Abstract
Gas foam injection offers a viable solution to challenges faced in oil reservoirs, yet ensuring optimal foamability and stability remains a pivotal hurdle in practical field operations. This study presents a novel synthesis procedure to create silica (SiO2) Janus nanoparticles (JNPs) and examines their potential to enhance gas foam stability for enhanced oil recovery (EOR) applications. Two variations of SiO2 JNPs were synthesized via a masking procedure, employing oleic acid and ascorbic acid within a Pickering emulsion, marking a pioneering approach. These nanoparticles underwent comprehensive analysis for a deeper understanding. The investigation sought to unravel the mechanisms behind these JNPs’ performance in the foam injection process, probing various operational parameters such as JNP type, concentration, and gas medium (air, CO2, and CH4) impact on surface tension reduction, foamability, and stability through static tests. Results uncovered remarkable efficiency in SiO2-oleic acid JNPs, showcasing a substantial edge in reducing surface tension compared to bare SiO2 nanoparticles. Specifically, at a concentration of 15,000 ppm, SiO2-oleic acid JNPs demonstrated a 25 mN/m greater reduction in surface tension than bare SiO2 within a CH4 medium. Notably, while the gas type had limited influence on surface tension under standard pressure, the synthesized JNPs showed superior foam stabilization in air compared to CO2 and CH4 environments. SiO2-oleic JNPs exhibited outstanding foamability, stabilizing 80% of the foam generator cell’s height and remaining stable for 122 min during the EOR process. Conversely, ascorbic acid-SiO2-oleic acid JNPs displayed elevated foamability but reduced stability compared to SiO2-oleic acid JNPs. Despite achieving full height in the foam generator cell, stability was limited to 26 min in the CO2 medium.
Similar content being viewed by others
Introduction
With the passage of time and the increase in oil production from reservoirs, coupled with the decline in the natural oil recovery capacity, the necessity for new EOR methods has become apparent1,2. Gas injection, encompassing both miscible and immiscible methods, stands out as a practical and cost-effective approach to stabilize reservoir pressure and extract oil from various oil fields. In the process of miscible gas injection, capillary pressure is minimized3,4. Consequently, dry gas within the reservoir rock’s pores is pushed towards the production well with sufficient pressure to facilitate extraction. Elevating the reservoir pressure through gas injection enhances the oil’s volumetric coefficient, ultimately increasing the ultimate oil recovery factor5,6.
The effectiveness of the gas injection process hinges on several factors, such as the reservoir’s geometric shape, permeability, oil type, reservoir pressure and temperature, gas type and composition, and fluid saturation within the reservoir7. The substantial mobility of the gas phase within the reservoir might limit the efficiency of this process8. Gas injection can accentuate the fingering phenomenon, diminishing sweeping efficiency, and leading to issues like gravity override and channelization. Consequently, this results in wastage of the injected fluid during the process, and over time, primarily gas is extracted from the production well, diminishing the overall efficiency of the EOR process. One prevalent solution to combat this issue is the Water Alternating Gas (WAG) method9. However, this method requires a significant amount of water, and in the case of using carbon dioxide (CO2) gas, the high miscibility of this gas in water results in substantial wastage8. In this scenario, employing foam emerges as a viable solution10.
The foam material is typically utilized to manage the high mobility of the gas phase. Formed from water, gas, and surfactant, injecting foam into the reservoir allows for sweeping more areas of the oil within it. The foam’s structure involves the gas phase in the form of scattered bubbles within a continuous liquid phase, separated from the liquid by thin films. Compared to gas, foam has higher viscosity and a lower mobility ratio. With the addition of a small amount of water and surfactant to create a stable foam, gas mobility significantly decreases, enabling substantial oil recovery within reservoirs11,12. Nonetheless, surfactants are not stable in harsh, saline conditions within oil reservoirs, leading to poor durability and stability10. This instability causes the foam to lose its properties after some time of injection, substantially reducing process efficiency. As a result, recent research has turned towards studying nanotechnology applications as a novel approach to stabilize foams13. Pseudoplastic character and viscoelasticity analyses of the stabilized foams exhibited promising oil mobilization ability into the porous medium14. Due to the action of various forces in a crude oil reservoir, surface modification of nanoparticles is a vital stage for the successful implementation of foam injection in porous mediums15,16. One effective method involves using bi-functional (Janus) particles achieved by modifying nanoparticle surfaces to convert the single-property nature of nanoparticles (either hydrophobicity or hydrophilicity) into dual or multiple properties17,18,19. Hydrophilic nanoparticles accelerate foam drainage, whereas partially hydrophobic nanoparticles inhibit foam rupture. Consequently, JNPs offer additional mechanisms for bubble interaction and coalescence inhibition, enhancing the efficiency of foam injection operations20.
The use of JNPs in Pickering emulsion significantly enhances emulsion stability compared to particles with a uniform surface21. JNPs demonstrate higher surface activity, reducing interfacial surface tension (IFT) more than particles with a uniform surface of the same size and chemical nature. Augmenting the amphiphilic properties of JNPs elevates their interfacial activity, enabling the formation of emulsions with increased stability22. Research indicates that JNPs can notably stabilize water-gas and water-oil interfaces. While a small percentage of polymer-based JNPs could maintain stability in oil-water emulsions for several months, they exhibited limited potential in stabilizing the water-gas interface23.
The application of JNPs in foam stability is an emerging technique that has recently piqued researchers’ interest. Despite various conducted studies, investigations have predominantly focused on the effect of limited JNPs like SiO2 and graphene oxide on foam stability24,25,26,27. Consequently, many aspects of this field remain unexplored, warranting diverse experimental and numerical studies. Water viscosity and foam stability decrease due to increased gas solubility in water28. Notably, the type of gas utilized is a pivotal factor affecting the efficiency of EOR processes29,30. Existing research predominantly concentrates on a limited range of gas mediums. Understanding how JNPs perform in different gas mediums is a significant research gap, necessitating an exploration of the influence of JNPs on foam stability with various gases.
Presently, numerous techniques have emerged for synthesizing JNPs, generally classified into three methods: masking, bottom-up assemblies, and controlled phase separations31,32. Identifying optimal conditions and establishing an efficient synthesis method for JNPs greatly impacts the economic viability of foam injection in the EOR process. The masking method stands out as a suitable procedure, offering a simple, rapid, and cost-effective approach20,33.
