Abstract
This study aims to examine the relationship between physical activity and inhibitory function in college students with depressive symptoms and to explore the role of resting EEG as a mediator in this relationship. Specifically, it analyzes how physical activity correlates with inhibitory function and identifies potential routes through which physical activity impacts inhibitory function. A cross-sectional study was conducted involving 154 college students with depressive symptoms. Participants completed the Basic Information Questionnaire, the Beck Depression Inventory-II, and the Physical Activity Rating Scale. Five-minute EEG signals were recorded, and participants performed behavioral tasks to assess inhibitory function. Data were analyzed using independent samples t-tests, χ2 tests, Pearson correlation analysis, and structural equation modeling. Significant differences were found between the adequate and inadequate physical activity groups in BDI scores and response inhibition (P < 0.05). There was a significant positive correlation between physical activity and the average accuracy rate on No-go tasks (r = 0.281, P < 0.001) in students with depressive symptoms. Delta and beta1 lateralization in the central, parietal, and temporal regions were significantly positively correlated with both physical activity and response inhibition (r = 0.20, r = 0.38, both P < 0.05). Both direct and mediating effects were significant, with direct effects accounting for 67.80% of the total effect (B = 0.141, 95% CI: 0.013–0.287) and mediating effects accounting for 32.20% of the total effect (B = 0.067, 95% CI: 0.029–0.136). Gender did not significantly moderate the relationship between physical activity and response inhibition (P > 0.05). There is a close relationship between physical activity, specific EEG indicators, and response inhibition. The central, parietal, and temporal regions serve as potential routes linking physical activity and response inhibition. Delta band lateralization (in the central, parietal, and temporal regions) may be the route through which physical activity influences response inhibition.
Similar content being viewed by others
Introduction
Depression is a serious mental disorder affecting over 264 million individuals globally, with a prevalence rate of approximately 5–10%1. The prevalence of depressive symptoms among college students has been increasing in recent years2, making it a common mental health problem in this demographic. Symptoms of depression include reduced volitional activity, decreased learning efficiency, difficulties in interpersonal relationships, and disturbances in appetite and sleep. In severe cases, individuals may experience thoughts or behaviors related to self-harm or suicide3. Depression has widespread adverse effects on individuals, families, and society. For individuals, it leads to low mood, loss of interest, fatigue, and a high risk of suicide4; for families, it results in strained relationships and increased financial burdens5; for society, it causes decreased productivity and increased healthcare costs6. Given its extensive impact and severe consequences, there is an urgent need to raise public awareness, increase investments in healthcare resources, and improve diagnostic and treatment methods.
Inhibitory function (IF) is a core component of executive function, typically divided into response inhibition and interference inhibition7. Response inhibition involves suppressing unnecessary or inappropriate behaviors, while interference inhibition involves suppressing conflicting or irrelevant stimuli7. Research indicates that individuals with depression exhibit impaired inhibitory function when dealing with negative emotions and thoughts8. This impairment makes it difficult for them to escape negative thought patterns, exacerbating emotional regulation difficulties. Improving inhibitory function is crucial for individuals with depression9. Studies have also found that college students with depressive symptoms exhibit impaired inhibitory function, making it difficult for them to control impulses and ignore distractions, thus affecting attention and increasing negative emotions10. A close relationship exists between depressive symptoms and inhibitory function, with higher depression scores associated with poorer performance on inhibitory function tasks11,12. Individuals with good IF are better able to adapt to changing environments, reducing their risk of depressive symptoms13.
Physical exercise, as an economical, green, and side-effect-free non-medical intervention, plays a crucial role in preventing and alleviating depressive symptoms14. Studies have found that college students who engage in more physical exercise have lower rates of depressive symptoms15. Physical exercise may alleviate depressive symptoms through various routes, such as increasing levels of neurotransmitters (e.g., dopamine and serotonin), reducing inflammatory responses, and regulating the hypothalamic-pituitary-adrenal axis16. Physical exercise is also closely related to inhibitory function, with regular exercise improving inhibitory function and enhancing emotional stability17. Exercise may improve self-control and response inhibition by increasing blood flow and oxygen supply to the prefrontal cortex, enhancing prefrontal cortex activity, and promoting neural plasticity18,19,20. While research has suggested that physical activity can improve depressive symptoms and inhibitory function, the specific routes through which physical activity enhances inhibitory function remain under exploration.
