Abstract
Pediatric non-alcoholic fatty liver disease (NAFLD) is emerging as a worldwide health concern with the potential to advance to cirrhosis and liver cancer. NAFLD can also directly contribute to heart problems through inflammation and insulin resistance, even in individuals without other risk factors. The pathological mechanisms of NAFLD are linked to functional differences of miRNAs in different biological environments. The miRNA in serum exosomes may reflect the pathological state of the liver and changes in systemic metabolism, while the miRNA in serum may be associated with physiological processes other than the liver. Pediatric non-alcoholic fatty liver disease (NAFLD) is emerging as a worldwide health concern with the potential to advance to cirrhosis and liver cancer. NAFLD can also directly contribute to heart problems through inflammation and insulin resistance, even in individuals without other risk factors. The pathological mechanisms of NAFLD are linked to functional differences of miRNAs in different biological environments. The miRNA in serum exosomes may reflect the pathological state of the liver and changes in systemic metabolism, while the miRNA in serum may be associated with physiological processes other than the liver. Pediatric non-alcoholic fatty liver disease (NAFLD) is emerging as a worldwide health concern with the potential to advance to cirrhosis and liver cancer. NAFLD can also directly contribute to heart problems through inflammation and insulin resistance, even in individuals without other risk factors. The pathological mechanisms of NAFLD are linked to functional differences of miRNAs in different biological environments. The miRNA in serum exosomes may reflect the pathological state of the liver and changes in systemic metabolism, while the miRNA in serum may be associated with physiological processes other than the liver. Our study identified 36 miRNAs with differential expression in the serum of NAFLD patients compared to the control group, including 21 miRNAs with significantly increased expression and 15 with decreased expression. Consistent with our previously reported data on serum-derived exosomal miRNA profiling, this study also observed a notable upregulation of serum miR-122-5p levels in NAFLD patients. PCR validation confirmed the differential expression of miR-122-5p identified through RNA sequencing. Functional analysis using GO and KEGG pathways revealed a diverse range of biological roles associated with these differentially expressed miRNAs. Notably, NAFLD significantly impacts heart health, with miR-122-5p playing a key role in regulating cardiovascular function. Furthermore, activation of the miR-122/Sirt-6/ACE2 axis may contribute to myocardial necrosis, highlighting its potential role in NAFLD-associated cardiovascular risks. Our study suggests that miR-122 plays a key role in the progression of NAFLD and its associated metabolic disturbances, which can increase the risk of cardiovascular disease. Targeting miR-122 may offer potential therapeutic benefits for improving both liver and heart health in individuals with NAFLD.
Similar content being viewed by others
Introduction
Childhood non-alcoholic fatty liver disease (NAFLD) is emerging as a leading contributor to long-term liver conditions linked to a significant overhaul of liver metabolism. Previously reported data demonstrated that deregulation of the expressions and activities of microRNAs (miRNAs) have a key role in metabolic disorders linked with NAFLD1. miRNAs are short endogenous non-coding RNA composed of about 20–30 nucleotides, which regulate about 90% of human genes and have a significant effect on gene expression in almost every biological process2,3. Serum exosome, a nano-sized extracellular vesicle with an endocytic origin, also contains a variety of proteins and miRNAs that mediate intercellular communication4. In our 2022 study, we demonstrated a marked variation in the expression of 80 miRNAs, including miR-122-5p, miR-27a, and miR‐335-5p, in exosomes derived from serum of children with NAFLD compared to the control group5. There is a significant association between NAFLD and cardiovascular disease (CVD), especially in the more severe forms of NAFLD6. This association may be related to metabolic abnormalities in patients with NAFLD, including insulin resistance, lipid metabolism disorders, and inflammatory responses7. In patients with NAFLD, the elevation of miR-122-5 may be associated with fat accumulation and inflammatory response in the liver, all of which may contribute to the development of cardiovascular disease8. In addition, the increased risk of cardiovascular events in patients with NAFLD may be related to the release of inflammatory factors and metabolites from their liver, which may affect the cardiovascular system through blood circulation6.
