal dose of MPH. Finally, the protocol followed during the two sessions was identical in respect of timing and tests administered, to ensure blinding of participants.
Participants were then prescribed the same long-acting MPH formulation (Concerta XL), following the usual clinical practice of medically supervised titration up to 54 mgs, as per indications of the UK British National Formulary (BNF; https://bnf.nice.org.uk/drugs/methylphenidate-hydrochloride/). During titration, telephone follow-up appointments were offered to monitor potential side effects and clinical response, and to adjust the dose if needed. Dose was considered as a covariate (see below). To evaluate treatment response, ADHD symptoms were measured at baseline and at follow-up using the Barkley Adult ADHD Rating Scale-IV (BAARS-IV)45. This provided three scores: ADHD Total score, ADHD Inattention, and ADHD Hyperactivity-Impulsivity. Next, we grouped ADHD participants into ‘responders’ and ‘non-responders’. We based the categorical definition of treatment response on an overall symptomatic improvement of at least 30%, as measured by the BAARS-IV total score at follow-up as compared to baseline symptom levels. We chose this approach because it is commonly used in pharmacological trials in ADHD8,46,47. It allowed us to identify a subgroup with a high average and another with a low average treatment response, which we respectively labelled as ‘responders’ and ‘non-responders’. In addition, we performed secondary analyses using a dimensional approach, to identify whether the identified brain characteristics were differentially associated with the individual degree of improvement on each symptom domain (i.e. inattention and hyperactivity/impulsivity) (see below). Of note, for our control participants, we only acquired baseline scans.
All participants received detailed information about the study and provided written informed consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The protocol was approved in 2012 by the Camden & Islington Research Ethics Committee (Ethical approval REC number: 12/LO/0630).
Structural and functional magnetic resonance imaging (MRI) data acquisition
Rs-fMRI data was acquired under PLC and after a single dose of MPH in participants with ADHD; and only once (without any capsule) in neurotypicals. Subjects were instructed to relax and keep their eyes open. T2-weighted echo-planar imaging (EPI) images with blood oxygenation level-dependent (BOLD) contrast were acquired with the following parameters: 39 slices, 3 mm thickness with a 0.3 mm gap, TR/TE = 2000/30 ms and flip angle 75°, for a total of 300 volumes and an acquisition time of 10 min per subject. High-resolution structural images were also acquired for each subject (196 slices, 1.2 mm thickness with a 1.2 mm gap, TR/TE = 7.312/3.016 ms, and flip angle = 11°).
Structural and functional MRI data processing
Analyses were conducted using in-house software, Conn v19.c48, and R version 4.1.1 (R Core Team). All scans were inspected manually to ensure adequate data quality and signal to noise ratio; and to exclude scans with obvious artefacts, e.g., blurring, distortions, ghosting, or warping. Structural scans were normalized to Montreal Neurological Institute (MNI) space, and segmented into grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF).
Functional scans were trimmed (first five functional volumes discarded to allow for magnetization equilibrium). The remaining functional volumes were realigned (first: within each subject, all 3D volumes [295 per subject] were aligned to that subject’s first 3D volume; second: all subjects’ 3D volumes were aligned to the first subject’s first 3D volume) and unwarped. We censored scans (to remove motion artifacts) using ARtifact detection Tools (ART) (nitrc.org/projects/artifact_detect) to identify functional outliers (global signal z-value threshold = 3 standard deviations and subject motion threshold = 1 mm; in line with previous studies, e.g.,49). Next, functional scans were segmented, normalized to MNI space, and smoothed using a Gaussian filter with a 6-mm full-width at half-maximum (FWHM) kernel. Moreover, as head motion can distort measures of functional connectivity50, we performed motion correction. Specifically, we applied a threshold of ≥ 3 mm/˚ movement in any dimension and excluded individuals with > 5% of volumes identified as outliers (two-step thresholding procedure, similar to previous studies, e.g.,51). Combined, these steps led to the exclusion of four individuals with ADHD and three neurotypicals. Next, in line with previously published recommendations52, we removed movement confounds (ART scrubbing parameters, and their second order derivatives and quadratic effects) and realignment parameters (as well as their first and second order derivatives) from our data using linear regression. Last, we excluded WM and CSF confounds and simultaneously filtered (band-pass: 0.008–0.09 Hz) and detrended (linear trend removal) our data. We also computed mean head motion (mean of ART-derived framewise motion) to be used as a covariate in our secondary analyses.
We then parcellated the brain into 132 regions using predefined and standardized atlases (cortical and subcortical regions of the FSL Harvard-Oxford atlas53,54,55,56 and cerebellar areas specified in the Automated Anatomical Labeling (AAL) atlas57); and extracted the mean time series of each region. Next, for each subject, we defined the connectivity between each pair of these regions as the (Pearson’s) correlation between their mean time series. Hence, each region served as seed/target region. Correlation coefficients were then Fisher-transformed to improve normality of the data. Next, individual functional connectivity maps were entered into standard analyses of variance to evaluate experimental effects across the brain (as detailed below). For those connections displaying significant effects, we extracted subject-level connectivity values under PLC and MPH. We then computed individual-level percentage-wise change scores (T2-T1)/T1. These values were cleaned (by removing values below Quantile 1-1.5*Interquartile range [IQR] and values above Quantile 3 + 1.5*IQR); and used in subsequent analyses of association with behavioural outcome. Hence, for these analyses, we retained values for 17 neurotypicals and 48 individuals in the ADHD group (14 non-responders, 34 responders).
Statistical analysis
Acute effect of MPH vs. PLC on rs-fc in ADHD
Within the ADHD group, we identified those brain networks (i.e., clusters of seed- and target-regions) whose rs-fc was significantly shifted by an acute dose of MPH. To this aim, we performed a repeated-measures analysis of variance with drug (MPH vs. PLC) as within-group factor, and brain-wide connections as dependent variable. We thresholded our results at the cluster-level using Threshold Free Cluster Enhancement (TFCE) non-parametric inference and Family-wise error (FWE) correction (pFWE<0.05); and additionally at the connection-level using False Discovery Rate (FDR) correction (pFDR<0.05)58. The identified networks were entered in the following analyses.