With the rapid development of neurophysiological technologies, resting EEG has been found to effectively reflect cognitive function21, providing technical support for exploring the relationship between physical activity and IF and their routes. EEG is a non-invasive technique for measuring cortical neural electrical activity changes, effectively reflecting pathological and physiological changes related to cognitive function. It is non-invasive, convenient, rapid, and has high resolution, making it widely used in psychology, sports science, and neurology22. Studies have shown that the frontal, parietal, and temporal cortical regions are closely related to exercise23. Whedon et al.24,25,26 found that the alpha band in the frontal region is closely related to IF, but evidence explaining delta, theta, alpha1, alpha2, beta1, and beta2 bands in the central, parietal, occipital, and temporal regions remains lacking. Regarding lateralization, increasing evidence shows that response inhibition exhibits frontal lateralization27,28, but whether other brain region bands show lateralization requires further exploration.
Reviewing previous literature, the routes through which physical activity affects IF primarily focus on the alpha band in the frontal region, with evidence of frontal lateralization. Over the past decade, under the leadership of Professor Xing Wang, our team has been committed to exploring the dose-response relationship between physical activity and inhibitory function. Preliminary research has shown that physical activity is closely related to inhibitory function and depressive symptoms, suggesting that physical activity may be an important means of improving inhibitory function and alleviating depressive symptoms29,30,31,32. Recent findings indicate that decreased delta power values in the parietal region and prefrontal alpha1 lateralization may be routes through which physical exercise alleviates depressive symptoms33,34. Based on these preliminary findings, this study aims to explore the potential routes between physical activity and inhibitory function through a cross-sectional study design, addressing the following questions: Is there a relationship between physical activity, EEG, and IF? If so, how does EEG mediate the relationship between physical activity and IF? Which brain regions and bands are primarily involved in EEG mediation? The goal is to provide evidence for clinical practice.
Subjects and methods
Subject recruitment
The study subjects were college students. Sample size estimation: Based on the principle of Monte Carlo power analysis for indirect effects, an online tool was used for sample size estimation (Monte Carlo Power Analysis for Indirect Effects, written by Alexander M. Schoemann, Aaron J. Boulton, and Stephen D. Short; website: https://schoemanna.shinyapps.io/mc_power_med/). According to previous reviews (Verburgh35and Chang36), the effect size was determined, and with a sample size of 140, the statistical power for indirect effects reached 0.80 or above. Considering a 10% sample attrition rate, 154 participants were planned for recruitment. The study recruited 154 college students with depressive symptoms from Songjiang University Town in Shanghai, using convenience sampling and voluntary participation principles.
Inclusion criteria: (1) Non-physical education majors; (2) Left-handed; (3) Good physical health; (4) Normal vision and hearing; (5) Normal psychological state, able to communicate verbally, and willing to complete the survey; (6) Willing to sign an informed consent form; (7) Beck Depression Inventory-II score of 14 or higher.
Exclusion criteria: (1) Severe cardiovascular or major organic diseases; (2) Severe muscular diseases that prevent long-term standing; (3) Presence of exercise contraindications; (4) Poor vision or hearing that prevents completing assessments; (5) Long-term or recent use of psychotropic drugs such as chlorpromazine or drugs affecting physical activity.
This study was approved by the Ethics Committee of Shanghai University of Sport (Ethics No.: 102772021RT004).
Experimental Procedure
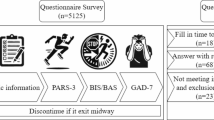
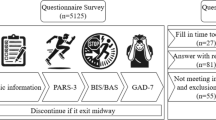
The experiment was conducted from September to December 2023 at the Sports and Health Promotion Research Center of Shanghai University of Sport. Testing times were from 2 PM to 5 PM. All participants were required to avoid strenuous exercise and the consumption of caffeine, alcohol, or other stimulants 12 h before the experiment. The testing procedure is shown in Fig. 1.
Experimental measurement tools and indicators
(1) Basic Information Questionnaire.
This questionnaire collects essential information about the participants, including their gender, age (years), height (meters), weight (kilograms), smoking habits (defined as smoking at least one cigarette per day for a continuous or cumulative period of six months), drinking habits (defined as drinking more than once a week for a continuous or cumulative period of six months), household registration location (rural/urban), whether they are an only child, and whether they come from a single-parent family.