SiO2 nanoparticles are among the most extensively used nanoparticles in EOR processes for stabilizing foams34,35,36,37,38. These nanoparticles are cost-effective, abundantly available in the earth’s crust, and compatible with numerous oil reservoirs such as sandstone. They have been extensively used in different sections of petroleum industry like cement slurry, drilling fluid, oil-well cementing and chemical flooding19. They efficiently facilitate oil recovery within reservoir rock pores without damaging formations during the EOR injection process. Additionally, SiO2 nanoparticles exhibit high mechanical and chemical resistance, making them suitable for harsh reservoir conditions characterized by high temperature, pressure, and salinity. Their hydrophilic nature fosters strong interaction with water molecules within reservoir rock, altering wettability from oil-wet to strongly water-wet. Moreover, these nanoparticles possess substantial potential for reducing surface tension between water and oil, improving crude oil movement within the reservoir.
The utilization of SiO2 nanoparticles as the primary foundation of JNPs, along with the provision of an optimal synthesis method and an investigation into their functioning in enhancing foam stability and reducing surface tension across various gases, offers a potential solution to address the challenges of foam injection in field-scale operations. Therefore, this study introduces an optimal approach to synthesizing SiO2 JNPs utilizing acid agents through a masking technique for the first time. Various methods were employed to characterize these nanoparticles. The study aims to provide insights into the potential of these JNPs in improving foamability, foam stability, and surface tension across different gas mediums (air, CO2, and CH4), addressing a notable gap in the current knowledge base.
Experimental section
Chemicals
Commercial SiO2 nanoparticles in powder form, with an average diameter size of 30 nm and a molecular weight of 60.08 g/mol, were selected as the fundamental material for producing JNPs in this study, provided by the Sigma Aldrich Company. Solid paraffin wax, characterized by a melting point ranging from 58 to 62 °C, a boiling point of 322 °C, and a density of 0.82 g/cm3 at 20 °C, was procured from Tetra-Chem Company. Two distinct acid types were employed in the synthesis of SiO2 JNPs: ascorbic acid, in powder form with a molecular weight of 176.12 g/mol and a density of 1.694 g/cm3 (sourced from Merck Company), and oleic acid in liquid form, with a molecular weight of 282.468 g/mol and a density of 0.895 g/cm3 (also provided by Merck Company). High-purity ammonia (NH3) and chloroform (CHCl3) were obtained from Merck Company and utilized as intermediates and washing solvents during the synthesis process. Deionized (DI) water was supplied by Zolal Company. Additionally, three high-purity gases—air, CO2, and CH4—were employed in the foam generation process.
Synthesis of SiO2 JNPs
SiO2 nanoparticles alone typically cannot stabilize water-gas emulsions due to their inability to effectively reach the interface between two fluids and their minimal impact on reducing the liquid’s surface tension. To address this limitation, the study employed a Pickering emulsion utilizing paraffin wax as the oil medium to synthesize asymmetrical SiO2 JNPs. The intention behind using paraffin and the Pickering emulsion was to selectively cover only the exposed portion of the nanoparticles with specific acids by dispersing the nanoparticles in paraffin wax. Pickering emulsions, stabilized by solid particles, create an environment where the particles’ surfaces are in contact with both oil and water phases. These solid nanoparticles work at the interface of the two liquids, reducing the solution’s overall surface energy. The arrangement of these particles at the interface creates an asymmetric surface, essential for selective nanoparticle modification processes39,40,41.
The synthesis procedure for producing SiO2 JNPs involved multiple steps. The initial step involved dissolving an appropriate quantity of SiO2 nanoparticles in 100 ml of DI-water using high-power sonication. The solution was then stirred at 400 rpm under a temperature exceeding the melting point of the paraffin wax (70 °C). Following this, a specific amount of paraffin wax (10 times the weight of SiO2 nanoparticles) was gradually incorporated into the mixture over a span of nearly 2 h to create a Pickering emulsion. This process continued until a homogeneous solution was achieved. After the addition of paraffin, the solution underwent an additional 1.5 h of stirring to complete the reaction, ensuring that the nanoparticles were entirely covered by the paraffin wax. Throughout the stirring process, any evaporated water was compensated for by the addition of DI-water. The subsequent step involved rapidly cooling the mixture in an ice water bath, causing the solution of nanoparticles and paraffin to solidify. Following this, the sample underwent suction filtration to remove excess paraffin and was then stored in the refrigerator to synthesize the JNPs. The schematic detailing the process of the solid Pickering emulsion can be found in Fig. 1.
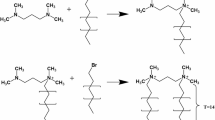
Oleic acid, known for its high hydrophobicity, was used to modify the surface of the hydrophilic SiO2 nanoparticles. Initially, a specific quantity of the prepared sample from the prior step was dissolved in 100 cc of DI-water at 200 rpm. Subsequently, the mixture’s pH was adjusted to 12 by adding five cc of ammonia solution. In the following step, oleic acid was slowly added to the solution drop by drop and stirred for 12 h. Upon completion of the reaction, the sample was centrifuged at 10,000 rpm for 20 min to remove excess water. Then, chloroform was gradually added drop by drop to the solution to separate the paraffin attached to the nanoparticles. Finally, the sample was dried using the freeze-drying method to obtain SiO2-oleic acid JNPs.
In this study’s second phase of JNP synthesis, the objective was to produce JNPs with half of their surface coated by oleic acid and the other half by ascorbic acid. The aim was to enhance amphiphilic properties by introducing both hydrophobic and hydrophilic characteristics to the surfaces of SiO2 nanoparticles. Using paraffin wax within the Pickering emulsion aimed to protect and cover the exposed portions of the SiO2 nanoparticles with the respective acids. Initially, the half-face of the hydrophilic SiO2 was coated with high-hydrophobicity oleic acid, modifying the dual-characteristic surface. Subsequently, the uncoated half-face was covered by hydrophilic ascorbic acid. To achieve this, a specific quantity of SiO2-oleic acid JNPs from the previous step was stirred in 100 cc of DI-water. The mixture’s pH was adjusted to 12.5 by adding seven cc of ammonia. Then, the solution was transferred to a three-neck flask, maintaining a temperature of 80 °C, and stirred at 500 rpm using a magnetic stirrer. Ascorbic acid at a 1:1 molar ratio was then added to the mixture. Stirring continued for 12 h to complete the reaction. Finally, the sample was dried in a freeze dryer to obtain ascorbic acid-SiO2-oleic acid JNPs. The synthesis process of SiO2 JNPs in this study is illustrated in Fig. 2.
Characterization of nanoparticles
One way to accurately determine the behavior and properties of materials is through comprehensive characterization tests and detailed analysis. This research employs four quantitative tests to identify the synthesized JNPs and compare them with the base nanoparticles. Fourier Transform Infrared Spectroscopy (FTIR) was conducted at room temperature within the range of 4000–400 cm−1 to characterize the functional groups on the JNPs’ surface. Dynamic Light Scattering (DLS) analysis was utilized to examine the size distribution of the synthesized JNPs. The Scanning Electron Microscopy (SEM) test was carried out after diluting the solution to analyze the shape and morphology of the synthesized JNPs, reducing the presence of agglomerated particles in the captured image. Finally, Zeta potential analysis was performed at room temperature to determine the surface charge of the nanoparticles, with this analysis focusing on studying the colloidal stability of the synthesized JNPs in a solution.