Association between MPH-induced shifts in rs-fc and treatment response in ADHD
Primary analyses
Within the ADHD group, we used binary logistic regressions (separately for each significant cluster of seed- and target-regions) to test if the identified acute drug-induced shifts in rs-fc were categorically associated with treatment response at two months (responders vs. non-responders). We limited our analyses to those functional connections that were significantly shifted by MPH in the previous step (rather than to all functional connections in the brain) in line with our hypotheses and to accommodate for our moderate sample size (see Limitations). We corrected our analyses for age, baseline total symptom severity, dose, weight, and full-scale IQ. We chose these covariates as they have previously been associated with treatment response59. Classification accuracy was computed as the number of correct predictions (true positives + true negatives) divided by the total number of observations (true positives + true negatives + false positives + false negatives). To establish the robustness of our results, we also (1) repeated our logistic regression analyses using bootstrapping (1000 iterations60), and (2) controlled for additional potential confounds (handedness and mean head motion).
Secondary analyses
First, to better understand the relationship between the drug-induced shifts in rs-fc and treatment response, we extended our analyses from the group-level (using a categorical approach) to the individual level (using a dimensional approach) and from studying overall symptom severity to examining individual symptom domains. Specifically, we used linear regression to examine if the association between the drug-induced shift in rs-fc and treatment response was driven by improvement on either inattentive and/or the impulsive/hyperactive symptoms (BAARS-IV Inattention and BAARS-IV Impulsivity/Hyperactivity scores). Analyses were corrected for baseline severity of each respective symptom domain.
Second, to aid the interpretation of MPH-induced changes, for the functional connections that were significantly shifted by acute MPH, we compared rs-fc between neurotypicals (representing the baseline measure) and the ADHD groups, both under PLC and MPH, using independent samples t-tests (p < .05). We did not compare all brain functional connections between adults with ADHD and neurotypicals, as this was beyond the purpose of our study (i.e. understanding single-dose MPH-induced changes related to treatment response in ADHD).
Results
Participants
Details on participant characteristics have been reported in33. Overall, 56 male adults with ADHD completed the study (79% medication-naïve). Most participants were treated with Concerta XL up to 54 mg as per protocol. The dose was modified in 34% of cases, mainly due to side effects. Dose at follow-up was included as a potential confounder in the statistical analyses (see Methods). Following MRI-data quality checks, the final analyses included 56 individuals with ADHD: 40 responders and 16 non-responders. These two subgroups did not significantly differ in the covariates of interest: age, full-scale IQ, baseline total symptom severity, weight, MHP dose, handedness, and mean head motion (Table 1). As expected, responders showed significantly greater symptom improvement at follow-up (compared to non-responders)(See Table 1 for full results). Neurotypicals did not differ from the ADHD group in age, IQ, sex or mean head motion. Neurotypicals were mostly right-handed (90%) (Table 1).
Acute effect of MPH versus PLC on rs-fc in ADHD
We observed significant effects of drug (MPH > PLC) on the functional connectivity within three clusters of seed/target regions (corrected for multiple comparisons). In Cluster 1, MPH increased rs-fc between the left posterior superior temporal gyrus and both the right and left thalamus (Cluster T(TFCE) = 74.28, pFWE = 0.030). (Table 2; Fig. 1a). In Cluster 2, MPH increased rs-fc between the vermis lobule 6 (Ver6, which is located in the most anterior part of the posterior cerebellar lobe) and the left postcentral and precentral gyrus (Cluster T(TFCE) = 72.19, pFWE = 0.034) (Table 2; Fig. 1b). In Cluster 3, MPH increased rs-fc between the right putamen and the left and right postcentral gyrus, the left and right precentral gyrus, and the left supplementary motor area. Similarly, MPH increased rs-fc between the left putamen and the left and right postcentral gyrus, and the left and right precentral gyrus. Also, MPH increased rs-fc between the left pallidum and the left and right postcentral gyrus, the left and right precentral gyrus, and the left supplementary motor area. Last, we observed an MPH-induced increase in rs-fc between the anterior cingulate cortex and the left postcentral gyrus (Cluster T(TFCE) = 69.89, pFWE = 0.045) (Table 2; Fig. 1c).
Drug-induced shift in functional connectivity within the three identified clusters (a–c) in the ADHD group (MPH > PLC). T-values are indicated by the color bar. Sample size N = 60. Abbreviations: AC, anterior cingulate; L, left; MPH, methylphenidate condition; PLC, placebo condition; PostCG, postcentral gyrus; PreCG, precentral gyrus; pSTG, posterior superior temporal gyrus; R, right; SMA, supplementary motor area; T, t-statistic; TFCE, threshold free cluster enhancement; Ver6, vermis 6.
Association between MPH-induced shifts in rs-fc and treatment response in ADHD
Primary analyses
We examined if the significant drug-induced acute shifts in rs-fc identified above were associated with treatment response (categorically defined) across the ADHD group. We observed no significant association between the drug-induced shift in rs-fc in Cluster 1 and treatment response (χ2(7) = 13.090, p = .070). Conversely, in Cluster 2, the drug-induced shift in rs-fc was significantly associated with treatment response (χ2(7) = 22.740, p = .002). The model explained 60% of the variance (Nagelkerke R2) and correctly classified 88% of cases. Specifically, a greater increase in rs-fc between Ver6 and the left precentral gyrus was associated with a greater likelihood of being a responder (p = .009). (Table 3). These results remained robust when applying Bootstrapping (1000 iterations) (Table S1); and when accounting for additional potential confounds (handedness and mean head motion) (Table S2). In Cluster 3, it was not feasible to examine all functional connections within the same statistical model due to missing data (following the exclusion of outliers, as described in Methods). Therefore, we performed the analyses described above separately for each seed region (multiple comparison correction: p = .05/(#clusters * #seed regions) = 0.004). For two seeds, we observed significant overall models, but no significant effect of the variables of interest (right putamen: χ2(10) = 28.091, p = .002; left pallidum: χ2(10) = 32.815, p < .001). For the remaining seeds (left putamen and anterior cingulate cortex), the overall models were not significant. Please note that, as we only included the connections significantly shifted by MPH (see previous step), and each analysis addressed an individual a priori hypothesis, we did not correct for multiple comparisons across these clusters. Nevertheless, the association between rs-fc change in cluster 2 and treatment outcome would have retained significance even if we had applied Bonferroni-correction both within and across clusters.