(2) Inhibitory Function Behavioral Tests.
The study employs the Go/No-go task to assess response inhibition and the Stroop task to evaluate interference inhibition, both of which are programmed and recorded using MATLAB software (refer to Fig. 2).
-
(1)
Response Inhibition Test: This is conducted using the Go/No-go task paradigm, which includes a practice module and a main experiment module. The practice module consists of 20 trials, each followed by feedback on performance and reaction time. Participants must achieve a 75% accuracy rate to proceed to the main experiment module; otherwise, they will repeat the practice module up to three times. After the practice module, participants take a 30-second break before starting the main experiment module, which comprises 120 trials with a 4 (96): 1 (24) Go to No-go stimulus ratio. In this task, “M” and “W” letters appear randomly in black. After a fixation point (“+”) appears, a letter (M or W) is displayed at the screen center. Participants should not respond to “M” but press the “F” key when “W” appears. The stimulus is presented for 50,800 ms. This task assesses response inhibition, measured by the difference in accuracy between Go and No-go trials—the smaller the difference, the better the inhibitory control.
-
(2)
Interference Inhibition Test: This test is conducted using the Stroop task, divided into a practice module and a main experiment module. In the practice module, participants familiarize themselves with the task procedures to prevent errors due to unfamiliarity. They can proceed to the main experiment module only after fully understanding the procedures. The Stroop task involves stimuli consisting of Chinese characters (“red,” “green,” “yellow,” “blue”) displayed in corresponding colors (red, green, blue, yellow). There are four congruent trials (character and color match) and twelve incongruent trials (character and color do not match), making up sixteen stimuli presented randomly. In incongruent trials (I trials), the character’s color does not match its meaning (e.g., “blue” written in green), whereas in congruent trials (C trials), the character’s color matches its meaning (e.g., “red” written in red). Participants need to press the key corresponding to the color they see (R for red, Y for yellow, B for blue, G for green) as quickly and accurately as possible. The main experiment module consists of 24 trials, each presented for 1500 ms, with inter-trial intervals ranging from 200 to 1000 ms. This task assesses interference inhibition, measured by the difference in accuracy and reaction times between congruent and incongruent trials—the smaller the Stroop interference effect, the better the interference inhibition.
(3) The Physical Activity Rating Scale-3 (PARS-3).
The PARS-3, developed by Kuniomi Hashimoto, was utilized in this study using its Chinese version, revised by Liang Deqing et al. The scale’s internal consistency reliability ranges from 0.80 to 0.86, with a test-retest reliability of 0.8237. This scale assesses the physical activity of non-sports majors over the past month, evaluating three dimensions: intensity, duration, and frequency. Intensity and frequency are scored from 1 to 5, while duration is scored from 0 to 4. Higher scores indicate greater intensity, higher frequency, and longer duration, corresponding to a greater amount of physical activity. The activity level is calculated as intensity × duration × frequency, with scores ranging from 0 to 100. Scores ≤ 19 indicate low physical activity (insufficient activity), 20–42 indicate moderate activity, and ≥ 43 indicate high activity.
(4) Beck Depression Inventory-II (BDI-II).
The Beck Depression Inventory-II, developed by psychologist Aaron Beck and colleagues, was adapted for this study using the Chinese version revised by Wang Zhen et al. The Chinese version’s internal consistency reliability is 0.94, demonstrating good test-retest reliability38. This scale measures the severity of depressive symptoms across 21 items, each scored from 0 to 3. The total score is the sum of these 21 items, with scores of 0–13 indicating no depression, 14–19 indicating mild depression, 20–28 indicating moderate depression, and 29–63 indicating severe depression.
(5) EEG Signal Collection.