Circulating miRNAs, along with exosomal miRNAs, have garnered significant attention as a possible diagnostic biomarker for clinical use. Deregulation in circulatory miRNA expressions causes a variety of chronic illnesses, including chronic liver disease. miRNA-34a was up-regulated in serum samples from patients with NAFLD, and its levels of expression were linked with disease severity9,10. Our goal in this study was to create a detailed microRNA profile of serum samples from individuals with non-alcoholic fatty liver disease (NAFLD) and explore how different microRNAs could affect cardiovascular well-being.
Materials and methods
Sample Collection and RNA extraction
The local Ethics Committee approved the ethics application (Ethics Committee of Shaoxing Maternal and Child Health Care Hospital, NO. 2018035). All participants provided written informed consent in accordance with the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations. Between July and December 2019, the Pediatric Endocrinology Department at our hospital selected three children with non-alcoholic fatty liver disease who were admitted to the hospital and matched them with three other patients for comparison purposes. Detailed inclusion and exclusion criteria have been published previously11. At the Shaoxing Maternal and Child Health Care Hospital, every patient underwent medical check-ups to ensure their well-being. Serum was extracted from 3 mL of blood for RNA extraction.
RNA library preparation and sequencing
To create the small RNA library, 3 µg of total RNA was isolated for each sample. Following the instructions provided by the manufacturer, libraries for sequencing were generated utilizing the NEBNext® Multiplex Small RNA Library Prep Set for Illumina® (NEB, USA), with unique index codes assigned to each sample for sequence identification. The samples indexed were grouped together using a cBot Cluster Generation System with TruSeq SR Cluster Kit v3-cBot-HS (Illumia) according to the instructions provided by the manufacturer. The library samples were examined using an Illumina Hiseq 2500/2000 system, resulting in the generation of 50 bp single-end reads following the formation of clusters.
The quality of the sequencing data was evaluated using Fast-QC software from http://www.bioinformatics.babraham.ac.uk/projects/fastqc/, which analyzed minimum quality standards, high-value region dispersion, GC content, PCR duplication content, and Kmer frequency. The DEGseq (2010) R package was utilized to analyze three samples for variations in expression. q-value was used to alter the P-value. P-value < 0. 01 and log2(fold change) > 1 were defined as the default significance threshold for variable expression Levels.
miRNA target genes were predicted using TargetScan, miRanda, and miRDB. DAVID was utilized for Annotation, Visualization, and Integrated Discovery to conduct GO enrichment and KEGG pathway enrichment analysis.
qPCR analysis
Extraction of miRNAs from serum was performed using a miRcute miRNA isolation kit from TIANGEN in Beijing, China. The miR-122-5p forward primer sequence is 5′-TATTCGCACTGGATACGACACAAAC-3′, while the reverse primer sequence is 5′-GCCCGTGGAGTGTGACAATGGT-3′. The RT-qPCR was conducted simultaneously using the StepOne Plus Real-Time PCR System from Applied Biosystems and the SYBR Green PCR master mix from Tiangen Biotechnology Co., Ltd. Beijing-based company Tiangen provided all miRNA primers.
Statistical analysis
Student t-test was utilized to analyse data from real-time qPCR. Data are presented as the means ± standard deviations (SD). Statistical analysis was performed using the hypergeometric distribution method; the most significant function was calculated, and P < 0. 05 was used as the threshold of significant gene enrichment.
Results
Characteristics of patients
Patients with obesity and NAFLD (n = 3) had a mean age of 12.1 ± 0.53 years and a BMI of 31.5 ± 3.06. In contrast, controls with obesity but without NAFLD had a mean age of 11.9 ± 1.79 years and a BMI of 28.38 ± 0.75. Age, weight, height, BMI, and triglyceride (TG) did not differ significantly between the groups. Demographic and clinical data for each subject are presented in Table 1, while the ultrasound examination results are shown in Figure S1. The results suggest enhanced liver echo, abnormal attenuation of ultrasound signals (i.e. poor display of deep liver and diaphragm), and loss of normal liver echo texture (including poor display of blood vessel wall).