Secondary analyses
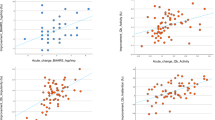
As a secondary analysis, where we observed an association between drug-induced shifts in rs-fc and treatment outcome, we tested dimensionally whether this association was driven by improvement at an individual level on either symptom domain (BAARS-IV Inattention and BAARS-IV Impulsivity/Hyperactivity). In Cluster 2, enhanced rs-fc between the Ver6 and the left precentral gyrus was significantly associated with a greater improvement on both inattentive (Model: F(7,32) = 6.061, p < .001, R = .755; p = .013) (Table 4) and impulsive/hyperactive symptoms (Model: F(7,32) = 9.909, p < .001, R = .827; p = .012) (Table 5) (Fig.S1).
Moreover, to aid the interpretation of MPH-induced shifts associated with treatment response, we compared the rs-fc of the identified connection (i.e., Ver6 - the left precentral gyrus) in the ADHD groups (both under PLC and under MPH) with the corresponding measures obtained in neurotypicals at baseline. Compared to this baseline measure, rs-fc increased under MPH in individuals with ADHD, and we observed a difference between rs-fc ADHD (under MPH) and rs-fc neurotypicals, but this did not reach statistical significance (p = .07) (Table S3, Fig. 2).
Functional connectivity of cluster 2 between the Vermis 6 and the left precentral gyrus in the neurotypicals and the ADHD group after placebo (PLC) and after methylphenidate (MPH) administration. Connectivity values (y-axis) are presented as Fisher Z-transformed Pearson’s Correlation coefficients. Violin plots include boxplots (indicating quartiles) and group means (solid black circles). FC, functional connectivity.
Discussion
Overview
This study shows that a single-dose MPH challenge (compared to placebo) administered to adults with ADHD before starting long-term treatment induced a shift in brain rs-fc, which was associated with the clinical treatment response at two months. Specifically, MPH (vs. PLC) significantly increased rs-fc within three subcortical-cortical-cerebellar clusters. Among these, enhanced connectivity between the cerebellar vermis (lobule 6) and the left precentral gyrus was significantly associated with a greater probability of responding to treatment after dose optimization. This association was driven by improvement in both inattentive and hyperactive/impulsive symptoms.
Acute effect of MPH on rs-fc in ADHD
Previous studies examining the effect of MPH on rs-fc largely focused on children and adolescents. Collectively, these studies demonstrated that MPH ‘shifts’ local and long-range rs-fc61, but they mostly included small samples. Studies in adults have been more limited and rarely implemented a placebo-controlled design. For instance, they included a study that scanned participants on and off their usual stimulant medication; and observed an alteration and re-organisation of rs-fc when on treatment27. Collectively, they have demonstrated that the effect of MPH on rs-fc varies within and across brain regions and networks and brain regions24,25,26,27. Our work extends this prior research by investigating the effect of an acute, single-dose MPH challenge in a relatively large group of adults with ADHD. We observed that a single-dose of MPH increased rs-fc in multiple cortical-subcortical-cerebellar clusters. These included cortical fronto-parietal-temporal regions, such as the pre- and post-central gyri and superior temporal gyrus; subcortical structures, such as the thalamus and the basal ganglia; and the vermis (lobule 6) of the cerebellum. Notably, these regions (and their connections) have been implicated in functions that are relevant to ADHD, including motor and cognitive processes33,62,63. For instance, connections between the cerebellum and the pre- and post-central gyri are part of the somatosensory motor network (SMN) and support motor coordination63. Also, the lobule 6 of the vermis is considered to be a transition zone between the motor and the cognitive regions of the cerebellum64 and is functionally connected to the several resting state networks, including the sensorimotor network65.
Similarly, the role of fronto-striatal circuits in ADHD aetiology is well established. These GABA-glutamatergic circuits modulated by dopamine help regulate motor, cognitive, and affective processing by connecting various parts of the cortex (e.g., sensorimotor, dorsolateral prefrontal, and anterior cingulate cortices) to the basal ganglia and thalamus, which in turn project back to the cortex. Thus, these circuits contribute to executive functioning and affective control, both of which are implicated in ADHD18. Moreover, the left superior temporal gyrus has been linked to language processing66; and functional alterations in this area have been reported in ADHD and other, frequently co-occurring, conditions, such as dyslexia67. Hence, MPH-induced shifts in these connections may improve brain functions that are affected in ADHD, such as cognitive and motor control68,69. Given the transdiagnostic nature of ADHD-related features, such as the attentional mechanisms underpinning executive functioning, our results may also be relevant to other neurodevelopmental conditions70.
Notably, the networks found as altered in our study have been implicated in ADHD in previous studies. For instance, previous studies in children reported lower rs-fc between the cerebellum (including the vermis) and frontal regions in ADHD compared to neurotypicals71,72. In contrast, studies in adults have observed greater rs-fc between the pre- and post-central gyri and either the DMN component of the cerebellum (also including the vermis) or the left cerebellar tonsil73,74. These diverging findings may be due to age and methodological differences; nonetheless, combined with our results, they suggest that cortico-cerebellar rs-fc plays a role in ADHD pathophysiology and/or treatment response.