Surface tension measurement
Studying the change in surface tension can significantly aid in understanding the behavior of fluids at the interface of two phases. This study investigated the Interfacial Tension (IFT) between two phases, specifically the nanofluid and gas, at varying concentrations of synthesized JNPs using the Pendant Drop method. The experimental setup for this analysis included a computer, an isolated glass cell to capture images of the hanging drop, and gas capsules for altering the gas type around the hanging drop. For this purpose, several nanofluids were initially prepared by dispersing different nanoparticle concentrations (ranging from 100 to 15000 ppm) in DI-water. Subsequently, the density of each nanofluid was measured using a pycnometer. The drop’s shape, suspended from a needle, was determined by the balance of forces in a bulk gaseous phase (utilizing three different mediums: air, CO2, and CH4). Finally, the IFT was calculated using the Eq. (4)2:
where γ represents the surface tension, Δρ is the density difference between fluids, g is the gravitational constant, D denotes the equatorial diameter, and H signifies the shape factor.
Foamability and foam stability analysis
Two crucial parameters that impact the efficiency of foam injection in EOR processes are foamability and foam stability43. These parameters dictate the movement of the foam within the porous medium and the sweeping efficiency during field operations. In this study, the assessment of variations in the height of the foam generated in the foam generator cell over time has been employed to determine foam stability. The setup for measuring foamability and foam stability is depicted in Fig. 3.
At first, specific amounts of synthesized JNPs (ranging from 100 to 15,000 ppm) were dispersed in 100 cc of DI water. The solution underwent sonication for 20 min to create a stable nanofluid. Subsequently, the solution was transferred to the transparent foam generator cell. The subsequent step involved injecting gas into the cell at a specified flow rate (30 mL/min). In this study, three different gas types—CH4, CO2, and air—were utilized to explore the impact of gas type on the potential of synthesized JNPs in foam stability. Each gas represents a distinct environment where foam stability might significantly contribute to enhancing oil recovery. The selection of these gases was based on their relevance to oil reservoir environments. Air, despite not being a typical choice in EOR, served as a control and a baseline in the experiments due to its commonality and inert nature. This comparison helped to assess the effectiveness of the other gases. CO2 flooding is a widely employed technique in EOR, known for its miscibility with oil, making it a prevalent choice to augment oil recovery. Lastly, CH4 was chosen owing to its organic nature and potential relevance to specific reservoir environments. Exploring its unique properties aimed to ascertain if it could offer any advantages in foam production.
In the subsequent stage, the cell’s outlet was sealed to prevent further gas entry into the cell. The criterion for studying foam stability was based on observing changes in the foam height over different time intervals—from the moment of ceasing gas entry until the foam dissipated. All experiments were conducted under ambient temperature and pressure.
Due to the time-intensive nature of the foamability and foam stability analyses in this study, involving several crucial and meticulous testing steps, conducting multiple test repetitions to check the measured results’ uncertainty was not feasible. However, a subset of the designed tests (approximately 50 randomly selected tests) was diligently repeated to ensure result reproducibility and calculate the standard deviation. The observed standard deviations fell within the range of 2–4%, signifying the excellent repeatability and reliability of the tests conducted in this research.
Results and discussion
Characterization analysis
FTIR spectra were conducted with the solids dispersed in the KBr matrix to elucidate the chemical bonds of oleic and ascorbic acid on the surface of the synthesized JNPs. The spectra of bare and SiO2 JNPs are depicted in Fig. 4. The absorption peaks at 471 cm−1, 1813 cm−1, and 1103 cm−1 in all graphs correspond to Si-O, Si-OH, and Si-O-Si bonds in SiO2 nanoparticles, respectively. The peak observed in the 2800 cm−1 to 3400 cm−1 range in all IR spectra signifies the stretching vibration of the O–H band due to some surface water in the sample. In Fig. 4b, the peaks at 723 cm−1 and 1456 cm−1 are attributed to C-H and CH3 alkane bonds, while the peak at 1712 cm−1 corresponds to the C = O bands of a carboxylic acid functional group. Additionally, the absorption peaks at 2855 cm−1 and 2926 cm−1 are attributed to the RCH2CH3 alkane bond. The presence of these peaks in Fig. 4b confirms the surface modification of the SiO2 JNPs by oleic acid. Furthermore, in addition to the peaks for the SiO2-oleic acid JNPs, Fig. 4c shows a minor peak around 3600 cm−1, which corresponds to the RCH2OH alcohol bond in the ascorbic acid present on the surface of the ascorbic acid-SiO2-oleic acid JNPs.
DLS analysis is a physical method employed to ascertain the size distribution of particles in solutions and suspensions. Figure 5 presents the DLS analysis of the synthesized SiO2 JNPs. The results indicate an average hydrodynamic diameter of approximately 100 nm. The presence of two distinct peaks in the size distribution reveals a bimodal pattern. The larger peak corresponds to the SiO2 JNPs that were successfully synthesized and surface-modified using acid agents, which enhanced their hydrophobic or amphiphilic properties due to the successful attachment of acid groups during the modification process.
In contrast, the smaller peak likely represents bare SiO2 nanoparticles that did not undergo surface modification with acid agents. This could be attributed to the inherent hydrophilic nature of silica nanoparticles, which resist interaction with hydrophobic modifying agents. The synthesis of these asymmetric SiO2 JNPs was achieved using the Pickering emulsion method. Pickering emulsions are stabilized by solid particles, where one portion of the particle surface interacts with the oil phase, while the other remains in contact with the aqueous phase44.
Not all nanoparticles effectively participate in the Pickering emulsion process for JNP synthesis due to variations in their surface properties and behavior. Successful involvement in this process relies on the nanoparticles’ ability to adsorb and stabilize at the oil-water interface. This is essential for the formation of JNPs and requires an asymmetric distribution of surface characteristics—specifically, a balance between hydrophilic and hydrophobic regions39,40,41. Particles lacking these amphiphilic properties are less likely to stabilize the interface, thereby remaining unmodified during the synthesis process45. The smaller peak in the DLS results can be explained by several factors:
-
Incomplete Surface Coverage: Some SiO2 nanoparticles may not have fully reacted with the acid agents, potentially due to limited accessibility of their surfaces during the masking process.
-
Aggregation Resistance: Bare SiO2 nanoparticles, with their strong hydrophilic properties (attributed to surface hydroxyl groups), may have resisted interaction with the acid-based modifiers, leaving them unaltered.