Analysis of NAFLD serum microRNAs
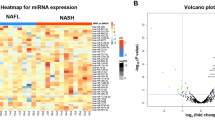
Before analyzing the raw sequencing data for quality control, it is possible to undertake a cursory inspection of the data. RNA sequencing and evaluation were performed on three serum samples from individuals with NAFLD and three samples from individuals without NAFLD. NAFLD patients’ serum contained an average of 80. 1 million valid reads, while healthy controls’ serum had 87. 0 million valid reads on average. Most of the accurate interpretations from the NAFLD and NON-NAFLD serum samples were effectively linked to the human non-coding RNA dataset, with percentages of 84. 6% and 90. 0% shown in Table 2. We discovered 1523 miRNAs by combining conserved and new miRNAs (Supplemental Table S1). A notable contrast was observed in serum miRNA concentrations between the NAFLD and control groups (P < 0. 05), showing an increase in 21 miRNAs and a decrease in 15 miRNAs (Supplemental Table S2).
The differential miRNA expression in NAFLD versus non-NAFLD samples was then investigated using hierarchical cluster analysis to construct a heat map evaluating the outcomes of the two groups as illustrated in Fig. 1. Based on the comparison between NAFLD and controls, we selected significantly 10 up-regulated microRNAs (hsa-miR-122-5p, hsa-miR-378d, hsa-miR-885-5p, hsa-miR-4686, hsa-miR-144-5p, hsa-miR-4485-3p, hsa-miR-122-3p, hsa-miR-885-3p, hsa-miR-15a-5p, and hsa-miR-192-3p) and 10 downregulated microRNAs (hsa-miR-206, hsa-miR-873-5p, hsa-miR-873-3p, hsa-miR-664b-5p, hsa-miR-409-5p, hsa-miR-1291, hsa-miR-195-3p, hsa-miR-370-3p, hsa-miR-370-3p, and hsa-miR-1246) for further investigation. miR-122-5p was detected in the serum of individuals with both NAFLD and non-NAFLD (Fig. 2).
Target genes predicted and their KEGG and GO annotations
Using target genes, the roles of these miRNAs with varying expression levels were hypothesized. miRanda was employed to forecast the target gene of microRNAs. Eighty miRNAs with differential expression were hypothesized to target 36,718 mRNAs. The GO analysis revealed the top 20 hits for biological process (BP), cellular component (CC), and molecular function (MF) in the presentation (Fig. 3). Enrichment analysis using KEGG pathways was conducted on miRNA targets that were differentially expressed to identify the top 20 enriched terms (Fig. 4). The KEGG pathway enrichment analysis shows that the miRNAs with differential expression are primarily associated with the vascular smooth muscle contraction, ubiquitin-mediated proteolysis, transcriptional misregulation in cancer and toll-like receptor signaling pathway.