EEG signals were collected using an electroencephalograph (NCERP-190012) from Shanghai Nuocheng Electric Co., Ltd., equipped with a pre-amplifier. A 16-lead monopolar setup was used, with a 500 Hz sampling frequency, a 50 Hz notch filter, a 30 Hz low-pass filter, and a 0.3 Hz high-pass filter. The EEG was divided into delta (1–4 Hz), theta (4–8 Hz), alpha1 (8–10.5 Hz), alpha2 (10.5–13 Hz), beta1 (13–20 Hz), and beta2 (20–30 Hz) bands based on frequency, using a left-closed, left-open interval. Testing was conducted in a quiet, ventilated darkroom, with no electronic devices allowed. Participants acclimated to the environment by sitting quietly. Once stabilized, they were fitted with an EEG cap and connected to the electroencephalograph. The tester adjusted the impedance below 5kΩ, following the 10/20 system electrode placement method specified by the International Federation of Clinical Neurophysiology, with leads at Fp1, Fp2, F3, F4, F7, F8, C3, C4, P3, P4, O1, O2, T3, T4, T5, and T6. The ground electrode was GND, and the reference electrodes were A1 and A2 on both earlobes. Participants were instructed to relax, place their hands naturally by their sides, close their eyes, stay awake, breathe steadily, and avoid swallowing or teeth-grinding. EEG signals were recorded for 5 min once stable.
Statistical analysis
Segments of data with significant artifact interference were filtered out using a toolbox. Each lead signal was assigned to the corresponding brain region: orbitofrontal (Fp1, Fp2), prefrontal (F3, F4), lateral frontal (F7, F8), central (C3, C4), parietal (P3, P4), occipital (O1, O2), temporal (T3, T4), and posterior temporal (T5, T6). The lateralization index of each pair of homologous electrodes in the left brain was calculated as (P_left − P_right) / (P_left + P_right), with higher values indicating greater right lateralization (P represents absolute power value; left and right represent the symmetric electrode points on the left and right sides). The power value indicators of each brain region were calculated as P_left + P_right.
Data processing and analysis were performed using SPSS 29.0 software, with structural equation modeling for route analysis established using Amos 24.0. Measurement data conforming to normal or approximate normal distribution were described by mean ± standard deviation (M ± SD), and intergroup comparisons were made using independent sample t-tests. Significantly skewed measurement data were described by median (interquartile range), with intergroup comparisons made using the Mann-Whitney U test. Count data were described by n (%), with intergroup comparisons made using the χ2 test. Pearson tests explored the relationship between physical activity, inhibitory function, and specific EEG indicators, with the association’s effect size represented by the goodness of fit R2. Structural equation model evaluation indicators included RMSEA (Root Mean Square Error of Approximation), RMR (Root Mean Square Residual), NFI (Normed Fit Index), GFI (Goodness of Fit Index), and CFI (Comparative Fit Index). Route analysis parameter estimates were obtained using the non-parametric percentile Bootstrap method (no strict requirements on variable distribution), with 5,000 resampling iterations. Mediation effects were statistically significant if the 95% confidence interval (95%CI) did not include 0. Statistical significance was indicated by P < 0.05, P < 0.01, and P < 0.001, with the test level α set at 0.05. All parameter statistical analyses were conducted using two-tailed tests.
Results
Basic Information
A total of 154 participants were included in this study, with an average age of 19.500 ± 1.080 years, height of 1.709 ± 0.063 m, weight of 60.241 ± 8.153 kg, and BMI of 20.275 ± 2.989 kg/m2. 24% had a smoking habit, 3.9% had a drinking habit, 72.7% were from urban households, 55.2% were only children, and 7.8% were from single-parent families. There were 59 males and 95 females, with an average physical activity score of 18.12 ± 16.167 points, and an average No-go accuracy rate of 64.870 ± 14.469%. There were 100 individuals in the insufficient physical activity group and 54 in the sufficient physical activity group. No statistically significant demographic differences were found between the groups except for gender and BDI scores (P > 0.05), as shown in Table 1.
Difference in IF between insufficient physical activity group and sufficient physical activity group
As shown in Table 2; Fig. 3, there existed significant differences in Go reaction time, No-go accuracy, the accuracy of congruent trial for Stroop task, the response time of congruent trial for Stroop task and the response time of incongruent trial for Stroop task between the insufficient physical activity groups and sufficient physical activity groups (P < 0.05) while the difference between Go accuracy and the accuracy of incongruent trial for Stroop task was not significant (P > 0.05), which suggested that physical activity might be an important factor for inhibitory function.
The correlation between physical activity and IF
As shown in Table 3, there was a significant positive correlation between the No-go accuracy and physical activity (r = 0.281, P < 0.001). The result showed that the higher the physical activity, the higher the No-go accuracy. Other indexes of IF were positively correlated with physical activity, but the difference was not statistically significant (P > 0.05). These results suggested that physical activity might be an important means to enhance response inhibition.