-
Process Limitations: Variations in mixing, emulsion formation, or exposure to modifying agents during the synthesis process could result in some nanoparticles being excluded from the reactive interface.
This bimodal distribution highlights the importance of optimizing synthesis parameters to ensure uniform surface modification and maximize the efficiency of the Pickering emulsion method.
Figure 6 displays the SEM images of the synthesized SiO2 JNPs. As depicted in the figure, coating the surface of nanoparticles with oleic and ascorbic acid does not appear to impact the morphology and shape of the synthesized JNPs, which have retained their spherical form. The dispersion of all nanoparticles is typical, and no agglomeration is observed. The average particle size of the nanoparticles can be calculated as 100 nm.
Zeta potential refers to the effective electric charge on the surface of nanoparticles, measured in millivolts (mV). A higher zeta potential in a solution signifies greater repulsive forces between the particles in the colloidal solution compared to their attractive forces46. Thus, zeta potential serves as an indicator of a solution’s stability1. The zeta potential of bare SiO2 nanoparticles measured − 15 mV, indicating a moderate stability owing to the inherent hydrophilicity of these particles in water. Through the surface modification with oleic acid to synthesize JNPs, the zeta potential increased to − 23.3 mV, signifying an improvement in the stability of these particles. Oleic acid, a long-chain unsaturated fatty acid, imbues the surface of silica nanoparticles with hydrophobic characteristics. This hydrophobicity prevents aggregation by repelling water molecules. When adsorbed onto the nanoparticles’ surfaces, the hydrophobic tails of oleic acid orient themselves away from water, creating a steric barrier. This repulsion keeps the particles dispersed and prevents aggregation.
However, upon synthesizing ascorbic acid-SiO2-oleic acid JNPs, the zeta potential increased further to − 36.8, indicating excellent colloidal stability of the nanofluids prepared with these nanoparticles. Ascorbic acid, being water-soluble, contributes hydrophilic or polar properties to the silica nanoparticles’ surface. This can improve dispersion by enhancing the affinity towards water molecules. Ascorbic acid’s interactions with the surface may influence the charge distribution, potentially affecting the electrostatic repulsion between particles and aiding in their stabilization. Employing both oleic acid and ascorbic acid provides a unique dual-functional surface. This combination of hydrophobic (from oleic acid) and hydrophilic (from ascorbic acid) properties might result in a more stable colloid by balancing interactions at the water interface. The hydrophobic tails of oleic acid repel water and prevent particle aggregation, while the hydrophilic nature introduced by ascorbic acid might improve interactions with the aqueous phase and prevent agglomeration. This tailored surface chemistry could enhance nanoparticle dispersion in water, reducing their tendency to aggregate and ensuring their stability in suspension. Such dual-surface functionalization with both hydrophobic and hydrophilic properties aims to create stable, well-dispersed nanoparticles in water, enhancing their colloidal stability and efficacy in various applications.
Surface tension measurements
Injecting fluids into a porous medium significantly impacts the capillary pressure and the stability of the two-phase interface by creating a pressure difference between the injected fluid and the oil at the interface47. As capillary pressure increases, oil droplets become trapped in the reservoir rock pores, leading to a reduction in the oil recovery factor. Therefore, the capacity of JNPs to reduce surface tension can substantially influence the stability and efficiency of injected foam in an EOR process. The ability of a JNP to decrease surface tension is crucial for the stability of injected foam. Furthermore, the efficiency of JNPs in various reservoirs can be influenced by the type of gas used to create the foam48. This study employed three different types of gas to measure surface tension. The results of varying concentrations of synthesized JNPs in an air medium are presented in Fig. 7.
The surface tension of DI-water alone measured between 71 and 72 mN/m. Upon adding nanoparticles to the water, these particles act at the interface of two phases, leading to a reduction in surface tension. SiO2 nanoparticles, owing to their inherent hydrophilicity, exhibit little inclination to interface between the two phases. Consequently, at low concentrations, these nanoparticles demonstrated limited ability to decrease surface tension. However, with an increase in nanoparticle concentration (beyond 1000 ppm), the surface tension decreased further and reached 59 mN/m at a concentration of 15,000 ppm49. Introducing a hydrophobic substance like oleic acid to cover a portion of the naturally hydrophilic SiO2 nanoparticle surface achieves a balanced surface chemistry. Oleic acid, an oil with an 18-carbon hydrocarbon chain, offers robust hydrophobic properties. By synthesizing SiO2 JNPs and coating their surface with oleic acid, a dual hydrophilic and hydrophobic composition is achieved, resembling that of a surfactant. Consequently, SiO2-oleic acid JNPs exhibited superior surface tension reduction compared to SiO2 nanoparticles. At a concentration of 15,000 ppm, the surface tension decreased to 37 mN/m. Ascorbic acid-SiO2-oleic acid JNPs possess a well-balanced hydrophilic-hydrophobic composition on their surface, rendering them highly stable in water. The elongated hydrophobic portion and the hydrophilic part comprising several carbons allow these nanoparticles to effectively interact at the interface of two phases. Hence, this type of nanoparticle displayed a greater reduction in surface tension compared to bare SiO2 nanoparticles. Specifically, at a concentration of 15,000 ppm, the surface tension decreased by an additional 16 mN/m compared to water alone. The ability of these SiO2 JNPs to reduce surface tension improves the spread and penetration of gas into the reservoir’s oil-wet pores, leading to better interaction between the displacing gas and the oil phase in EOR operation. Figure 8 presents the results of different concentrations of synthesized JNPs on surface tension in a CO2 medium.
When investigating changes in surface tension under a CO2 gas medium at atmospheric pressure, no significant variations in surface tension were observed at different nanoparticle concentrations compared to the air medium30. This is because at this pressure, CO2 gas partially dissolves in water. Nonetheless, in measurements conducted under atmospheric pressure, a slight reduction in surface tension compared to the state under an air medium was observed. For instance, at a concentration of 1500 ppm SiO2-oleic acid JNPs, the surface tension in the CO2 medium was 2 mN/m lower than that in the air medium. This reduction might be attributed to interactions between CO2 gas and the droplet surface, the partial dissolution of CO2 gas in water, and the creation of slightly acidic conditions within the nanofluid droplet. Significant surface tension reduction is expected when pressure is increased, leading to enhanced CO2 gas solubility in water. As pressure rises, more gas molecules can dissolve in the liquid due to stronger intermolecular interactions. In the case of CO2, increased pressure amplifies its solubility in water. As more CO2 dissolves in the water, it forms carbonic acid (H2CO3), increasing the overall acidity of the solution. The elevated concentration of carbonic acid disrupts hydrogen bonding among water molecules at the liquid-gas interface, causing surface tension to decrease. This reduction is a consequence of changes in the interactions between water molecules and CO2, ultimately affecting the liquid’s capacity to form cohesive bonds at its surface. This phenomenon is known as a decrease in surface tension resulting from alterations in intermolecular forces at the liquid-gas interface and is directly linked to the increased solubility of CO2 in water at higher pressures. Figure 9 displays the results of different concentrations of synthesized JNPs on surface tension in a CH4 medium.