Discussion
Non-alcoholic fatty liver disease (NAFLD) has emerged as one of the most prevalent chronic conditions among children. The early onset of NAFLD poses a significant risk, as prolonged exposure to the disease and its metabolic complications can lead to severe health outcomes over time12. This highlights the critical need for early diagnosis and timely intervention to reduce long-term risks and improve outcomes in children with NAFLD. In recent times, there has been an increased focus on the role of miRNA in regulating genes related to liver health and diseases. Earlier studies have indicated that miRNAs dysregulated networks play a part in the development of NAFLD, including factors like oxidative stress, hepatic inflammation, and lipid buildup13,14. By employing high-throughput sequencing methods, we discovered 36 miRNAs with varying levels in the serum of children with NAFLD compared to the control group. Our previous data indicated a notable variation of 80 exosomal miRNAs, with 30 showing an increase and 50 showing a decrease in the same group of children compared to the control group5. Consistent with our earlier findings, we demonstrated a novel association between elevated serum miR-122-5p levels and competition with serum exosomal microRNAs in pediatric NAFLD patients, suggesting a potential role in predicting disease advancement. Our findings using real-time PCR indicate that there are consistent differences in serum miR-122-5p expression levels between children with NAFLD and control subjects, suggesting that it could serve as a more sensitive diagnostic tool for NAFLD. Our discovery regarding miR-122-5p aligns with a recent study that conducted miRNA sequencing on plasma samples from individuals with NAFLD15. Furthermore, we identified variations in the expression levels of several crucial miRNAs, including 10 up-regulated microRNAs (hsa-miR-122-5p, hsa-miR-378d, hsa-miR-885-5p, hsa-miR-4686, hsa-miR-144-5p, hsa-miR-4485-3p, hsa-miR-122-3p, hsa-miR-885-3p, hsa-miR-15a-5p, and hsa-miR-192-3p) and 10 significantly downregulated microRNAs (hsa-miR-206, hsa-miR-873-5p, hsa-miR-873-3p, hsa-miR-664b-5p, hsa-miR-409-5p, hsa-miR-1291, hsa-miR-195-3p, hsa-miR-370-3p, hsa-miR-370-3p, and hsa-miR-1246) in the comparison between the NAFLD and control groups. We also pick the above miRNAs to construct a heat map, using hierarchical cluster analysis. Heat maps clearly showed differences in miRNA expression between NAFLD and control groups. According to our recent research, previous studies have shown that miR-378 is crucial in the progression of liver inflammation and fibrosis through its positive control of the NF-κB-TNFα pathway16. In the same way, blood microRNA analysis indicated a direct correlation between levels of hsa-miR-122-5p and hsa-miR-885-5p with fatty liver and associated lipoprotein metabolism17. In addition, the levels of hepatic miR-122-3p, miR-140-5p, and miR-148b-5p are associated with serum cytokeratin-18 levels in metabolic-associated fatty liver disease18. The present research revealed enrichment of the vascular smooth muscle contraction, ubiquitin-mediated proteolysis, transcriptional misregulation in cancer, and toll-like receptor signaling pathway through KEGG analysis. Newly released information shows that toll-like receptor signaling exacerbates alcoholic liver disease and liver damage in non-alcoholic steatohepatitis, which aligns with the findings of KEGG pathway enrichment analysis19,20. Likewise, strong evidence has shown that the hedgehog pathway’s imbalance plays a crucial role in the progression of hepatic steatosis and the transition from hepatic steatosis to more severe liver conditions21.
NAFLD has a notable impact on heart health, with miR-122-5p playing a key role in regulating lipid metabolism, inflammation, and endothelial function22,23. In our study, apolipoprotein A levels in the NAFLD group were slightly lower, and common carotid artery thickness was marginally higher compared to the non-NAFLD group. Although these differences were not statistically significant, likely due to the small sample size, a trend of change was evident. These findings highlight potential distinctions between the groups and suggest that elevated miR-122-5p expression could increase the risk of cardiovascular disease in children with NAFLD. High levels of miR-122-5p are linked to higher lipid buildup and changes in inflammatory reactions, which play a role in the advancement of atherosclerosis and negative changes in heart structure. The significance of this microRNA in preserving vascular integrity and reducing cardiovascular complications is highlighted by its impact on endothelial function24,25. Notably, miR-122 targets the sirtuin-6 (Sirt-6) gene, which is an essential regulator of cardiovascular function and is believed to be a partial angiotensin-converting enzyme 2(ACE2) activator. Modulation of this axis is thought to contribute to the pathogenesis of myocardial infarction26. New research has emphasized the various ways it affects cardiovascular disease, indicating that miR-122-5p could be a valuable tool for diagnosing and treating cardiovascular conditions associated with NAFLD27.