Selection of EEG Specific indicators for No-go Accuracy
Pearson correlation tests were used to explore the relationship between various EEG indicators and No-go accuracy. As shown in Table 4, significant positive correlations were found between No-go accuracy and the following EEG bands: delta (central, parietal, occipital, lateral frontal, temporal, and posterior temporal regions), theta (central and temporal regions), alpha2 (central, parietal, occipital, temporal, and posterior temporal regions), beta1 (orbital frontal, prefrontal, central, parietal, occipital, temporal, and posterior temporal regions), and beta2 (prefrontal, central, parietal, occipital, temporal, and posterior temporal regions) (all P < 0.05). This suggests that EEG specific indicators in different bands and brain regions may serve as biomarkers for response inhibition.
Selection of EEG specific indicators for physical activity
To further investigate EEG specific indicators sensitive to physical activity, Pearson correlation tests were conducted to examine the relationship between physical activity and EEG specific indicators. As shown in Tables 4 and 5, lateralization phenomena were observed in the delta (central, parietal, occipital, temporal, and posterior temporal regions) and beta1 (occipital and posterior temporal regions) bands, both of which were significantly positively correlated with physical activity (P < 0.05). Although the theta band showed lateralization in the posterior temporal region and had a statistically significant correlation with physical activity, it was not among the specific EEG indicators for No-go accuracy. The correlation coefficients of the other indicators with physical activity were not statistically significant (P > 0.05). This suggests that EEG specific indicators in the delta and beta1 bands may serve as common biomarkers reflecting the relationship between physical activity and response inhibition.
The mediating effect of the specific indexes of EEG and the moderating effect of gender
As shown in Fig. 4, to explore the mediating effect of specific indexes of EEG on the correlation between physical activity and No-go accuracy, a hypothesis model was constructed with physical activity as the independent variable, 7 specific indexes of EEG as the mediator, and No-go accuracy as the dependent variable.
The results of the structural equation model are as follows: CMIN/DF = 2.642, RMR = 0.060, RMSEA = 0.104, GFI = 0.921, NFI = 0.950, and CFI = 0.968. The 95% confidence intervals (CIs) for the total effect, mediating effect, and direct effect did not include zero. As shown in Table 6, the direct effect accounted for 67.67% of the total effect [B = 0.157, 95% CI (0.013, 0.287)], while the mediating effect through the lateralization of the delta band (central, parietal, occipital, temporal, and posterior temporal regions) and the beta1 band (occipital and posterior temporal regions) accounted for 32.33% of the total effect [B = 0.075, 95% CI (0.029, 0.136)]. Among these, the lateralization of the delta band (central, parietal, and temporal regions) ranked highest in the weight of EEG specific indicators. This suggests that specific EEG indicators can mediate the relationship between physical activity and No-go accuracy, with the lateralization of the delta band (central, parietal, and temporal regions) likely playing a significant role in the route between physical activity and response inhibition.(See Fig. 5).
As the differences in physical activity between different genders were found above, in order to further explore whether gender had a moderating effect on the relationship between physical activity and No-go accuracy, a structural equation model was constructed with physical activity as the independent variable, gender as the moderator, and No-go accuracy as the dependent variable. As shown in Table 7, gender didn’t have the moderating effect on the correlation between physical activity and No-go accuracy (P > 0.05).
Discussion
The findings of this study indicate significant differences in depression scores and response inhibition between students with adequate physical activity and those with insufficient physical activity. There is a positive correlation between physical activity and the accuracy of response inhibition among college students with depressive symptoms—more physical activity is associated with better response inhibition. This is consistent with previous research35,39,40. Prior studies suggest that physical exercise can enhance inhibitory function through various mechanisms. Exercise can boost the activity of the brain’s prefrontal cortex and promote neuroplasticity, thereby improving self-control and response inhibition41. It also stimulates the production of brain-derived neurotrophic factor, vital for the growth, differentiation, and survival of neurons, thus strengthening neural network connectivity18 and enhancing response inhibition.
