Abstract
Microplastics (MPs) are emerging pollutants that threaten the aquatic ecosystem. Aquatic insects may play a crucial role in moving MPs into different trophic levels within and across the ecosystems. However, field-level evidence is still insufficient globally despite its tremendous ecological significance. Thus, for the first time in Bangladesh, MPs were explored in six species of aquatic insects along with water and sediment of the Daleshwari River. Digestion and density separation methods were used for the extraction of MPs. Microscopic inspection and Fourier transform spectroscopy (FT-IR) were done to identify and quantify MPs. The average concentration of MPs in sediment and water is 143.1 ± 28.52 of MPs/L and 30153.8 ± 2313.62 of MPs/kg, respectively. In aquatic species, the highest MPs found in D. rusticus (57.82 ± 14.98 MPs/g), followed by B. contaminate (38.53 ± 6.87 MPs/g), Ranatra sp. (34.05 ± 5.39 MPs/g), C. servilia (26.99 ± 7.88 MPs/g), D. annulatus (16.44 ± 6.95 MPs/g), and O. sabina (14.13 ± 4.52 MPs/g). A total of eight types of polymers have been identified. It was important to notice that the studied aquatic insects bear similar MPs (size, shape, and color) found in water and sediments from the river. It reveals the potential for the insects (accumulators of MPs) to be a driving factor for the transport of the MPs across different ecosystems. It has also been found that Aquatic insect’s size, weight, feeding habitat, and host reserviour could be responsible for MPs ingestion. In addition, ecological risk assessment (Contamination Factor, Nemerrow Pollution Index, Pollution Load Index, Polymer Hazard Index) indicates different levels of risk for the pertaining river ecosystem.
Similar content being viewed by others
Introduction
Plastic goods are commonly employed in commercial and residential contexts because they are lightweight, affordable, and durable1,2. This is persistent because of their slow breakdown, making it easier to spread over long distances from their sources1. Plastic production began commercially in the 1940s and has increased rapidly. Currently, plastic output climbed from 330 million metric tonnes in 2016 to 400 million metric tonnes in 20223and is projected to double by 20394. Various kinds of studies have revealed evidence that plastic debris reaches the marine environment every year in amounts between 4.8 and 12.7 million tonnes, whilst freshwater receives more than 8 million tonnes globally4,5. Plastic waste can take on a variety of forms and dimensions, but the smallest particles are referred to as microplastics (MPs).
There is no universally accepted definition of MPs, however, an upper limit of 5 mm is commonly accepted in the literature6. In general, MPs are classified as either primary or secondary. For example, primary MPs can be discovered in various products such as bath gels, shaving lubricants, eye makeup, personal care products, blushing powders, foundation, eyeliner, shaving foam, newborn goods, bubble bath creams, hair color, nail paint, insect repellant, and moisturizer. Secondary MPs are produced through physical, chemical, and biological processes that break big waste plastics7,8,9. Secondary MPs are found in various environments, including bottle caps, bags, drinking straws (plastic/foam), food packaging, throwaway bottles, and plastic beverage bottles7. Consequently, secondary MPs are a major cause of plastic pollution due to their small size and potential to be consumed by organisms in aquatic environments7. The physical properties mainly consist of the color, shape, size, density, and crystallinity of MPs4. MPs come in various forms such as fibers, films, foams, fragments, or pellets. Among these, fibers are described in various ways by different studies. Typically, they are over twice as long as they are wide, threadlike, and extremely thin10. MPs can consist of various types of chemicals such as Polypropylene (PP), Polystyrene (PS), polyvinyl chloride (PVC), polythene terephthalate (PET), polyamide (PA), nylon, polyester (PES), polyurethanes (PU), poly(methyl methacrylate) (PMMA), epoxy resins, etc., each with different densities or specific gravities4,7.
These MPs particles have been detected in various environmental settings such as rivers, lakes, oceans, terrestrial areas, islands, river channels, atmosphere, and ice sheets, raising worries about plastic pollution in aquatic ecosystems10,11,12. MPs primarily become part of aquatic food chains by being consumed by plankton or zooplankton and filter feeders13,14,15. But it is mostly ingested directly or indirectly by aquatic organisms like fish, crabs, snails, and aquatic insects16,17,18. These fish, crabs, and snails serve as human food. In this process, they also entered into the human food chain. Aquatic insects also act as recyclers of nutrients, decomposers of organic compounds19, connectors of trophic level transfer20,21, and bioindicators22,23. The frequency and importance of aquatic insects in the food chain among producers and higher consumers have a substantial impact on the distribution, bioaccumulation, and risk assessment of microplastics (MPs) in habitats.
MPs directly or indirectly affect the human body by entering into the food chain. It has also impacts on wild organisms in the natural environment24,25. Reproduction success, growth, metabolic alterations, behavior, and histopathology are all negative impacts of MPs toxicity26. The ability of MPs to produce immunological changes, disruptions of gene expression, genotoxicity, endocrine difficulties, and neurotoxicity is the source of these adverse effects17,24.In addition, MPs also act as a reservoir or carrier of several pollutants like Heavy Metals (HMs), organic pollutants, and antibiotics17,27. That also increases the risk of negative impact because each of the pollutants has also provided negative impacts.