Conclusion
In summary, the miRNA expression profiles in the serum of children with NAFLD are markedly different from those in the control group. The cognition of these miRNAs can help to identify key factors and risks for disease prognosis and treatment. Additionally, our research revealed an increase in serum miR-122-5p levels in children diagnosed with NAFLD, aligning with our prior findings on serum exosomal microRNAs in this group of patients. In addition, our research emphasizes the important regulatory function of miR-122-5p in maintaining cardiovascular well-being, connecting NAFLD to cardiovascular issues. Our results may also provide mechanistic insights into the development of cardiovascular diseases in NAFLD patients.
Data availability
Sequence data that support the findings of this study can be accessed through the NCBI SRA resource: PRJNA821449.
References
C, Y. et al. Comparison of serum exosome isolation methods on co-precipitated free microRNAs. PeerJ 8 https://doi.org/10.7717/peerj.9434 (2020).
Zm, Z. Regulation of cellular miRNA expression by human papillomaviruses. Biochim. Biophys. Acta. 1809 https://doi.org/10.1016/j.bbagrm.2011.05.005 (2011).
M S, B S, N T, et al. Widespread changes in protein synthesis induced by microRNAs. Nature ; 455. (2008). https://doi.org/10.1038/nature07228
Bischoff, J. P., Schulz, A. & Morrison, H. The role of exosomes in intercellular and inter-organ communication of the peripheral nervous system. FEBS Lett. 596, 655–664. https://doi.org/10.1002/1873-3468.14274 (2022).
Zhang, J-W. & Pan, H-T. microRNA profiles of serum exosomes derived from children with nonalcoholic fatty liver. Genes Genomics. 44, 879–888. https://doi.org/10.1007/s13258-021-01150-8 (2022).
Targher, G., Byrne, C. D. & Tilg, H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 69, 1691–1705. https://doi.org/10.1136/gutjnl-2020-320622 (2020).
Hk, M. & A, K. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metabol. 15 https://doi.org/10.1016/j.cmet.2012.04.004 (2012).
Liu, X-L. et al. Lipotoxic hepatocyte-derived exosomal MicroRNA 192-5p activates macrophages through Rictor/Akt/Forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology 72, 454–469. https://doi.org/10.1002/hep.31050 (2020).
Yamada, H. et al. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin. Chim. Acta. 424, 99–103. https://doi.org/10.1016/j.cca.2013.05.021 (2013).
Salvoza, N. C. et al. Association of Circulating Serum miR-34a and miR-122 with dyslipidemia among patients with non-alcoholic fatty liver disease. PLoS One. 11, e0153497. https://doi.org/10.1371/journal.pone.0153497 (2016).
Zhang, J. et al. Prolactin is a key factor for nonalcoholic fatty liver disease in obese children. Horm. Metab. Res. 55, 251–255. https://doi.org/10.1055/a-2043-1044 (2023).
Draijer, L. et al. A natural history study of paediatric non-alcoholic fatty liver disease over 10 years. JHEP Rep. 5, 100685. https://doi.org/10.1016/j.jhepr.2023.100685 (2023).
Torres, J-L. et al. Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease. World J. Gastroenterol. 24, 4104–4118. https://doi.org/10.3748/wjg.v24.i36.4104 (2018).
Zhang, T. et al. MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-κB-TNFα pathway. J. Hepatol. 70, 87–96. https://doi.org/10.1016/j.jhep.2018.08.026 (2019).
Hu, Y. et al. MicroRNA-122-5p inhibition improves inflammation and oxidative stress damage in Dietary-Induced non-alcoholic fatty liver Disease through Targeting FOXO3. Front. Physiol. 13, 803445. https://doi.org/10.3389/fphys.2022.803445 (2022).
Raitoharju, E. et al. Blood hsa-mir-122-5p and hsa-mir-885-5p levels associate with fatty liver and related lipoprotein metabolism-the Young finns Study. Sci. Rep. 6, 38262. https://doi.org/10.1038/srep38262 (2016).
Gn, L-S. et al. Hepatic mir-122-3p, mir-140-5p and mir-148b-5p expressions are correlated with cytokeratin-18 serum levels in MAFLD. Ann. Hepatol. 27. https://doi.org/10.1016/j.aohep.2022.100756 (2022).