MPH-induced shifts in rs-fc associated with treatment response in ADHD
The MPH-induced increase in rs-fc between the vermis lobule 6 and the precentral gyrus was significantly associated with an greater probability of being a responder at two months. These results align with previous reports in children/adolescents with ADHD. For example, one of these studies reported a correlation between acute MPH-induced reduction in the degree of regional homogeneity (i.e., local connectivity) in the right parietal cortex and symptom reduction at two months in a sample of 7 children22. Other studies investigated rs-fc before and after 3–6 months of MPH treatment and reported MPH-induced shifts in rs-fc associated with symptom improvement in the DMN and left putamen75; pre- and post-central gyri76; and cerebellar networks28. Considering adults with ADHD, previous studies have observed an MPH-induced enhancement in rs-fc of the SMN and DMN, thus supporting the notion that MPH can shift rs-fc in adults29,30. However, those studies tested individuals after varying lengths of MPH treatment and did not investigate or identify associations between a shift in rs-fc and treatment response. Thus, their comparability with our work is limited. In sum, our results add to previous research by investigating the effect of an acute MPH challenge on rs-fc before treatment initiation – and how this effect relates to post-titration clinical response in adults.
Neurobiological underpinnings
How MPH induces the observed shifts in rs-fc and how these relate to clinical improvement is poorly understood. Preclinical and nuclear medicine studies have shown that MPH optimises dopamine and norepinephrine transmission5. This, in turn, may modulate the cortico-subcortical activation and functional connectivity supporting brain functions such as attention and inhibition19,61. Our results of increased rs-fc between the vermis and the precentral gyrus following MPH administration suggest an enhancement in the interaction between the nodes of this functional network, which may contribute to the beneficial effects of treatment in responders. Previous systematic reviews suggested that ADHD medications may improve functioning both by ‘normalising’ atypical neural processes or inducing compensatory mechanisms19,61. Our results showed that MPH did not ‘normalize’ rs-fc but, instead, made it more different from that of neurotypicals. This increase in rs-fc may represent a compensatory mechanism. In line with our findings, a prior fMRI study showed no difference between cerebellar activation in adolescents with ADHD and neurotypicals under placebo, but a significant enhanced activation of the cerebellar vermis under MPH in the ADHD group, which was associated with improved working memory19. The reported direction of MPH effects is consistent with our findings, although we did not observe a significant difference compared to the neurotypicals. This might be related to the fact that stimulants have a reduced effect in adults compared to younger individuals7. Nevertheless, our results and those of others suggest that MPH may induce a compensatory recruitment of cerebellar networks.
Strength, methodological considerations, and future directions
Our study had several strengths, including the longitudinal design and the use of a data-driven whole-brain approach. Nonetheless, we acknowledge several methodological considerations.
For instance, considering sample selection, we only included male participants. This was because ADHD is more often diagnosed in males35 and, consequently, previous similar studies have largely focused on males23,24. Selecting men therefore enabled us to integrate our results into the context of this previous work. However, there is a growing recognition that females with ADHD are more poorly understood than males, leading to delayed or more limited access to clinical care77; and this highlights the urgent need for more research into ADHD, underlying mechanisms, and treatments in females. Such research should consider the effect of sex differences on rs-fc of brain networks36,37,38,62 and on stimulant treatment response39 to ensure the generalizability of results to females with ADHD. This research may be informed by recent work that demonstrated an effect of MPH on rs-fc in neurotypical (non-ADHD) women; which was related to ADHD symptoms (impulsivity)78.
Also, we only included unmedicated participants. This was motivated by our desire to avoid potential treatment related confounders; and to ensure that observed effects were due to the acute dose of MPH rather than regular MPH administration. Moreover, we solely included participants without co-occurring conditions; and this was due to the fact that co-occurring conditions may alter both brain function and treatment response62. Nonetheless, future studies should aim to expand our research to establish the robustness of our results in the general ADHD population. Considering study design, PLC and MPH capsules were administered in a prefixed, non-randomised order. Randomising the order of administration would have controlled for potential order effects, thus improving the study’s rigor. However, this study was not designed to be a clinical trial comparing the effects of PLC and MPH. Instead, we aimed to assess whether an acute response to medication was associated with treatment response at two months; and our study design was appropriate to answer this research question.
Further, we examined a relatively short follow-up period (two months), because this is the median duration of pharmacological trials in ADHD79 and we were interested in post-titration treatment response. However, recent evidence suggests that long-term treatment in ADHD through MPH can lead to adverse neuropsychiatric effects such as tic emergence or sleep disturbance; and impact the likelihood of the occurrence of other neuropsychiatric conditions80. This suggests that MPH has long-term effects on brain function; and these may also confound the association between acute challenge-induced changes in rs-fc (prior to titration) and subsequent clinical outcome post titration. Moreover, in younger (e.g., child and adolescent) populations, neurodevelopmental changes that occur during the clinical treatment may further confound the link between response to MPH pre- and post-titration. Future studies should therefore test how acute MPH-induced shifts in rs-fc are associated with treatment response at variable ages and durations.
Moreover, we implemented a single-blind design (participants were blinded, researchers/clinicians were unblinded). This step was taken to counter-act potential placebo-effects in participants. However, there is a growing awareness in psychiatry that placebo effects (including placebo effects on the clinician), e.g., those linked to baseline symptom severity, participation in other studies, and unbalanced randomization, may confound the results of (randomized placebo-controlled) drug trials81,82. The nature and magnitude of these effects, including on rs-fc, remain unclear; and are challenging to investigate (e.g., due to the infeasibility of placebo treatments in clinical practice82). Accordingly, future studies extending our work are required to explore potential approaches to examining how placebo (vs. MPH) effects may influence both rs-fc and treatment response in participants. We unblinded clinicians and researchers to ensure optimal care of the participants during their study participation; and to facilitate the statistical analysis (note that the data collection and the primary statistical analysis were conducted by separate people and this further reduced bias). However, future studies should consider fully blinded designs to mitigate potential biases more effectively.
The sample size of our study (N = 56) was based on a power calculation conducted for the original investigation into the effects of MPH on the ADHD brain33. While this sample size could be considered modest, to our knowledge, ours was among the largest neuroimaging studies to date investigating MPH-induced effects on rs-fc in ADHD in conjunction with a subsequent clinical response. For instance, one of the few prior studies investigating the association between single-dose induced functional connectivity changes and longer-term response followed up only 7 children22.