Realizing the dangers of microplastic pollution in river ecosystems, MPs pollution in river ecosystems has attracted global attention. Following the trend, a wide range of inspection and impact studies of MPs in sediment, water, and fish had already been conducted in Bangladesh. However, there are other components that require attention, such as aquatic insects in river ecosystems. Aquatic insects are the most abundant and diverse groups that have adapted to living in lentic or lotic water at least part of their life cycle. They are essential to ecosystems for their roles in food webs, water quality, nutrient cycling, and pollination. They provide a critical food source for fish, amphibians, birds, and other wildlife, thus maintaining biodiversity28,29,30. They can be found in almost every type of aquatic habitat like lakes, ponds, swamps, springs, streams, and rivers31. Many aquatic insects serve as excellent bioindicators of water and environmental quality due to their wide range of sensitivity to environmental contamination32. Despite having tremendous importance on the river ecosystem no research has been conducted on the pollution of MPs in aquatic insects in Bangladesh to date. To overcome this and create a new dimension of MPs study in Bangladesh and worldwide, this study was conducted on six species of aquatic insects along with water and sediment from the Dhaleshwari River. They serve as bioindicators, recyclers of nutrients, and connectors of trophic-level transfer. This study’s particular goals are to (1) Identify, quantify, and characterize microplastics (MPs) in sediment, water, and aquatic insects; (2) Identify the factors of MPs consumption by aquatic insects along with Bioaccumulation concentration (3) Ecological risk assessment.
Methodology
Study area
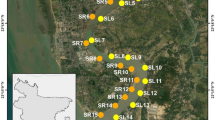
The study was conducted on the Daleshwari River of Dhaka, Bangladesh (Fig. 1). This river flows in the central part of Dhaka. Once, this river served as a source of irrigation water, fish, bathing, and sometimes as a drinking water source. But day by day over the year, due to the dumping of industrial waste, household waste, and tannery waste, this river lost its natural flow of water and degraded its water quality. Nowadays, it has become a misery for them. However, it has the potential to serve the locality in various ways. Sample locations are represented by S-1 to S-10 points on the map, while water and sediment samples were labelled as (W.1 to W.10) and (S.1 to S.10), respectively. Sample locations were chosen using a purposive random sampling method. This approach was adopted to ensure the sampling sites are representative of the study area and also aligned with the research goal, such as potential point sources of contamination (urban settlements, industrial discharge, waste dumpsites, and agricultural discharge etc.). In addition, the random aspect minimized any selection bias for statistical analysis.
Sample collection
A total of 100 samples of six aquatic organisms, 30 samples of water from 10 sampling stations, and 30 samples of sediment from 10 sampling points were collected from the Dhaleshwari River. Water samples were collected using Pyrex bottles using the grab sampling method24. At first, all the Pyrex bottles were rinsed with distilled water and then dried up. After that, the sample bottle was again rinsed with water from each location and then filled with 500 ml water at least 5 cm below the surface water by following the grab sampling method. After collecting, 1 ml HNO3 was added and stored with proper labelling. In the case of the sediment sample, Ekman dredge was used to collect sediment. After collection, it was wrapped with aluminium fuel paper and stored with proper labelling. Sampling location of water and sediment was similar.
A total of six species of aquatic insects were collected, including three species of aquatic heteroptera—Diplonychus annulatus, Diplonychus rusticus, and Ranatra sp.—which complete their entire life cycle in water. We also included three species of odonates—Brachythemis contaminata, Crocothemis servilia, and Orthetrum sabina—whose immature stages are aquatic, while adults inhabit terrestrial and aerial environments. All six species were abundant across all sampling stations, providing us with the necessary number of samples for our analysis. Notably, this study marks the first report of D. annulatus and D. rusticus in Bangladesh.
These insects were collected using a D-framed aquatic nets and a sweeping net. Specific routes were followed to collect the individuals. Primary identification was done in the field, and by using CCl4,specimens were rendered dead through jarring. The samples were subsequently kept in tiny triangle paper envelopes for careful preservation before being placed in Pyrex bottles. To prevent mold and other insects from growing on the specimens, naphthalene and silica gel were kept in the pots containing the tissues. The specimens that were gathered taken to the laboratory for identification. The taxonomic key that provided made identification easier33,34,35,36,37. Since the colors of the specimens can change or fade after preservation, Olympus, Xiaomi Redmi Note 13 phones, and Nikon digital cameras were used to take pictures of the specimens in the field or shortly after they were collected.
Sample preparation
All of the digestion and other MPs identification processes were conducted in the Laboratory of Ecotoxicology and Environmental Health, Department of Environmental Sciences, Jahangirnagar University, Bangladesh.