The findings of this study indicated that, at ambient pressure, the type of gas had minimal impact on surface tension. When comparing nanofluids prepared at different concentrations under a CH4 gas medium, there was a slight reduction in surface tension compared to air. Specifically, at a concentration of 15,000 ppm, the surface tension of ascorbic acid-SiO2-oleic acid JNPs in the air, CO2, and CH4 mediums were recorded as 43 mN/m, 41 mN/m, and 40 mN/m, respectively. This observation might be attributed to the organic nature of CH4 gas and the heightened affinity of ascorbic acid-SiO2-oleic acid JNPs to position themselves at the interface between two phases.
Foamability and foam stability analysis
Surfactants are well-known for their role in creating foam and enhancing its stability during gas injection processes in oil reservoirs. However, several factors, such as surfactant adsorption on reservoir rock, loss due to diffusion in oil, and decomposition under harsh reservoir conditions, limit the cost-effectiveness of using surfactants. Similarly to surfactants, nanoparticles bolster lamellar stability by residing at the thin interface between gas and water phases50. Through the Gypsum-Marangoni effect, these particles, with high surface-to-volume ratios, a strong surface charge, and both hydrophilic and hydrophobic characteristics, are positioned at the two-phase interface, preventing lamellae collapse51. JNPs exhibit greater adhesive force compared to surfactants at the fluid–fluid interface52, offering higher potential to enhance foam stability. Moreover, nanoparticles exert varying effects depending on the type of gas present53, and optimum foam stability may be achieved at different nanoparticle concentrations. As such, this study investigates the efficacy of synthesized SiO2 JNPs on foam stability at varying concentrations and in three different gas mediums. The variations in foam height induced by the synthesized JNPs in the presence of air are depicted in Fig. 10.
The SiO2-oleic acid JNPs demonstrated remarkable foamability and sustained the generated foam for an extended duration. Foam production commenced at a concentration of 100 ppm. At concentrations of 10,000 and 15,000 ppm, the generated foam reached 80% of the column height, showing final stabilities of 110 and 122 min, respectively. These findings indicate that the surface stabilization mechanism of nanoparticles differs slightly from that of surfactants. According to the Gibbs-Marangoni theory involving two miscible fluids, surfactants congregate at the nodes in the foam, positioned at the interface of the two fluids. Consequently, as the volume increases, the generated foam reaches its half-life sooner, leading to decreased stability. However, due to their significant surface area, placement at the interface of two immiscible fluids, and possessing a high zeta potential value, nanoparticles disperse over the entire interface between the two phases. As a result, increasing nanoparticle concentration significantly boosts the stability of the generated foam.
Also, it was observed that the ascorbic acid-SiO2-oleic acid JNPs exhibited higher foamability but lower foam stability compared to SiO2-oleic acid JNPs. Foamability commenced at a concentration of 500 ppm of nanoparticles. With increasing nanoparticle concentration in the fluid, foamability intensified, and at 15,000 ppm, the entire column height was filled with foam. An elevated nanoparticle concentration elevates the likelihood of nanoparticles being present at the fluid interface. These nanoparticles function as a physical barrier, impeding gas escape and stabilizing the lamellae. However, it’s essential to note that while an optimal concentration of JNPs optimizes foam stabilization by enhancing interfacial properties, exceeding this concentration could lead to overcrowding, aggregation, and changes in interfacial properties, reducing foam stability.
Beyond the optimum concentration, excessive JNPs may overcrowd the gas-liquid interface, forming a densely packed layer that restricts the adjustment or deformation of the foam lamellae. Higher JNP concentrations might promote agglomeration, reducing their effective surface area and uniform spread at the gas-liquid interface. Excessive concentrations can significantly alter the interface’s composition and structure, disrupting the delicate balance of hydrophilic and hydrophobic interactions essential for foam stabilization. This imbalance can affect interfacial tension and compromise stability. High nanoparticle concentrations may impact the bulk properties of the liquid phase, influencing the overall rheology of the foam, potentially interfering with its ability to resist drainage or coalescence36,54,55,56. Therefore, there appears to be an optimal concentration at which JNPs significantly affect foamability and foam stability in real EOR processes. The generated foam by ascorbic acid-SiO2-oleic acid JNPs at concentrations of 10,000 and 15,000 ppm filled 90% and 100% of the column height, respectively. The final stability times at these concentrations were 38 and 42 min, respectively. Figure 11 demonstrates the percentage changes in the foam height generated by the synthesized SiO2 JNPs in the CO2 medium.
In general, the efficiency of synthesized JNPs in CO2 gas was lower than in air. The foam generated by the SiO2-oleic acid JNPs at concentrations of 10,000 and 15,000 ppm occupied 90% and 100% of the cell’s height, respectively. The increased foamability of this nanoparticle in CO2 gas compared to air can be attributed to its superior capability to reduce surface tension, resulting in the production of more foam by decreasing the surface tension of the thin foam layer. However, the stability of the generated foam was low. At concentrations of 1,000 and 15,000 ppm, the produced foam remained stable for 24 and 26 min, respectively. As for the ascorbic acid-SiO2-oleic acid JNPs, the generated foam was quickly destroyed up to a concentration of 4,000 ppm. At concentrations of 1,000 ppm and 15,000 ppm, when this JNP reached its maximum foamability, the produced foam remained stable for only 10 min. Finally, Fig. 12 illustrates the percentage changes in the height of the foam generated by the synthesized JNPs in the CH4 medium.
Compared to ascorbic acid-SiO2-oleic acid JNPs, SiO2-oleic acid JNPs performed better in the CH4 medium, maintaining the generated foam more stably. The increase in the concentration of both nanoparticles in water enhanced their foamability. The stability of foam in the CH4 medium was higher than in CO2 gas. This could be attributed to the low rate of dissolution in water and the high reactivity of CH4. When gases have low solubility in water, as is the case with CH4, they tend to exist more as discrete gas bubbles rather than dissolving extensively into the liquid phase. This encourages the formation of stable gas pockets within the liquid matrix, which supports foam stability. These discrete gas bubbles help maintain the structural integrity of the foam by reducing gas release and coalescence. Moreover, lower reactivity enhances foam stability over time by preserving the integrity of the gas-liquid interface, reducing the tendency for rapid gas release or coalescence. The foam produced by SiO2-oleic acid JNPs at a concentration of 15,000 occupied 85% of the height of the foam generator cell and remained stable for 94 min. In contrast, the foam generated by SiO2 nanoparticles at the same concentration filled the entire cell height but remained stable for only 32 min. Enhanced foam stability counters the effects of gravity override, where gas tends to rise to the top of the reservoir. By maintaining a stable foam barrier, SiO₂ JNPs help evenly distribute the gas phase throughout the reservoir.