Moreover, our sample included a relatively small number of non-responders (N = 16; compared to the overall N = 56), but this proportion was in line with previous randomized clinical trials46 and representative of the real-world ADHD clinical population. Similarly, we included only 17 neurotypicals. This was because the study aimed to understand the association between single-dose and longer-term response in adults with ADHD, and neurotypicals were only included to facilitate the interpretation of treatment-related effects on rs-fc. The resulting sample size, as well as missing data, precluded us from using more complex machine-learning approaches, e.g. using support vector machines, to identify characteristics associated with treatment response83. However, given the challenges inherent to collecting repeated-measures neuroimaging and behavioural data within a pharmacological study, especially in neurodivergent participants, our sample size was appropriate to provide initial proof-of-concept that the brain response to single-dose MPH relates to clinical response post dose-optimization in ADHD; while providing insights into the underlying biological mechanisms. In fact, post-hoc power analyses revealed that our sample was sufficiently powered to detect drug-induced shifts in rs-fc within clusters 1 and 2 (power ranging from 0.76 to 0.92, alpha < 0.05); but not consistently in cluster 3 (power ranging from 0.34 to 0.85). Hence, our results should be considered preliminary and replicated in future, larger, cohorts.
Considering the choice of imaging techniques, we relied on fMRI-derived measures of rs-fc to study the effect of MPH on the brain. We selected rs-fc because previous studies have established this as an objective measures of brain responsivity to pharmacological modulation51,84,85. Note that future studies are required to further evaluate the effect of imaging artifacts (e.g., head motion) and pre-processing strategies (e.g., motion correction) on the observed results to ensure their robustness.
To increase the potential translatability of our work to routine care settings, future studies should aim to validate our results using more cost-effective approaches. Specifically, such studies could leverage the proof-of-concept provided by our study and build on this by examining how acute MPH-induced shifts in other aspects of brain function (e.g. regional/global activity and connectivity), measured through cheaper and more available techniques (such as electroencephalography or functional near-infrared spectroscopy) relate to subsequent treatment response. Combined, such research may pave a way for the future development of accessible neuroimaging-based tools to support clinical care.
Future studies should also explore the neurobiological underpinnings of the observed association between rs-fc shifts and treatment response; and investigate the potential role of other brain networks. ADHD is a highly (biologically and clinically) heterogeneous condition; hence, our results should be interpreted as some of several potential mechanisms through which MPH exerts its effect on brain and behaviour.. Moreover, we need to understand better how other characteristics (e.g., clinical profiles and demographic factors, such as co-occurring conditions, lifestyle factors, and medication adherence) may affect an individual's response to MPH. For example, a previous study reported that MPH changed cerebellar blood flow; but that the direction of this change was dependent on baseline hyperactivity levels86. Additionally, future studies should test if associations between rs-fc shifts and MPH treatment response vary across the age span, as this may help understand the age-related biological mechanisms underlying reduced response rates in adults. Collectively, this research may help improve the generalizability of our results to the wider ADHD community. Also, additional studies may wish to investigate the brain’s response to alternative ADHD medications – and the relationship between that response and future clinical outcome. For instance, initial studies suggest that MPH and the non-stimulant drug atomoxetine have both shared and distinct effects on brain function in ADHD31; and a better understanding of how these effects relate to long-term outcome may, in the future, guide research into more targeted (personalised) interventions. Finally, future studies should expand our research to examining the relationship between MPH’s effect on rs-fc and on neuropsychological features in ADHD. For instance, recent studies have revealed beneficial effects of MPH on cognitive skills frequently altered in ADHD, including attention, working memory, and inhibition87. A better understanding of how drug-induced changes in brain function relate to variability in treatment-induced improvement in these features may further inform the development of personalised medicines.
In sum, we provided proof-of-concept that an acute MPH challenge induces functional biological changes (i.e., shifts in cortico-cerebellar rs-fc) that are associated with post-titration clinical drug response in adults with ADHD. These results need to be replicated and extended in independent samples88. Only once these steps have been completed according to the principles of precision psychiatry12,88 can we explore their potential clinical utility, for instance to help identify likely non-responders in the context of clinical trials of new medications. Although neuroimaging does not currently have applicability in clinical practice, our observations may encourage further research into a more personalized and cost-effective approach to clinical care, where the most suitable medication is identified before starting treatment. Ultimately, such research may help to optimise clinical outcomes and improve wellbeing within the ADHD community, while reducing costs and waiting times associated with trial-and-error approaches.
Data availability
The data that support the findings of this study are available from the authors (VP) upon reasonable request.
References
Polanczyk, G., de Lima, M. S., Horta, B. L., Biederman, J. & Rohde, L. A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 164, 942–948. https://doi.org/10.1176/ajp.2007.164.6.942 (2007).
Sibley, M. H., Mitchell, J. T. & Becker, S. P. Method of adult diagnosis influences estimated persistence of childhood ADHD: A systematic review of longitudinal studies. Lancet Psychiatry 3, 1157–1165. https://doi.org/10.1016/S2215-0366(16)30190-0 (2016).
Arnsten, A. F. & Pliszka, S. R. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 99, 211–216. https://doi.org/10.1016/j.pbb.2011.01.020 (2011).
Berridge, C. W. et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry 60, 1111–1120. https://doi.org/10.1016/j.biopsych.2006.04.022 (2006).
Faraone, S. V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav Rev. 87, 255–270. https://doi.org/10.1016/j.neubiorev.2018.02.001 (2018).
Seixas, M., Weiss, M. & Muller, U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J. Psychopharmacol. 26, 753–765. https://doi.org/10.1177/0269881111412095 (2012).
Cortese, S. et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 5, 727–738. https://doi.org/10.1016/S2215-0366(18)30269-4 (2018).
Cortese, S. Pharmacologic treatment of attention deficit-hyperactivity disorder. N. Engl. J. Med. 383, 1050–1056. https://doi.org/10.1056/NEJMra1917069 (2020).