Water sample digestion
Initially, 2 mm sieves were used to filter water samples, and later, 100 mL water samples were placed in a conical flask by using a measuring cylinder (Pyrex). Then, it was digested with 20 ml hydrogen peroxide (H2O2) (Merck German) to decompose organic matter38,39. After that, samples were kept in a dark environment for three days to get better results. Subsequently, the water sample was filtered by using 0.45 μm Whatman Microfiber glass filter paper40. The filter paper was then preserved in a clean Petri dish for microscopic observation17,41.
Soil sample digestion
The sediment sample was first oven-dried at 60 °C in a petri dish. Five grams of the sediment were put in a glass beaker once it had dried. 20 ml hydrogen peroxide (Merck German) was added, and the mixture was let to settle for six hours in order to decompose the organic detritus38. For density separation, the sample was mixed with a saturated 50 ml NaCl solution (1.2 g cm3) and shaken with glass rood for 30 min. Then, it is allowed to settle down for two days. After that, the supernatant was transferred to a test tube, and then it went through a centrifugation process at 2000 rpm for 10 min to completely separate the sediment from the supernatant solution (v). Then, the supernatant solution was filtered by using Whatman Microfiber glass filter paper, whose pore size was 0.45 μm42,43. The process was repeated with the remaining sediment sample by adding 50 ml ZnCl2(d = 1.70 g/ml) for better accuracy17,41. Finally, these two filter papers were preserved for microscopic observation in a clean Petri dish with proper labeling.
Aquatic insect sample digestion
The aquatic insect was first separated according to their species and then separated according to their taxonomy in different Pyrex glass beakers. Then, each of the insect’s size and weight were measured. After that, a 1 gm sample and individual species were taken for digestion. Each sample was initially treated with 50 ml hydrogen peroxide (30%) (Merck German) and left in a dark environment for 15 days in order to aid in digestion. After that, each sample was heated for five hours at a temperature of 600C and a speed of 2000 rpm using a magnetic stirrer hot plate. Next, 0.45 μm Whatman Microfiber glass filter paper to filter was used to filter the entire digested sample. After that, the filter paper was kept in a clean Petri dish for microscopic inspection.
Microscopic inspection by stereo microscope
Leica®EZ4 HD stereo microscope (with a Built-in Digital Camera range of magnification 8x-35x) was used to examine microplastic from the filter paper. With its assistance, pictures of these microplastic particles were also taken. This stereo microscope did not have an internal facility for measuring microplastic particle size. For that reason, the MPs particle sizes were calculated by using ImageJ (version 2022) software. MPs were categorized based on their morphology, size, and colour after being visually identified. Based on size, it was separated into three groups. Less than 0.5 mm falls into Category 1, 0.5–1 mm into Category 2, and 1–5 mm into Category 317,41. It is separated into six categories based on colour: green, blue, black, red, white, and pink. Five categories were used to categorize morphology: fiber, fragment, pellet, and film17,41.
Fourier transformed-infrared (FT-IR) spectrometry analysis
By utilizing wavelength, Fourier Transformed-Infrared (FT-IR) spectroscopy was used to confirm the type of MPs. For that, IR Prestisize-21 was employed. The higher concentration of microplastic concentration makes it difficult to analyzed all the samples in FTIR. To minimized that problem, 500 water and sediment, and 250 aquatic insects MPs were randomly selected. After that, 20% of the selected MP was again chosen randomly for FTIR analysis. This is one of the limitations of this study. Then, KBr pellet was initially made by combining MPs particles with KBr salt. After that, it passed through FTIR analysis process17,44,45. That technique measured wavelengths between 400 and 4000 cm−146. MPs polymer types were detected by paralleling with published research: Hummel Polymer and Additives, Polymer Laminate Films, Cross Sections Wizard, HR Spectra IR Demo, and Aldrich Vapor Phase Sample Library17,44,45.
Scanning electron microscope (SEM) analysis
For any type of morphological examination, the scanning electron microscope (SEM), which produces high-resolution images of the materials, is frequently used. This research used a high vacuum SEM (model no. EVO18, Carl Zeiss AG, UK) to evaluate the surface morphology of the identified MPs at 1 and 5 X magnification17,41.
Ecological risk assessment of MPs
Nemerow pollution index (NPI)
To assess the degree of pollution in MPs, the Nemerow Pollution Index (NPI) is frequently utilized. Once the abundance and composition of MPs have been extensively examined, Eq. (1) presents the calculation47.
Where, Qi reflects the abundance of MPs in samples. Si max and Si ave represent the typical maximum and average values for MPs abundance.
Pollution load index (PLI)
For evaluating ecological risk in both terrestrial and aquatic ecosystems, the pollutant load index (PLI) is a commonly used measure2. The ecological danger in this study was evaluated using the MPs percentage found in the water and sediment of Dhaleshwari River. The PLI was calculated using the following formulas2,47.