This study identified EEG indicators that are associated with both physical activity and response inhibition, primarily in the delta band lateralization (central, parietal, occipital, temporal, and posterior temporal regions) and beta1 band lateralization (occipital and posterior temporal regions), with the delta band lateralization (central, parietal, and temporal regions) showing the highest weight. These areas should be the focus of attention. The central region is linked to perception and movement42, the parietal region to somatosensory perception43, and the temporal region to perception, hearing, and memory44. This suggests that the central, parietal, and temporal regions can be starting points for optimizing exercise programs to improve response inhibition, offering a basis for more precise exercise prescriptions. For instance, the focus on the parietal and temporal regions during exercise could enhance individuals’ awareness of themselves and their environment. The delta band is closely related to sleep45, while the beta1 band is associated with high stress, anxiety, or excitement46,47. This indicates that physical activity may improve response inhibition by enhancing sleep and reducing stress and anxiety. Brain lateralization refers to the neuropsychological differentiation between the brain’s left and right hemispheres (with the left hemisphere responsible for syntactic and phonetic analysis, and the right hemisphere for recognizing and understanding intonation, non-verbal signals, and pragmatics). Left lateralization implies that the left hemisphere dominates in processing information related to response inhibition, such as intonation, non-verbal signals, and pragmatics48,49. This suggests that enhancing response inhibition through physical activity should focus on recognizing and understanding intonation, non-verbal signals, and pragmatics, and college students should be encouraged to increase physical activity to improve response inhibition.
The study also found that the correlation between physical activity and conflict inhibition was not significant, which differs from previous meta-analyses35,40. This discrepancy may be due to differences in the subject population and the intensity and duration of exercise interventions. Moreover, gender does not moderate the relationship between physical activity and response inhibition, indicating a stable mechanism for improving inhibitory function through physical exercise that is unaffected by gender. Recently, researchers have been identifying specific neuroelectrophysiological indicators of physical activity and inhibitory function through EEG50, providing some guidance for enhancing response inhibition. However, there is still no consensus on the different brain regions and frequency bands. Future research should further explore specific electrophysiological indicators and investigate the effects of different exercise programs on these indicators.
This study has some limitations: (1) It is a cross-sectional design, which limits causal inferences. Future studies should conduct longitudinal research to verify the route of delta band lateralization (central, parietal, and temporal regions) proposed in this paper. (2) Physical activity in this study was measured using a scale, which introduces subjectivity. Future research should use objective measurement tools. (3) A larger sample size is needed to further elucidate the relationship between physical activity and response inhibition. Future studies should test the effectiveness of improving response inhibition through specific EEG indicators and consider these as outcome measures for designing precise exercise prescriptions. (4) The study participants were predominantly aged 17 to 24 years. Future research should further investigate different age groups and populations.
Conclusion
There is a close relationship between physical activity, specific EEG indicators, and response inhibition. The central, parietal, and temporal regions are potential routes through which physical activity influences response inhibition. Delta band lateralization (central, parietal, and temporal regions) may be the mechanism by which physical activity enhances response inhibition. College students should be encouraged to increase physical activity to improve response inhibition.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
ELBE, A.-M. et al. Is regular physical activity a key to mental health? Commentary on “Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: A cross-sectional study”, by Chekroud et al., published in Lancet Psychiatry [J]. J. Sport Health Sci. 8(1), 6–7 (2019).
SMITH K. Mental health: a world of depression [J]. Nature 515 (7526), 181 (2014).
PARK L T, ZARATE C A, J. R. Depression in the Primary Care setting [J]. N Engl. J. Med. 380 (6), 559–568 (2019).
BLACKWELL C, K. et al. Longitudinal Changes in Youth Mental Health from before to during the COVID-19 pandemic [J]. JAMA Netw. Open. 7 (8), e2430198 (2024).
OGDEN C, L. et al. Depression and diet Quality, US Adolescents and Young Adults: National Health and Nutrition Examination Survey, 2015-March 2020 [J] (J Acad Nutr Diet, 2024).
KöHLER-FORSBERG, D. A. M. V. H. & OZENNE, K. B, et al. Effect of Antidepressant Treatment on 5-HT(4) Receptor Binding and Associations with Clinical Outcomes and Verbal Memory in Major Depressive Disorder [J] (Biol Psychiatry, 2024).
DIAMOND A. Executive functions [J]. Annu. Rev. Psychol. 64, 135–168 (2013).
JOORMANN, J. & GOTLIB I, H. Emotion regulation in depression: relation to cognitive inhibition [J]. Cogn. Emot. 24 (2), 281–298 (2010).