Chang, Z. et al. Risks and benefits of attention-deficit/hyperactivity disorder medication on behavioral and neuropsychiatric outcomes: A qualitative review of pharmacoepidemiology studies using linked prescription databases. Biol. Psychiatry 86, 335–343. https://doi.org/10.1016/j.biopsych.2019.04.009 (2019).
Pitts, M., Mangle, L. & Asherson, P. Impairments, diagnosis and treatments associated with attention-deficit/hyperactivity disorder (ADHD) in UK adults: Results from the lifetime impairment survey. Arch. Psychiatr Nurs. 29, 56–63. https://doi.org/10.1016/j.apnu.2014.10.001 (2015).
Cortese, S., Newcorn, J. H. & Coghill, D. A. Practical evidence-informed Approach to managing stimulant-refractory attention deficit hyperactivity disorder (ADHD). CNS Drugs 35, 1035–1051. https://doi.org/10.1007/s40263-021-00848-3 (2021).
Michelini, G., Norman, L. J., Shaw, P. & Loo, S. K. Treatment biomarkers for ADHD: Taking stock and moving forward. Transl Psychiatry 12, 444. https://doi.org/10.1038/s41398-022-02207-2 (2022).
Myer, N. M., Boland, J. R. & Faraone, S. V. Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Mol. Psychiatry 23, 1929–1936. https://doi.org/10.1038/mp.2017.234 (2018).
Sari Gokten, E. et al. Predictive value of slow and fast EEG oscillations for methylphenidate response in ADHD. Clin. EEG Neurosci. 50, 332–338. https://doi.org/10.1177/1550059419863206 (2019).
Ogrim, G. et al. Predicting the clinical outcome of stimulant medication in pediatric attention-deficit/hyperactivity disorder: Data from quantitative electroencephalography, event-related potentials, and a go/no-go test. Neuropsychiatr Dis. Treat. 10, 231–242. https://doi.org/10.2147/NDT.S56600 (2014).
Hong, S. B. et al. Functional dysconnectivity of corticostriatal circuitry and differential response to methylphenidate in youth with attention-deficit/hyperactivity disorder. J. Psychiatry Neurosci. 40, 46–57. https://doi.org/10.1503/jpn.130290 (2015).
Pagerols, M. et al. Integrative genomic analysis of methylphenidate response in attention-deficit/hyperactivity disorder. Sci. Rep. 8, 1881. https://doi.org/10.1038/s41598-018-20194-7 (2018).
Parlatini, V., Bellato, A., Murphy, D. & Cortese, S. From neurons to brain networks, pharmacodynamics of stimulant medication for ADHD. Neurosci. Biobehav Rev. 164, 105841. https://doi.org/10.1016/j.neubiorev.2024.105841 (2024).
Rubia, K. et al. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Biol. Psychiatry 76, 616–628. https://doi.org/10.1016/j.biopsych.2013.10.016 (2014).
Hart, H., Radua, J., Nakao, T., Mataix-Cols, D. & Rubia, K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: Exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 70, 185–198. https://doi.org/10.1001/jamapsychiatry.2013.277 (2013).
Peterson, B. S. et al. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am. J. Psychiatry 166, 1286–1294. https://doi.org/10.1176/appi.ajp.2009.08050724 (2009).
An, L. et al. Methylphenidate normalizes resting-state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology 38, 1287–1295. https://doi.org/10.1038/npp.2013.27 (2013).
Silk, T. J., Malpas, C., Vance, A. & Bellgrove, M. A. The effect of single-dose methylphenidate on resting-state network functional connectivity in ADHD. Brain Imaging Behav. 11, 1422–1431. https://doi.org/10.1007/s11682-016-9620-8 (2017).
Kaiser, A. et al. Effects of a single-dose methylphenidate challenge on resting-state functional connectivity in stimulant-treatment naive children and adults with ADHD. Hum. Brain Mapp. 43, 4664–4675. https://doi.org/10.1002/hbm.25981 (2022).
Farr, O. M. et al. The effects of methylphenidate on resting-state striatal, thalamic and global functional connectivity in healthy adults. Int. J. Neuropsychopharmacol. 17, 1177–1191. https://doi.org/10.1017/S1461145714000674 (2014).
Mowinckel, A. M. et al. Increased default-mode variability is related to reduced task-performance and is evident in adults with ADHD. NeuroImage: Clin. 16, 369–382 (2017).
Cary, R. P. et al. Network structure among brain systems in adult ADHD is uniquely modified by Stimulant Administration. Cereb. Cortex 27, 3970–3979. https://doi.org/10.1093/cercor/bhw209 (2017).
Yoo, J. H., Kim, D., Choi, J. & Jeong, B. Treatment effect of methylphenidate on intrinsic functional brain network in medication-naive ADHD children: A multivariate analysis. Brain Imaging Behav. 12, 518–531. https://doi.org/10.1007/s11682-017-9713-z (2018).
Cary, R. P. et al. Network structure among brain systems in adult ADHD is uniquely modified by stimulant administration. Cereb. Cortex 27, 3970–3979 (2017).
Picon, F. A. et al. Methylphenidate alters functional connectivity of default mode network in drug-naive male adults with ADHD. J. Atten. Disord. 24, 447–455. https://doi.org/10.1177/1087054718816822 (2020).
Kowalczyk, O. S. et al. Methylphenidate and atomoxetine normalise fronto-parietal underactivation during sustained attention in ADHD adolescents. Eur. Neuropsychopharmacol. 29, 1102–1116. https://doi.org/10.1016/j.euroneuro.2019.07.139 (2019).
Rubia, K. et al. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology 57, 640–652. https://doi.org/10.1016/j.neuropharm.2009.08.013 (2009).
Parlatini, V. et al. Poor response to methylphenidate is associated with a smaller dorsal attentive network in adult attention-deficit/hyperactivity disorder (ADHD). Transl Psychiatry 13, 303. https://doi.org/10.1038/s41398-023-02598-w (2023).
Parlatini, V. et al. Cortical alterations associated with lower response to methylphenidate in adults with ADHD. Nat. Mental Health https://doi.org/10.1038/s44220-024-00228-y (2024).