Where, A sample station is denoted by ‘i’, while the total number of sampling stations is represented by ‘n’, and CFi is the Contamination factor and it’s determined by dividing the MP concentration (Ci) at each location by the background concentration (Coi). This study utilized the lowest observed abundance of MPs in water (101 MPs/L) and sediment (13456 MPs/kg) as the baseline value due to limited background values.
Polymer hazard index (PHI)
MPs’ environmental impact is assessed by examining the chemical toxicity of various polymers48. The following formula is used to estimate the PHI49:
Where, Pn shows the average percentage of each MPs polymer in all samples, whereas Sndenotes the risk score of each polymer based on the prior study by49.
Bioaccumulation factor (BAF)
Bioaccumulation factor (BAF) of aquatic insects means the accumulation rate of different pollutants in any aquatic insect species. BCF of aquatic insects has been calculated through the following equation:
Here, \(\:{C}_{i}\) indicates the MPs concentration in aquatic insects and \(\:{C}_{W}\) indicates MPs concentration in water (MPs/L) and sediments (MPs/kg) of Dhaleshwari river. BCF > 1 indicates hyperaccumulators and BCF = 1 is excluder.
Statistical analysis
The entire statistical analysis was performed using Microsoft Excel 2020. For generating visual representations such as bar charts with error bars, stacked columns, pie charts, and graphs for FT-IR data plotting, Origin Pro-2022 was utilized. Study area map was created by using Qgis desktop 3.22.1 (https://ftp.osuosl.org/pub/osgeo/download/qgis/windows/QGIS-OSGeo4W-3.22.1-1.msi).
Quality control
Sample collection tools, such as Ekman Dredge, Pyrex bottle, D-framed aquatic nets, and a sweeping net, were made of steel, wood, pyrex, and organic fiber. After collecting samples, they were preserved in a cold box. Then, as soon as possible, all of the samples were refrigerated at − 40C temperature in the Laboratory of Environmental Health and Ecotoxicology, Department of Environmental Sciences, Jahangirnagar University, Dhaka 1342. To avoid any kind of external contamination, a cotton suit apron and hand gloves were worn during the entire experiment. Further, all of the apparatus used in this experiment were made of pyrex. In addition, all beakers, Petri dishes, and Pyrex bottles used in the entire analysis were rinsed three times with deionized water and then heated for four hours at 120 °C. For every sample, three duplicates were examined. Three blank samples were also analyzed to prevent contamination from the chemicals used in this study. In addition, to prevent contamination in each case, blank filter paper was also examined during microscopic observation. SEM and FTIR were also done with great care to avoid any kind of external contamination.
Results and discussion
Abundance of MPs in water, sediment and aquatic species
This study investigated microplastics (MPs) pollution in water, sediment, and aquatic species of Dhaleshwari River, Dhaka. In water samples, the highest concentration of MPs was found in the sampling station at W.3 (195 ± 18.95 MPs/L) and the lowest at W.5 (101 ± 31.45 MPs/L), with an average concentration was 143.1 ± 28.52 of MPs/L (Fig. 2A). The abundance of MPs in water samples of Dhaleshwari river was comperatively higher than the previous studies of MPs in Buriganga Rivers water 117 to 250 MPs/L2, Ganges to Meghna River in Bangladesh 50.9 ± 24.4 to 64.1 ± 26.3 MPs/L47, Xiangjiang River in China 7.32 ± 2.36 to 11.0 ± 3.08 MPs/L50. In sediment samples, the highest concentration of MPs was found in the sampling station at S.4 (46789 ± 3124.24 MPs/kg) and the lowest at S.8 (13456 ± 1123.44 MPs/kg), with an average concentration was 30153.8 ± 2313.62 of MPs/kg (Fig. 2B). The abundance of MPs in sediment samples of Dhaleshwari river was also found higher than the previous studies of MPs in Buriganga Rivers sediment 3500 to 8170 MPs/kg2, Ganges to Meghna River 2953.49 ± 1670.52 to 4014.66 ± 1717.59 MPs/kg47, Karnaphuli River 22.29 to 59.5 MPs/kg51, Turag River in Bangladesh (19.2 ± 2.44 MPs/kg5, Major tributaries of Yellow River in China (430.30, 145.09, 253.33 MPs/kg52, Xiangjiang River in China (150 ± 75.6 to 249 ± 182 MPs/kg50. This variation may be due to sampling location, sampling volume, geographical position, and detection method. The abundance of MPs in a different compartment of Dhaleshwari River varied, which could be the different sources of pollution, such as industrial waste dumping, discharges of industrial wastewater, fishing activities, disposal of urban waste, thrown of domestic wastewater, stormwater, and so on53,54. Pearson correlation was used to determine the relation between water and sediment contamination in Dhaleshwari river. A negative relationship was discovered (r = − 0.22), with MPs contamination in the water and sediment of the Dhaleshwari River (Fig. 4A).