DISNER S G et al. Neural mechanisms of the cognitive model of depression [J]. Nat. Rev. Neurosci. 12 (8), 467–477 (2011).
VILGIS, V. & SILK T J, V. A. N. C. E. A. Executive function and attention in children and adolescents with depressive disorders: a systematic review [J]. Eur. Child. Adolesc. Psychiatry. 24 (4), 365–384 (2015).
CHEN M H et al. Cortical inhibition function is associated with baseline suicidal symptoms and post-ketamine suicidal symptom reduction among patients with treatment-resistant depression and strong suicidal ideation [J]. J. Psychiatr Res. 161, 48–53 (2023).
LEWIS, G. et al. Inhibitory control of positive and negative information and adolescent depressive symptoms: a population-based cohort study [J]. Psychol. Med. 52 (5), 853–863 (2022).
AïTE, A. et al. Adolescents’ inhibitory control: keep it cool or lose control [J]. Dev. Sci., 21(1). (2018).
PEARCE, M. et al. Association between Physical Activity and Risk of Depression: a systematic review and Meta-analysis [J]. JAMA Psychiatry. 79 (6), 550–559 (2022).
HEISSEL, A. & HEINEN, D. Exercise as medicine for depressive symptoms? A systematic review and meta-analysis with meta-regression [J]. Br. J. Sports Med. 57 (16), 1049–1057 (2023).
XIA, H. S. et al. The brain mechanism of physical exercise promoting cognitive function [J]. Adv. Psychol. Sci. 26 (10), 1857–1868 (2018).
CHEN C C, RINGENBACH S D, C. R. E. W. S. D. et al. The association between a single bout of moderate physical activity and executive function in young adults with Down syndrome: a preliminary study [J]. J. Intellect. Disabil. Res. 59 (7), 589–598 (2015).
ZHAO, J. L. et al. Exercise, brain plasticity, and depression [J]. CNS Neurosci. Ther. 26 (9), 885–895 (2020).
LUDYGA, S. et al. Neurophysiological correlates of interference control and response inhibition processes in children and adolescents engaging in open- and closed-skill sports [J]. J. Sport Health Sci. 11 (2), 224–233 (2022).
KANDOLA, A. et al. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity [J]. Neurosci. Biobehav Rev. 107, 525–539 (2019).
MUSHTAQ, F. & WELKE, D. GALLAGHER A, et al. One Hundred Years of EEG for Brain and Behaviour Research [J] (Nat Hum Behav, 2024).
MARINO, M. MANTINI D. Human brain imaging with high-density electroencephalography: techniques and applications [J]. J. Physiol., (2024).
STILLMAN, C. M. et al. Effects of Exercise on Brain and Cognition Across Age Groups and Health States [J]. Trends Neurosci. 43 (7), 533–543 (2020).
YU, J., ABDULLAH, M. & MANSOR, N. S. EEG components of inhibitory control ability in internet gaming disorder: a systematic review and meta-analysis of randomized controlled trials [J]. Brain Behav. 14 (1), e3388 (2024).
WHEDON, M. & PERRY N B, BELL, M. A. Relations between frontal EEG maturation and inhibitory control in preschool in the prediction of children’s early academic skills [J]. Brain Cogn. 146, 105636 (2020).
CHOO, Y. et al. Right inferior frontal gyrus damage is associated with impaired initiation of inhibitory control, but not its implementation [J]. Elife, 11. (2022).
LUCON-XICCATO T, MONTALBANO, G. et al. Lateralization correlates with individual differences in inhibitory control in zebrafish [J]. Biol. Lett. 16 (8), 20200296 (2020).
CIPOLOTTI, L. et al. Inhibition processes are dissociable and lateralized in human prefrontal cortex [J]. Neuropsychologia 93 (Pt A), 1–12 (2016).
LI, S. et al. Correlation of exercise participation, behavioral inhibition and activation systems, and depressive symptoms in college students [J]. Sci. Rep. 13 (1), 16460 (2023).
LI, S. et al. Correlates of physical activity levels, muscle strength, working memory, and cognitive function in older adults [J]. Front. Aging Neurosci. 15, 1283864 (2023).
SUN, W. X. et al. Physical exercise participation level and depressive symptoms in college students: mediating role of social support [J]. Chin. J. Health Stat. 40 (03), 421–424 (2023).