K, M. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139. https://doi.org/10.1053/j.gastro.2010.03.052 (2010).
N D, L W, B K, et al. A single nucleotide polymorphism of toll-like receptor 4 identifies the risk of developing graft failure after liver transplantation. J. Hepatol. ; 53. (2010). https://doi.org/10.1016/j.jhep.2009.12.044
Machado, M. V. & Diehl, A. M. The hedgehog pathway in nonalcoholic fatty liver disease. Crit. Rev. Biochem. Mol. Biol. 53, 264–278. https://doi.org/10.1080/10409238.2018.1448752 (2018).
Machado, M. V. & Diehl, A. M. Hedgehog signaling in liver pathophysiology. J. Hepatol. 68, 550–562. https://doi.org/10.1016/j.jhep.2017.10.017 (2018).
Ar, L-P. et al. Concerted regulation of non-alcoholic fatty liver disease progression by microRNAs in apolipoprotein E-deficient mice. Dis. Models Mech. 14 https://doi.org/10.1242/dmm.049173 (2021).
Yang, K. et al. Would Combination be Better: Swimming Exercise and Intermittent Fasting Improve High-Fat Diet-Induced nonalcoholic fatty liver disease in obese rats via the miR-122-5p/SREBP-1c/CPT1A pathway. Diabetes Metab. Syndr. Obes. 17, 1675–1686. https://doi.org/10.2147/DMSO.S448165 (2024).
Wu, X. et al. Inhibition of miR-122 reduced atherosclerotic lesion formation by regulating NPAS3-mediated endothelial to mesenchymal transition. Life Sci. 265, 118816. https://doi.org/10.1016/j.lfs.2020.118816 (2021).
Zhao, H. et al. MiR-122-5p as a potential regulator of pulmonary vascular wall cell in idiopathic pulmonary arterial hypertension. Heliyon 9, e22922. https://doi.org/10.1016/j.heliyon.2023.e22922 (2023).
Abdel-Nasser, Z. M., Zaafan, M. A. & Abdelhamid, A. M. Modulation of the miR-122/Sirt-6/ACE2 axis on experimentally-induced myocardial infarction. Chem. Biol. Interact. 369, 110276. https://doi.org/10.1016/j.cbi.2022.110276 (2023).
Gaddam, R. R. et al. γ peptide nucleic acid-based miR-122 inhibition rescues vascular endothelial dysfunction in mice Fed a High-Fat Diet. J. Med. Chem. 65, 3332–3342. https://doi.org/10.1021/acs.jmedchem.1c01831 (2022).
Funding
This work was supported by the National Natural Science Foundation of China (82071729), the Science Technology Department of Zhejiang Province, China (LTGY23H040004, LTGY23H040005, LTGY24H040006), the Health Commission of Zhejiang Province, China (2022KY1306), the Health Commission of Shaoxing, China (2022KY040, 2023SKY047), the Science Technology Department of Shaoxing (2022A14006, 2023A14032).
Author information
Authors and Affiliations
Contributions
Jian-Wei Zhang and Hai-Tao Pan performed experiments. Jian-Wei Zhang, Kamran Ullah, and Hai-Tao Pan wrote the main manuscript text. Jian-Wei Zhang and Nauman Khan prepared Figs. 1, 2, 3 and 4. Jian-Wei Zhang and Kamran Ullah prepared Table 1, and 2. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
For research involving human participants who are minors, including tissue sample donors, informed consent was obtained from a parent and/or legal guardian.
Ethical approval
This study received approval from the Shaoxing Maternal and Child Health Care Hospital’s Ethics Committee. Everyone who participated in the study gave informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, JW., Ullah, K., Khan, N. et al. Comprehensive profiling of serum microRNAs in normal and non-alcoholic fatty liver disease (NAFLD) patients. Sci Rep 15, 3766 (2025). https://doi.org/10.1038/s41598-025-87791-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87791-1