In aquatic species of Dhaleshwari river, the highest MPs was found in D. rusticus (57.82 ± 14.98 MPs/g; 2.91 ± 1.78 MPs/ind), followed by B. contaminata (38.53 ± 6.87 MPs/g; 5.45 ± 3.21 MPs/ind), Ranatra sp. (34.05 ± 5.39 MPs/g; 2 ± 2.89 MPs/ind), C. servilia (26.99 ± 7.88 MPs/g; 4.11 ± 3.21 MPs/ind), D. annulatus (16.44 ± 6.95 MPs/g; 7.97 ± 4.78 MPs/ind), and O. sabina (14.13 ± 4.52 MPs/g;1.98 ± 0.91MPs/ind) (Fig. 3).
Over 600 genera and 5,740 species of odonates have been reported globally. There are currently 179 species of Odonataknown to exist in Bangladesh, including 90 species of dragonflies and 89 species of damselflies55. Among them D. rusticus and D. annulatus are in similar genera (Diplonychus) and family (Belostomatidae); B. contaminata, C. servilia, O. sabina, and are from the Libellulidae family but different genous Brachythemis, Crocothemis, Orthetrum; and Ranatra sp. is from Ranatra genus and Nepidae family. The MPs distribution among genous was Brachythemis (38.53 ± 6.87 MPs/g; 5.45 ± 3.21 MPs/ind), Diplonychus (37.13 ± 10.96 MPs/g, 5.41 ± 3.28 MPs/ind ), Ranatra (34.05 ± 5.39 MPs/g; 2 ± 2.89 MPs/ind), Crocothemis (26.99 ± 7.88 MPs/g; 4.11 ± 3.21 MPs/ind), Orthetrum (14.13 ± 4.52 MPs/g;1.98 ± 0.91MPs/ind). In case of family the distribution of MPs pattern is Belostomatidae (38.53 ± 6.87 MPs/g; 5.45 ± 3.21 MPs/ind), Nepidae (34.05 ± 5.39 MPs/g; 2 ± 2.89 MPs/ind), Libellulidae (26.55 ± 6.42 MPs/g, 3.84 ± 2.44 MPs/ind.
Among this species two groups of insects are well-known bioindicators of water quality. One group is dragonflies, and another is aquatic Heteroptera. Dragonflies spend one part of their life cycle (nymph/wrigglers) in water and another in terrestrial and aerial environments. The ditch jewel, Brachythemis contaminata, is a well-known bioindicator of pollution that is abundantly found in polluted ditches and drains. The high abundance of B. contaminataindicates elevated pollution levels56,57. Orthetrun sabinais has a wide distribution range, can live in diverse habitat types and is tolerant to disturbed habitats that experience pollution56,58. Crocothemis serviliais one of the common dragonflies frequently found in ponds, puddles, rivers, big wells, tanks, ditches and paddy fields59. Among the aquatic heteroptera, several species of gerrids have been reported as well-known bioindiocators of heavy metal pollution60. However, data on the potential of bioindicator of Diplonychus rusticus, D. annulatus and Ranatra sp. is completely lacking.
This study’s findings reflected the first-ever assessment in terms of MPs found in aquatic species of Bangladesh. However, a recent study was conducted in Osun and Ogun Rivers, Nigeria by Akindele et al., (2020) reported that identified MPs pollution in three aquatic insects such as Siphlonurus sp. (62.36 ± 3.53 MPs/g), Lestes viridis (43.29 ± 43.29 MPs/g) and Chironomus sp.(291.76 ± 26.55 MPs/g)21. Another study was done on aquatic insects living in rice fields in Thailand by Maneechan & On Prommi, (2022)61. In this study, MPs were detected in the whole body, gastrointestinal tract (GIT), and body without the GIT of Pantala sp.(1.34 ± 1.11, 1.06 ± 0.77, and 0.73 ± 0.51 items/g, respectively)62. Variations in these results might be due to the nature of species, number of species, habitat of species, feeding habitat, and detection methods. The study findings showed that MPs were present in every single species. The presence of MPs in the water and sediment of the Daleshawari River could be the possible pathway of MPs in Aquatic species. In addition, The aquatic heteropteran bugs or water bugs of the genus Diplonychus Laporte, like D. annulatus (Fabricius) and D. rusticus (Fabricius) are well known to prey upon mosquito larvae63,64,65. They are also known to feed on chironomids, aquatic crustaceans, small fishes and amphibians. Another water bug, Ranatrasp. is also a sit-and-wait predator of small insects and crustaceans66. Moreover, Aquatic nymphal stage of B. contaminata is omnivorous and eats a variety of foods, including debris and chlorophyceae. They are also known to be effective predators of mosquito larvae, including those of Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti67,68. The aerial stage of C. serviliafeeds on mosquitoes, flies and other insects in mid-air69,70. This might be the source of MPs as they act as insect food sources. Aquatic animals that consume MPs can also facilitate the transfer of hydrophobic and persistent organic pollutants, such as dichlorodiphenyltrichloroethane, polychlorinated biphenyls, and dioxins, to higher levels in the food chain. This is because MPs act as carriers for these pollutants21. This multiplies the effects of MPs on the environment and humankind.