QIU, S. L. et al. Effects of single high-intensity interval exercise on emotion and lateralization of frontal lobe α frequency band in depressed college students [J]. Acad. J. Naval Med. Univ. 43 (10), 1211–1216 (2022).
WANG, X. et al. Relationship among physical activity, mild depressive symptoms and frontal alpha power asymmetry in college students [J]. Chin. Mental Health J. 38 (02), 180–185 (2024).
WANG, P. et al. The relationship between physical activity level and depressive symptoms in college students: a pathway analysis based on resting EEG [J]. J. Shanghai Univ. Sport. 47 (04), 51–60 (2023).
VERBURGH, L. & KöNIGS, M. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis [J]. Br. J. Sports Med. 48 (12), 973–979 (2014).
CHAN Y S, JANG J T, HO C, S. Effects of physical exercise on children with attention deficit hyperactivity disorder [J]. Biomed. J. 45 (2), 265–270 (2022).
YANG, G. et al. Physical activity influences the mobile phone addiction among Chinese undergraduates: the moderating effect of exercise type [J]. J. Behav. Addict. 10 (3), 799–810 (2021).
WANG, Z. et al. Reliability and validity of the Chinese version of Beck Depression Inventory-II among depression patients [J]. Chin. J. Mental Health. 25 (06), 476–480 (2011).
ZHANG, D. et al. Enhanced response inhibition in experienced fencers [J]. Sci. Rep. 5, 16282 (2015).
AMATRIAIN-FERNáNDEZ, S., EZQUERRO GARCíA-NOBLEJAS, M. & BUDDE, H. Effects of chronic exercise on the inhibitory control of children and adolescents: a systematic review and meta-analysis [J]. Scand. J. Med. Sci. Sports. 31 (6), 1196–1208 (2021).
MEEUSEN R. Exercise, nutrition and the brain [J]. Sports Med., 44 Suppl 1(Suppl 1): S47–56. (2014).
FALTINE-GONZALEZ D, HAVRILAK, J. & LAYDEN M, J. The brain regulatory program predates central nervous system evolution [J]. Sci. Rep. 13 (1), 8626 (2023).
CASPERS, S. Microarchitecture and connectivity of the parietal lobe [J]. Handb. Clin. Neurol. 151, 53–72 (2018).
RICE G E, HOFFMAN, P. & LAMBON RALPH M, A. Graded specialization within and between the anterior temporal lobes [J]. Ann. N Y Acad. Sci. 1359 (1), 84–97 (2015).
DING, F. et al. Effect of exercise on cravings levels in individuals with drug dependency: A systematic review [J]. Addictt. Behav. 158, 108127 (2024)
BARRY R J, K. A. R. A. M. A. C. O. S. K. A. D. et al. Intrinsic EEG and task-related changes in EEG affect Go/NoGo task performance [J]. Int. J. Psychophysiol. 125, 17–28 (2018).
MEMON A R et al. Sleep and physical activity in university students: a systematic review and meta-analysis [J]. Sleep. Med. Rev. 58, 101482 (2021).
GüNTüRKüN, O. OCKLENBURG S Ontogenesis Lateralization [J] Neuron, 94(2): 249–263. (2017).
DUBOC, V. et al. Asymmetry of the brain: development and implications [J]. Annu. Rev. Genet. 49, 647–672 (2015).
CHU, C.-H. et al. Effects of acute aerobic exercise on motor response inhibition: An ERP study using the stop-signal task [J]. J. Sport Health Sci. 4(1), 73–81 (2015)
Funding
None.
Author information
Authors and Affiliations
Contributions
Jinlei Zhao: Conceptualization, Writing – original draft, Data curation, Investigation. Yanran Si: Data curation, Methodology, Writing – original draft. Feng Ding: Data curation, Investigation. Shuqi Jia: Data curation, Investigation. Cong Liu: Investigation. Haiyan Wen: Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent and institutional review board approval
For experiments involving human participants, informed consent has been obtained from all participants (all adults) in this study. Our study was approved by the ethical committee of Shanghai University of Sport (102772021RT004), All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jinlei, Z., Yanran, S., Feng, D. et al. Exploring the relationship between physical activity and Inhibitory Function in College Students with depressive symptoms through EEG. Sci Rep 15, 3149 (2025). https://doi.org/10.1038/s41598-025-87213-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87213-2