Nanoparticles exhibit exceptional resilience in high-temperature and saline reservoir conditions. They effectively adhere to the gas-liquid interface, impeding bubble coalescence. Due to their robust adhesion, solid nanoparticles establish a durable and permanent connection at the liquid surface, forming a resilient foam. Their adhesion is so tenacious that separating them under reservoir conditions is virtually impossible. Not only do nanoparticles reduce the rate of liquid drainage, but they also increase the maximum capillary pressure, limiting gas diffusion and retarding coalescence. They act as a barrier to mitigate coalescence and elevate liquid viscosity, resulting in longer-lasting foam. Capillary pressure, a critical factor influencing foam stability, is diminished by the addition of nanoparticles, particularly enhancing the maximum pressure at which bubbles rupture. This interaction enhances stability by reducing pressure differentials and preventing bubble coalescence.
Stable foams created using SiO2 JNPs increase the effective viscosity of the gas phase, which helps mitigate issues related to the inherently low viscosity of injection gases (e.g., CO2 or CH4). This reduces the tendency of gas to flow uncontrollably, ensuring a more uniform displacement front. This allows sustained gas mobility control over longer durations, which is crucial for effective EOR.
The adhesion force plays a critical role in the stability and foamability of fluid systems in the presence of silica JNPs. The unique amphiphilic nature of JNPs allows them to adsorb strongly at the gas-liquid interface, creating a robust barrier against lamellae collapse. This high adhesion force arises from the asymmetric surface chemistry of JNPs, which enhances their interfacial activity and ability to anchor firmly at the interface. Stronger adhesion forces lead to improved lamellar stability by reducing the drainage rate of the liquid film and suppressing coalescence between adjacent bubbles. This effect directly enhances foamability, as the stabilized lamellae can support the formation and retention of foam structures for extended periods. In contrast, lower adhesion forces would result in weaker interfacial stabilization, leading to faster lamellae thinning and eventual collapse, reducing both foamability and foam stability55,56.
The synthesized SiO2 JNPs, possessing an amphiphilic structure, exhibit increased effectiveness at the water-gas interface. Their presence leads to thicker lamellae, reducing liquid drainage and averting bubble coalescence, further aiding in sustained gas blocking and displacement in EOR processes. SiO2-oleic acid JNPs, with a better balance of hydrophilic and hydrophobic characteristics, tend to position themselves more favorably at the water-gas interface compared to ascorbic acid-SiO2-oleic acid JNPs. This results in the development of a robust layer with high adhesion energy, contributing to the creation of long-lasting stable foam.
Future work directions and challenges
The investigation in this study highlights the substantial potential of JNPs in improving Foamability and foam stability for applications in crude oil recovery processes. However, this approach is in its nascent stage of study and holds several unknown aspects. Therefore, numerous challenges confront this method, necessitating consideration by researchers and industrial practitioners for its viable implementation in large-scale field operations. Below, some of these challenges were outlined to be addressed in future studies:
-
Despite substantial efforts, the study on the application of JNPs in enhancing foam stability has been largely confined to silica nanoparticles. The exploration of other nanoparticle types or modifications could offer insights into potentially more effective or cost-efficient alternatives to JNPs.
-
A comprehensive investigation should be conducted to delve deeper into the synthesis of JNPs, exploring various synthesis methodologies to optimize the structure, composition, and surface properties. This optimization should specifically focus on tailoring the nanoparticle properties to enhance stability in the gas foam process for EOR.
-
The operating conditions of injection and reservoir conditions, such as temperature, pressure, and salinity, may influence foam stability, an aspect that has not been extensively examined. It’s crucial to investigate how environmental conditions, such as varying reservoir pressures and temperatures, impact foam stability utilizing JNPs. Dynamic tests, such as injection into glass micromodels, sandpacks, or core samples, can provide valuable insights into the efficacy of stabilized foam for enhancing crude oil recovery. This research will elucidate the nanoparticles’ performance in real reservoir conditions.
-
Understanding the optimal concentration and the thresholds beyond which stability reduces is crucial for practical application. Further research should delve into an in-depth analysis of the effect of nanoparticle concentration on foam stability. Utilizing design of experiment (DOE) methods to reduce the number of required tests and acquire accurate and comprehensive information from the process can be a viable solution.
-
Extensive analysis is essential to comprehend the relationship between nanoparticle structure, surface chemistry, and foam stability. Advanced characterization techniques should be employed to correlate nanoparticle properties with foam stability, potentially addressing the challenges of achieving optimal stability under varying conditions.
-
The application of JNPs in EOR operations raises concerns about economic feasibility and industrial-scale production due to potential nanoparticle costs. The economic viability of employing JNPs in oil recovery processes can be evaluated from various perspectives. Nanoparticles may initially be relatively expensive, impacting large-scale implementation feasibility. Nonetheless, cost efficiency could improve over time through advancements in production methods, economies of scale, or the discovery of cost-effective synthesis routes. Despite these costs, JNPs’ utilization can significantly enhance EOR performance. If nanoparticles notably enhance oil recovery rates, the additional extracted oil might outweigh the initial investment, rendering it cost-effective in the long term. Improving oil recovery efficiency with JNPs can yield multiple economic benefits, including enhanced oil production from existing wells, potentially extending reservoir lifespan, and reducing the need for costly new well drilling. Hence, a comprehensive cost-benefit analysis is essential to assess the economic feasibility of JNPs in EOR processes.
Conclusions
This study introduced a novel method for synthesizing two variants of SiO2 JNPs using a masking approach involving oleic acid and ascorbic acid. Characterization methods such as FTIR, DLS, SEM, and Zeta potential were employed to analyze these nanoparticles. The research primarily focused on assessing the influence of the synthesized SiO2 JNPs in enhancing foamability, foam stability, and reducing surface tension in various gas mediums, particularly air, CO2, and CH4. The main results obtained are as follows:
-
The SiO2-oleic acid JNPs notably reduced surface tension, achieving a decrease from 59 to 34 mN/m in a CH4 medium at a concentration of 15,000 ppm, demonstrating strong interfacial activity.
-
SiO2-oleic acid JNPs exhibited outstanding foam stability, maintaining 80% column height and remaining stable for 122 min, highlighting their potential for sustaining foam structures in challenging reservoir conditions.