MPs pollution assessment in Dhaleshwari River
MPs pollution assessment according to shape, color, and size
MPs comparison according to shape.
A total of five different shapes, including fibers, fragments, pellets, films, and foams, were discovered in the aquatic species, water, and sediment samples of Dhaleshwari river (SF. 1 A). Fibers were the most dominant MPs in all the samples, including aquatic species (74%), water (65%), and sediment (55%) followed by fragments in sediment (23%), water (21%), aquatic species (12%), foams in sediment (15%), aquatic species (7%), water (6%), pellets in water (5%), aquatic species and sediment (4%), and films in aquatic species, water, and sediment (3%) (SF. 1 A). Fibers are expected MPs in freshwater environments of previous scientific1,5,21,71kinds of literature, which supported current study findings. The relatively long fibers in river streams can originate from a number of things, including urban debris, fishing gear, roping, clothing, and industrial wastewater discharges. The relatively long fibers in river streams can originate from a number of things, including urban debris, fishing gear, roping, clothing, and industrial wastewater discharges1,5. Tire wear is the direct cause of MPs exposure in the Dhaleshwari River test locations because they are surrounded by automobile roadways. According to Baensch-Baltruschat et al. (2020), tire wear produces 6.1 million tons of MPs annually, of which 75.87% are fibers72. Fragments and foam indicate discarded packing materials and shattered plastic debris. The pellet is primarily found in skincare products, including face cleansers47.
MPs comparison according to color.
A total of five different colors, including black, blue, red, green, and white, were identified in the aquatic species, water, and sediment samples of Dhaleshwari river (SF. 1B). Black was the most dominant MPs colors in all the samples, including aquatic species (41%), water (39%), and sediment (32%) followed by red in sediment (27%), water (21%), aquatic species (15%), blue in water and sediment (25%), aquatic species (23%), green in aquatic species (14%), sediment (10%), water (9%), and white in aquatic species (7%), water and sediment (6%) (SF. 1B). Studies73,74,75have found a high prevalence of black plastic in freshwater environments worldwide. However, some investigations come to different results. For instance, Gabriel & Bacosa, (2024) and Haque et al. (2023) found that blue was the most dominant color in freshwater river water and sediments17,76, and Khedre et al. (2023) found that blue was dominant in aquatic organisms18. Bangladeshi textiles, commercial bags, packed bags, and aquatic organisms are mostly made of translucent or brightly colored plastics that mimic the food that zooplankton and other aquatic animals eat. Sunlight also stains plastic materials17.
MPs comparison according to size.
Three MPs sizes were found: < 0.5 mm, 0.5–1.0 mm, and 1.0–5.0 mm groups in the aquatic species, water, and sediment samples of Dhaleshwari river (SF. 1 C). The < 0.5 mm in size of MPs were dominant in all the samples including aquatic species (72%), sediment (65%), and water (55%) followed by 0.5–1 mm in water (25%), sediment (21%), aquatic species (14%), and 1–5 mm in water (20%), aquatic species and sediment (14%) (SF. SF. C). A recent study by Haque et al., (2023) found that less than 0.5 mm of water (42.38%), and sediment (49.92%) dominated their analysis of the Buriganga River in Dhaka City, which is consistent with the current study findings. MPs sizes play a significant role in their ingestibility by aquatic creatures51. In this present study, the majority portion of MPs were less than 0.5 mm in size. Consequently, it is easier for aquatic species of Dhaleshwari river to eat because of its size. Since plastic elements deteriorate gradually in the presence of sunshine17, it suggests that the Dhaleshwari river has been polluted with plastic for a long time. MPs size plays a vital role in MPs consumption by aquatic insects. Small particles can easily falsify aquatic organisms, and they can easily consume it rather than large particles. For that reason, small particle rate is higher in aquatic insects. MPs accumulated inside their prey as well as within the surrounding environment among predatory species, indicating that the MPs capacity to select food is restricted by size.
Factor responsible for aquatic insect’s MPs consumption
Insect’s weight, height, feeding habit, and host reservoir may be responsible for the MPs consumption among aquatic insects. Pearson correlation was used to ascertain any relationship between MPs abundance in water, sediment, and aquatic species with their length and weight samples of the Dhaleshwari River followed by linear regression (Fig. 4).
MPs consumption relation with aquatic species length and weight
A negative relation (r = − 0.61) was observed between the MPs abundance and their existing height Fig. 4D. That indicates MPs consumption is decreasing with their length. On the other hand, positive relation (r = 0.03) has been observed from the weight and MPs abundance. That indicates MPs consumption is increasing with their weight. This may be due to the physical characterestics of the aquatic insects. For example, Orthetrum sabina, Ranatra Sp. Crocothemis servilia has higher body length or height, while in the case of weight, they are lowest. However, in the case of Brachythemis contaminata, Diplonychus rusticus and Diplonychus rusticus showed opposite chracterestics. Details description of aquatic insects’ height and weight are given in ST.1.