Young, S. et al. Females with ADHD: an expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/ hyperactivity disorder in girls and women. BMC Psychiatry. 20, 404. https://doi.org/10.1186/s12888-020-02707-9 (2020).
Dupont, G., van Rooij, D., Buitelaar, J. K., Reif, A. & Grimm, O. Sex-related differences in adult attention-deficit hyperactivity disorder patients—An analysis of external Globus Pallidus functional connectivity in resting-state functional MRI. Front. Psychiatry. 13, 962911. https://doi.org/10.3389/fpsyt.2022.962911 (2022).
Chai, Y. et al. Sex-specific frontal-striatal connectivity differences among adolescents with externalizing disorders. Neuroimage Clin. 32, 102789. https://doi.org/10.1016/j.nicl.2021.102789 (2021).
Park, B. Y. & Park, H. Connectivity differences between adult male and female patients with attention deficit hyperactivity disorder according to resting-state functional MRI. Neural Regen Res. 11, 119–125. https://doi.org/10.4103/1673-5374.175056 (2016).
Kok, F. M., Groen, Y., Fuermaier, A. B. M. & Tucha, O. The female side of pharmacotherapy for ADHD-A systematic literature review. PLoS One. 15, e0239257. https://doi.org/10.1371/journal.pone.0239257 (2020).
American Psychiatric Association. (ed. American Psychiatric Association) (American Psychiatric Association, 2013).
Wechsler, D. Wechsler abbreviated scale of intelligence (1999).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113. https://doi.org/10.1016/0028-3932(71)90067-4 (1971).
Muller, U. et al. Plasma level-dependent effects of methylphenidate on task-related functional magnetic resonance imaging signal changes. Psychopharmacol. (Berl) 180, 624–633. https://doi.org/10.1007/s00213-005-2264-9 (2005).
Parlatini, V. et al. Cortical alterations associated with lower response to methylphenidate in adults with ADHD. Nat. Mental Health 2, 514–524 (2024).
Barkley, R. A. Barkley Adult ADHD Rating Scale-IV (BAARS-IV) (Guilford Press, 2011).
Biederman, J. et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 59, 829–835. https://doi.org/10.1016/j.biopsych.2005.09.011 (2006).
Rosler, M., Fischer, R., Ammer, R., Ose, C. & Retz, W. A randomised, placebo-controlled, 24-week, study of low-dose extended-release methylphenidate in adults with attention-deficit/hyperactivity disorder. Eur. Arch. Psychiatry Clin. Neurosci. 259, 120–129. https://doi.org/10.1007/s00406-008-0845-4 (2009).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. https://doi.org/10.1089/brain.2012.0073 (2012).
Redcay, E. et al. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front. Hum. Neurosci. 7, 573. https://doi.org/10.3389/fnhum.2013.00573 (2013).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018 (2012).
Pretzsch, C. M. et al. Modulation of striatal functional connectivity differences in adults with and without autism spectrum disorder in a single-dose randomized trial of cannabidivarin. Mol. Autism 12, 49. https://doi.org/10.1186/s13229-021-00454-6 (2021).
Satterthwaite, T. D. et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256. https://doi.org/10.1016/j.neuroimage.2012.08.052 (2013).
Makris, N. et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 83, 155–171. https://doi.org/10.1016/j.schres.2005.11.020 (2006).
Frazier, J. A. et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am. J. Psychiatry 162, 1256–1265. https://doi.org/10.1176/appi.ajp.162.7.1256 (2005).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021 (2006).
Goldstein, J. M. et al. Hypothalamic abnormalities in schizophrenia: Sex effects and genetic vulnerability. Biol. Psychiatry 61, 935–945. https://doi.org/10.1016/j.biopsych.2006.06.027 (2007).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. https://doi.org/10.1006/nimg.2001.0978 (2002).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061 (2009).
Simonoff, E. et al. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J. Child. Psychol. Psychiatry 54, 527–535. https://doi.org/10.1111/j.1469-7610.2012.02569.x (2013).
Wang, H., Zhu, R. & Ma, P. Optimal subsampling for large sample logistic regression. J. Am. Stat. Assoc. 113, 829–844. https://doi.org/10.1080/01621459.2017.1292914 (2018).
Pereira-Sanchez, V. et al. Systematic review: Medication effects on Brain intrinsic functional connectivity in patients with attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry 60, 222–235. https://doi.org/10.1016/j.jaac.2020.10.013 (2021).
Parlatini, V. et al. White matter alterations in attention-deficit/hyperactivity disorder (ADHD): A systematic review of 129 diffusion imaging studies with meta-analysis. Mol. Psychiatry 28, 4098–4123. https://doi.org/10.1038/s41380-023-02173-1 (2023).
Castellanos, F. X. & Proal, E. Large-scale brain systems in ADHD: Beyond the prefrontal-striatal model. Trends Cogn. Sci. 16, 17–26. https://doi.org/10.1016/j.tics.2011.11.007 (2012).
Stoodley, C. J. & Schmahmann, J. D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46, 831–844. https://doi.org/10.1016/j.cortex.2009.11.008 (2010).
Sang, L. et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 61, 1213–1225. https://doi.org/10.1016/j.neuroimage.2012.04.011 (2012).
Karnath, H. O. New insights into the functions of the superior temporal cortex. Nat. Rev. Neurosci. 2, 568–576 (2001).
Richlan, F., Kronbichler, M. & Wimmer, H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 30, 3299–3308. https://doi.org/10.1002/hbm.20752 (2009).
Bernard, J. A. et al. Resting state cortico-cerebellar functional connectivity networks: A comparison of anatomical and self-organizing map approaches. Front. Neuroanat. 6, 31. https://doi.org/10.3389/fnana.2012.00031 (2012).
Park, I. S., Lee, N. J. & Rhyu, I. J. Roles of the declive, folium, and tuber cerebellar vermian lobules in sportspeople. J. Clin. Neurol. 14, 1–7 (2018).