-
The efficiency of JNPs varied with gas type, with superior performance observed in air compared to CO2 and CH4. Foam stability in CO2 was notably lower, with stability limited to 26 min, underscoring the importance of selecting appropriate JNPs based on reservoir gas composition.
-
Ascorbic acid-SiO2-oleic acid JNPs exhibited higher foamability but reduced stability compared to SiO2-oleic acid JNPs, reflecting the critical balance required for practical applications.
The results highlight the potential of SiO2 JNPs to address key challenges in foam-based EOR, particularly by enhancing foam stability under varied operational conditions. These findings contribute valuable insights into optimizing nanoparticle-based formulations for industrial oil recovery processes, offering a cost-effective and efficient alternative to conventional surfactants. Further studies under dynamic reservoir conditions are recommended to validate scalability and real-world applicability.
Data availability
All data generated or analysed during this study are included in this article. Email for contact: asaeedi@modares.ac.ir.
References
Gharibshahi, R., Omidkhah, M., Jafari, A. & Fakhroueian, Z. Experimental investigation of nanofluid injection assisted microwave radiation for enhanced heavy oil recovery in a micromodel system. Korean J. Chem. Eng. 39, 562–575 (2022).
Saeedi Dehaghani, A. H. & Ghalamizade Elyaderani, S. M. Application of ion-engineered Persian Gulf seawater in EOR: effects of different ions on interfacial tension, contact angle, zeta potential, and oil recovery. Pet. Sci. 18, 895–908 (2021).
Lake, L. W., Johns, R. T., Rossen, W. R. & Pope, G. A. Fundamentals Enhanced Oil Recovery (2014).
Saeedi Dehaghani, A. H., Sefti, M. V. & Amerighasrodashti, A. The application of a new association equation of state (AEOS) for prediction of asphaltenes and resins deposition during CO2 gas injection. Pet. Sci. Technol. 30, 1548–1561 (2012).
Sheng, J. J. Enhanced oil recovery in shale reservoirs by gas injection. J. Nat. Gas Sci. Eng. 22, 252–259 (2015).
Mogensen, K. & Masalmeh, S. A review of EOR techniques for carbonate reservoirs in challenging geological settings. J. Pet. Sci. Eng. 195, 107889 (2020).
Bahadori, A. Fundamentals of Enhanced Oil and Gas Recovery from Conventional and Unconventional Reservoirs (Gulf Professional Publishing, 2018).
Du, F. & Nojabaei, B. A review of gas injection in shale reservoirs: enhanced oil/gas recovery approaches and greenhouse gas control. Energies 12, 2355 (2019).
Vishnyakov, V., Suleimanov, B., Salmanov, A. & Zeynalov, E. 11—Water Altering Gas Injection 127–139. https://doi.org/10.1016/B978-0-12-817632-0.00022-0 (Gulf Professional Publishing, 2020).
Afifi, H. R. et al. A comprehensive review on critical affecting parameters on foam stability and recent advancements for foam-based EOR scenario. J. Mol. Liq. 116808 (2021).
Skauge, A., Solbakken, J., Ormehaug, P. A. & Aarra, M. G. Foam generation, propagation and stability in porous medium. Transp. Porous Media 131, 5–21 (2020).
Ahmadi, A., Saeedi Dehaghani, A. H. & Saviz, S. Experimental study of SDS foam stability in the presence of silica nanoparticle. J. Chem. Pet. Eng. 1, 1 (2022).
Zhang, Y. et al. Nanoparticles as foam stabilizer: mechanism, control parameters and application in foam flooding for enhanced oil recovery. J. Pet. Sci. Eng. 202, 108561 (2021).
Pal, N., Verma, A., Ojha, K. & Mandal, A. Nanoparticle-modified gemini surfactant foams as efficient displacing fluids for enhanced oil recovery. J. Mol. Liq. 310, 113193 (2020).
Chaudhry, A. U. et al. Recent advancements in novel nanoparticles as foam stabilizer: prospects in EOR and CO2 sequestration. J. Mol. Liq. 1, 125209 (2024).
Jia, H. et al. Performance evaluation and formation mechanism of viscoelastic surfactant fracturing fluids with moderate interfacial activity enhanced by Janus-SiO2 nanoparticles. Langmuir 39, 11448–11458 (2023).
Tohidi, Z., Teimouri, A., Jafari, A., Gharibshahi, R. & Omidkhah, M. R. Application of Janus nanoparticles in enhanced oil recovery processes: current status and future opportunities. J. Pet. Sci. Eng. 208, 109602 (2022).
Wu, H. et al. Silica-based amphiphilic Janus nanofluid with improved interfacial properties for enhanced oil recovery. Colloids Surf. Physicochem. Eng. Asp. 586, 124162 (2020).
Jia, H. et al. Potential application of novel amphiphilic Janus-SiO2 nanoparticles stabilized O/W/O emulsion for enhanced oil recovery. Colloids Surf. Physicochem. Eng. Asp. 622, 126658 (2021).
Saeedi Dehaghani, A. H., Gharibshahi, R. & Mohammadi, M. Utilization of synthesized silane-based silica Janus nanoparticles to improve foam stability applicable in oil production: static study. Sci. Rep. 13, 18652 (2023).
Binks, B. P. & Fletcher, P. D. I. particles adsorbed at the oil–water interface: a theoretical comparison between spheres of uniform wettability and Janus particles. Langmuir 17, 4708–4710 (2001).
Glaser, N., Adams, D. J., Böker, A. & Krausch, G. Janus particles at liquid–liquid interfaces. Langmuir 22, 5227–5229 (2006).
Schröder, J. H. et al. Interfacial stabilization by soft Janus nanoparticles. Polymer (Guildf.) 106, 208–217 (2016).
Sun, N. et al. Janus nanographene oxide with aerophilic/hydrophilic characteristics for enhancing foam stability in high-temperature reservoirs. J. Mol. Liq. 371, 121087 (2023).
Chen, X. et al. Ultra-stable CO2-in-water foam by generating switchable Janus nanoparticles in-situ. J. Colloid Interface Sci. 630, 828–843 (2023).
Wang, D. et al. Investigation on the stability of Janus SiO2-n nanoparticle-assisted nonionic surfactant-stabilized foam and its application in enhanced oil recovery. Energy Fuels 36, 10205–10212 (2022).
Chen, L. et al. The foam reinforced with Janus amphiphilic graphene oxide to control steam channeling in heavy oil reservoir. Colloids Surf. Physicochem. Eng. Asp. 1, 132627 (2023).
Yu, J., Liu, N., Li, L. & Lee, R. L. Generation of nanoparticle-stabilized supercritical CO2 foams. In Carbon Management Technology Conference (2012).