MPs intake in relation to the feeding behavior of aquatic insects
One of the key elements influencing MPs consumption is feeding patterns. Two types of feeding habits are studied in this study. One type is aerial, and the other is predatory. The mean abundance of MPs in predatory and aerial species were 36.10 ± 5.14 and 26.55 ± 1.71 MPs/g, respectively (SF. SF.D). Besides, the abundance of MPs in predatory and aerial species was not statistically significant (P = 0.529, p> 0.05). The results show that species can consume and store MPs in their bodies at varying rates depending on their feeding strategy. A recent study was done in Egypt18 found that predatory aquatic insects (Aeshna sp.: 37 MPs/g) accumulated significant levels of MPs, which is consistent with current study findings. Predatory aquatic species contained more MPs than aerial aquatic species in this investigation. In their natural habitat, predatory species typically capture zooplankton and phytoplankton and feed on organic debris on the sediment surface. Given sediment’s role as a repository for pollutants in the freshwater system, settled MPs can likely be readily ingested. In contrast, aerial species usually inhabit bodies of water and are greatly affected by MPs on the water’s surface.
MPs consumption relation with insect host reservoir abundance
This insect spends their total life in the water and sediment of Dhaleshwari river. For that reason, its contamination is one of the major factors for MPs consumption in aquatic insect’s body. A positive relation has been observed with the MPs abundance in aquatic insects with sediment (r = 0.71) and water (r = 0.16) MPs contamination in Dhaleshwari river (Fig. 4C, D). That indicates MPs consumption increased with the contamination of the host reservoir.
FTIR analysis
FTIR analysis had been performed on a representative number of samples. Of them, 95% of the samples had plastic polymer identified in them. Eight different types of polymers were found in the water, sediment, and aquatic species of Dhaleshwari river using FTIR analysis (Fig. 5). These polymers include HDPE, Low Density Polyethylene (LDPE), PVC, Latex, EVA, PP, PS, and Nylon (Fig. 5). The most prevalent of them in aquatic insects is HDPE, which is followed in distribution pattern by PS (13%), PP (15%), PVC (20%), EVA (24%), and HDPE (28%). In the case of the sediment sample, the most prevalent polymer type was also HDPE, which was distributed as follows: HDPE (31%), PVC (21%), EVA (18%), Nylon (14.5%), PS (6.5), PP (4.5) and LDPE (3.5%). LDPE were the most prevalent polymers in the water sample, and their distribution pattern was distributed as follows: LDPE (38%), PVC (27%) Latex (17%), PS (11.5%), PP (6.5%).
Details description of polymer peak and identical characteristics are provided in (ST 2). Accurately predicting the most common polymer groups in MPs from a limited sample size investigation is difficult, though. Therefore, there’s a good likelihood that more polymers will be found in the Dhaleshwari River’s water, sediment, and aquatic insect species.
All of the polymer impacts on aquatic insects have not been studied yet. Only a few preliminary impacts of this type of polymer have been reported77,78,79,80. According to Lei et al. (2018), PVC and PP show significant effects on mortality, reduction of body length, inhibition in reproduction, and decreasing intestinal Ca levels in Caenorhabditis elegans77. PS shows negative impacts on Dreissena polymorpha, Daphnia magna, Gammarus pulex, Hyalella Azteca, Asellus aquaticus, Sphaerium corneum, Lumbriculus variegatus, Tubifex sppin case of mortality, growth, feeding rate, cellular stress, oxidative damage, neuro genotoxicity81,82,83,84.
MPs surface analysis by SEM
SEM is frequently used to describe or identify MPs because it produces a high-resolution image that amply reveals its surface. A representative number (15%) of MPs-like particles were taken to conduct SEM analysis. Figure 6 shows SEM for representative MPs. However, it can be quite challenging to recognize the MPs surface. The SEM results showed that the MP fiber contained flake, crack, linear fracture, crescentic fracture, protrusions, and scratches (Fig. 6)17,44,85. Furthermore, the SEM image clearly shows that the MPs have adequately changed into nano plastic, which presents another challenge for us. It will undoubtedly have a disastrous impact on the ecosystem.
Bioaccumulation factor (BAF) analysis
MPs bioaccumulation in aquatic insects refers to the accumulation of MPs from water and sediment of the host river. According to the bioaccumulation factor from water and sediment to aquation insects, the total species of insects showed hyper accumulation from water. In the case of sediment, 50% of species were hyperaccumulators. The other 50% also shows accumulation from sediment but not hyperaccumulator (Table 1).