Sadozai, A. K. et al. Executive function in children with neurodevelopmental conditions: A systematic review and meta-analysis. Nat. Hum. Behav. https://doi.org/10.1038/s41562-024-02000-9 (2024).
Kim, S. M. et al. Balance deficit and brain connectivity in children with attention-deficit/hyperactivity disorder. Psychiatry Investig. 14, 452–457. https://doi.org/10.4306/pi.2017.14.4.452 (2017).
Mizuno, Y. et al. Catechol-O-methyltransferase polymorphism is associated with the cortico-cerebellar functional connectivity of executive function in children with attention-deficit/hyperactivity disorder. Sci. Rep. 7, 4850. https://doi.org/10.1038/s41598-017-04579-8 (2017).
Kucyi, A., Hove, M. J., Biederman, J., Van Dijk, K. R. & Valera, E. M. Disrupted functional connectivity of cerebellar default network areas in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 36, 3373–3386. https://doi.org/10.1002/hbm.22850 (2015).
Hoekzema, E. et al. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum. Brain Mapp. 35, 1261–1272. https://doi.org/10.1002/hbm.22250 (2014).
Battel, L. et al. Intrinsic brain connectivity following long-term treatment with methylphenidate in children with attention-deficit/hyperactivity disorder. J. Child. Adolesc. Psychopharmacol. 26, 555–561. https://doi.org/10.1089/cap.2015.0221 (2016).
Shang, C. Y. et al. Differential effects of methylphenidate and atomoxetine on intrinsic brain activity in children with attention deficit hyperactivity disorder. Psychol. Med. 46, 3173–3185. https://doi.org/10.1017/S0033291716001938 (2016).
Martin, J. Why are females less likely to be diagnosed with ADHD in childhood than males? Lancet Psychiatry 11, 303–310 (2024).
Daood, M. et al. Graph analysis uncovers an opposing impact of methylphenidate on connectivity patterns within default mode network sub-divisions. Behav. Brain Funct. 20, 15. https://doi.org/10.1186/s12993-024-00242-1 (2024).
Lin, Q. et al. Sex differences in microstructural alterations in the corpus callosum tracts in drug-naïve children with ADHD. Brain Imaging Behav. https://doi.org/10.1007/s11682-021-00556-y (2022).
Krinzinger, H. et al. Neurological and psychiatric adverse effects of long-term methylphenidate treatment in ADHD: A map of the current evidence. Neurosci. Biobehav Rev. 107, 945–968. https://doi.org/10.1016/j.neubiorev.2019.09.023 (2019).
Weimer, K., Enck, P., Dodd, S. & Colloca, L. Editorial placebo and nocebo effects in psychiatry and beyond. Front. Psychiatry 11, 801. https://doi.org/10.3389/fpsyt.2020.00801 (2020).
Faraone, S. V. et al. Placebo and nocebo responses in randomised, controlled trials of medications for ADHD: A systematic review and meta-analysis. Mol. Psychiatry 27, 212–219. https://doi.org/10.1038/s41380-021-01134-w (2022).
de Salazar, G. et al. Individualized prediction models in ADHD: A systematic review and meta-regression. Mol. Psychiatry https://doi.org/10.1038/s41380-024-02606-5 (2024).
Gordon, I. et al. Intranasal oxytocin enhances connectivity in the neural circuitry supporting social motivation and social perception in children with autism. Sci. Rep. 6, 35054. https://doi.org/10.1038/srep35054 (2016).
Pretzsch, C. M. et al. The effect of cannabidiol (CBD) on low-frequency activity and functional connectivity in the brain of adults with and without autism spectrum disorder (ASD). J. Psychopharmacol. 269881119858306 https://doi.org/10.1177/0269881119858306 (2019).
Anderson, C. M., Polcari, A., Lowen, S. B., Renshaw, P. F. & Teicher, M. H. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. Am. J. Psychiatry 159, 1322–1328. https://doi.org/10.1176/appi.ajp.159.8.1322 (2002).
Parlatini, V., Bellato, A., Roy, S., Murphy, D. & Cortese, S. Association between single-dose and longer term clinical response to stimulants in attention-deficit/hyperactivity disorder: A systematic review of randomized controlled trials. J. Child. Adol Psychop. 34, 337–345 (2024).
Fusar-Poli, P., Hijazi, Z., Stahl, D. & Steyerberg, E. W. The science of prognosis in psychiatry: A review. JAMA Psychiatry 75, 1289–1297. https://doi.org/10.1001/jamapsychiatry.2018.2530 (2018).
Acknowledgements
We thank all the participants who took part into the study, the Adult ADHD Clinic, and the Pharmacy Team at the Maudsley Hospital.
Funding
This research project is part of a larger trial funded by Shire (project IST-ALB-000217) and included support from the NIHR Maudsley Biomedical Research Centre. The design of the study, data collection and analysis, and decision to publish were not influenced by the funding bodies. Further, the work by CMP and DGMM was supported in part by the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777394 for the project AIMS-2-TRIALS. This Joint Undertaking receives support from the European Union's Horizon 2020 Research and Innovation Programme, EFPIA, AUTISM SPEAKS, and SFARI. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results. The views expressed are those of the author(s) and not necessarily those of the funders (IHI-JU2, NIHR).
Author information
Authors and Affiliations
Contributions
All authors gave substantial contributions to the study. All authors contributed to the conception of the research question, study design, and writing/revision of the manuscript. In addition, VP was responsible for the recruitment of participants and data acquisition, and CMP conducted the imaging and statistical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pretzsch, C.M., Parlatini, V. & Murphy, D. Single–dose methylphenidate induces shift in functional connectivity associated with positive longer term clinical response in adult attention–deficit/hyperactivity disorder. Sci Rep 15, 5794 (2025). https://doi.org/10.1038/s41598-025-87204-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87204-3
Keywords
This article is cited by
-
From rest to focus: pharmacological modulation of the relationship between resting state dorsal attention network dynamics and task-based brain activation
Neuropsychopharmacology (2026)
-
Clinical response to a single-dose methylphenidate challenge is indicative of treatment response at two months in adults with ADHD
Translational Psychiatry (2025)