Wang, Y. et al. The stability study of CO2 foams at high pressure and high temperature. J. Pet. Sci. Eng. 154, 234–243 (2017).
Aarra, M. G., Skauge, A., Solbakken, J. & Ormehaug, P. A. Properties of N2-and CO2-foams as a function of pressure. J. Pet. Sci. Eng. 116, 72–80 (2014).
Khezrian, S., Khoee, S. & Caceres, M. Synthesis of combinatorial Janus nanoparticles based on EpCAM-PEG/PCL for targeted therapy of human colorectal adenocarcinoma. J. Biomed. Mater. Res. A 108, 2291–2304 (2020).
Khoee, S. & Jalaeian Bashirzadeh, M. Preparation of Janus-type superparamagnetic iron oxide nanoparticles modified with functionalized PCL/PHEMA via photopolymerization for dual drug delivery. J. Appl. Polym. Sci. 138, 49627 (2021).
Ghanbarinia Firozjah, R., Sadeghi, A. & Khoee, S. Ultrasonic de-cross-linking of the pH-and magneto-responsive PHEMA/PMMA microgel to Janus nanoparticles: a new synthesis based on grafting from/grafting to polymerization. ACS Omega 5, 27119–27132 (2020).
Alcorn, Z. P. et al. Pore-and core-scale insights of nanoparticle-stabilized foam for CO2-enhanced oil recovery. Nanomaterials 10, 1917 (2020).
Zhao, J., Torabi, F. & Yang, J. The synergistic role of silica nanoparticle and anionic surfactant on the static and dynamic CO2 foam stability for enhanced heavy oil recovery: an experimental study. Fuel 287, 119443 (2021).
Harati, S., Bayat, A. E. & Sarvestani, M. T. Assessing the effects of different gas types on stability of SiO2 nanoparticle foam for enhanced oil recovery purpose. J. Mol. Liq. 313, 113521 (2020).
Rattanaudom, P., Shiau, B. J., Suriyapraphadilok, U. & Charoensaeng, A. Effect of pH on silica nanoparticle-stabilized foam for enhanced oil recovery using carboxylate-based extended surfactants. J. Pet. Sci. Eng. 196, 107729 (2021).
Rezaei, A., Derikvand, Z., Parsaei, R. & Imanivarnosfaderani, M. Surfactant-silica nanoparticle stabilized N2-foam flooding: a mechanistic study on the effect of surfactant type and temperature. J. Mol. Liq. 325, 115091 (2021).
Li, X. et al. Preparation and application of Janus nanoparticles: recent development and prospects. Coord. Chem. Rev. 454, 214318 (2022).
Fu, J. et al. Janus nanoparticles for cellular delivery chemotherapy: recent advances and challenges. Coord. Chem. Rev. 422, 213467 (2020).
Milian, Y. E., Claros, M., Ushak, S. & Vallejos, S. Silica based Janus nanoparticles: synthesis methods, characterization, and applications. Appl. Mater. Today 34, 101901 (2023).
Noorizadeh Bajgirani, S. S. & Saeedi Dehaghani, A. H. Experimental investigation of wettability alteration, IFT reduction, and injection schemes during surfactant/smart water flooding for EOR application. Sci. Rep. 13, 11362 (2023).
AlYousef, Z., Almobarky, M. & Schechter, D. Enhancing the stability of foam by the use of nanoparticles. Energy Fuels 31, 10620–10627 (2017).
Chevalier, Y. & Bolzinger, M. A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. Physicochem. Eng. Asp. 439, 23–34 (2013).
Bhattacharjee, S. DLS and Zeta potential—what they are and what they are not? J. Control Release 235, 337–351 (2016).
Gharibshahi, R., Omidkhah, M., Jafari, A. & Fakhroueian, Z. Hybridization of superparamagnetic Fe3O4 nanoparticles with MWCNTs and effect of surface modification on electromagnetic heating process efficiency: a microfluidics enhanced oil recovery study. Fuel 282, 118603 (2020).
Hadler, K. & Cilliers, J. J. The effect of particles on surface tension and flotation froth stability. Min. Metall. Explor. 36, 63–69 (2019).
Talebian, S. H., Tan, I. M., Sagir, M. & Muhammad, M. Static and dynamic foam/oil interactions: potential of CO2-philic surfactants as mobility control agents. J. Pet. Sci. Eng. 135, 118–126 (2015).
Cheraghian, G. & Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part A: effects of nanoparticles on interfacial tension. Int. Nano Lett. 6, 129–138 (2016).
Fu, C., Yu, J. & Liu, N. Nanoparticle-stabilized CO2 foam for waterflooded residual oil recovery. Fuel 234, 809–813 (2018).
Lin, X. et al. Marangoni effect-driven transfer and compression at three-phase interfaces for highly reproducible nanoparticle monolayers. J. Phys. Chem. Lett. 11, 3573–3581 (2020).
Perro, A., Reculusa, S., Ravaine, S., Bourgeat-Lami, E. & Duguet, E. Design and synthesis of Janus micro-and nanoparticles. J. Mater. Chem. 15, 3745–3760 (2005).
Alooghareh, M. H., Kabipour, A., Sisakht, S. M. M. & Razavifar, M. Effects of different gases on the performance of foams stabilized by Cocamidopropyl betaine surfactant and silica nanoparticles: a comparative experimental study. Petroleum 1, 1 (2021).
Ab Rasid, S. A., Mahmood, S. M., Kechut, N. I. & Akbari A review on parameters affecting nanoparticles stabilized foam performance based on recent analyses. J. Pet. Sci. Eng. 208, 109475 (2022).
Phukan, R. & Tiwari, P. Chapter 13—CO2 Foams for Enhanced Oil Recovery 229–250. https://doi.org/10.1016/B978-0-323-90540-4.00012-0 (Gulf Professional Publishing, 2022).
Issakhov, M., Shakeel, M., Pourafshary, P., Aidarova, S. & Sharipova, A. Hybrid surfactant-nanoparticles assisted CO2 foam flooding for improved foam stability: a review of principles and applications. Pet. Res. 7, 186–203 (2022).
Acknowledgements
The authors would like to thank Tarbiat Modares University for supporting this research.
Author information
Authors and Affiliations
Contributions
Amir Hossein Saeedi Dehaghani: Supervision, Funding acquisition, Resources. Reza Gharibshahi: Formal analysis, Visualization, Writing–original draft. Mohammad Mohammadi: Conceptualization, Investigation, Methodology, Validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saeedi Dehaghani, A.H., Gharibshahi, R. & Mohammadi, M. Synthesis and performance analysis of novel SiO2 Janus nanoparticles for enhancing gas foam injection in oil reservoirs. Sci Rep 15, 2994 (2025). https://doi.org/10.1038/s41598-025-87367-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87367-z