Ecological risk assessment
Contamination factor (Ci), Nemerow pollution index (NPI), Pollution Load Index (PLI), and Pollution Zone Index (PLIzone) are the most usable tools for ecological risk assessment. All of these parameters for both sediment and water have been analyzed. According to the contamination factor (Ci), all of the sediment and water samples indicate moderate ecological threats to us (ST.3). NPI values lower than 2 indicate lower bioavailability86. This study found that the sediment and water of the Dhaleshwari river pose a moderate threat as the value of NPI was close to value 2. In the case of the Pollution Load Index (PLI), it poses a threat to category 1 (ST. 3). The Polymer Hazard Index (PHI) is created from the existing polymer hazard score87. PHI value of sediment and water of Dhaleshwari river revealed that Bansghi river poses risk category 5 (ST.3). That indicating extreme danger towards us. All of the risk assessment tools show higher or potential danger towards us, which may be because the ecosystem of the Dhaleshwari river is highly contaminated with MPs.
Ecological implipication
This study detected MPs in six species of aquatic insects. That raises concern about the impacts of MPs in aquatic ecosystems. This MPs has several direct effects on aquatic insects and indirect effects on their environment. MPs in aquatic insects reduce the weight of aquatic insects by hindering digestive tracts and nutrient absorption88. Rojo et al., (2020) reported detritivore mortality and reduced leaf litter decomposition due to MPs contamination89. Fudlosid et al. (2022) also reported the reducing growth effect of MPs contamination in Gryllodes Sigillatus.
species90. MPs have also shown effects on aquatic insect’s Intestinal permeability91, Locomotion behaviour91, Reproduction (PE)79, Emergence rate92, Assimilation efficiency84.
MPs can also coat in water and reduce suitable habitats or smother insect eggs for certain species88. It also mostly affects in the aquatic food chain by entering into the insect body and being transferred to predators further up the food chain, eventually fish, birds, and even humans17,93,94. As MPs pollution had negative impacts on the growth and population of aquatic insects78,82. It may cause cascading effects or disrupt the entire aquatic ecosystem.
Management plan for reducing microplastic pollution
Bangladesh is a riverine country. It lacks of proper drainage system and wastewater treatment facilities in the urban areas. All of the domestic wastewater goes into the improper drainage system, along with industrial wastewater and landfill leachate. Finally, this water is introduced into the river through several canals. In Bangladesh, the destination of untreated wastewater is either ponds or rivers95,96. That makes the rivers of Bangladesh a major reservoir of any kinds of contaminants and makes it difficult to manage. In addition, as a downstream deltaic country, Bangladesh shares 54 transboundary rivers from neighbouring countries like India and Myanmar, which makes the management more complex. However, in Bangladesh, the DoE (Department of Environment) under the Ministry of Environment, Forest and Climate Change is responsible for regulating any point sources of pollution that ultimately lead to any management initiatives. They set guidelines and standards for any kind of wastewater discharge97. Unfortunately, in Bangladesh’s guidelines, still there is no standard for microplastic pollution. To manage this microplastic, Bangladesh first has to reduce its production and disposal. Recently, the Government of Bangladesh has taken steps to phase out the single-use plastics from the country by imposing restrictions on the production value chain as well as at the consumer end98,99. However, proper implementation of this restriction may not be possible without creating and introducing alternatives to single-use plastic. At first, alternatives to plastic products must be introduced and popularized. Point sources of plastic and microplastic pollution must be identified and monitored. Drainage water from the drainage system must be treated before being introduced into the river. A standard for microplastic can be fixed for wastewater and household water discharge into the drainage system. An independent advisory and monitoring committee must be formed to reduce plastic pollution. Through public campaigns and advertisements, people must be aware of the destructive effects of microplastics in our food chain and ecosystem. This management plan must be improved continuously through the PDCA (Plan-Do-Check-Act) cycle.
Conclusions
The presence of microplastics (MPs) has been detected in every component (sediment, water, and aquatic insects) of Dhaleshwari river. That highlights the pervasive nature of this pollutant and its potential ecological risks. Aquatic insects, serving as bioindicators, reflect the bioavailability and possible trophic transfer of these pollutants, indicating a direct threat to higher trophic levels and the overall health of the ecosystem. For that reason, further research is required on the impacts of MPs on these insect’s bodies, as they are a significant part of our ecosystem. In addition, polymer-based impact study, source identification, and research on various taxonomic order, age, and seasonal studies are required for better understanding of MPs relation with aquatic insects and environmentally friendly decision-making. It will help Bangladesh’s river management authority and policymakers. In addition, it will create a new dimension for MPs monitoring studies on aquatic systems.
Data availability
The analyzed datasets during the current study are available from the corresponding author upon reasonable request.
References
Shahnewaz, H. M., Hasan, J., Manik, M. & Ahmed, M. Pervasiveness of microplastics in the gastrointestinal tract of some selected fi sh species from Turag River alongside the capital city of Bangladesh. Emerg. Contam. 10, 100309 (2024).
Haque, M. R. et al. Assessment of microplastics pollution in aquatic species (fish, crab, and snail), water, and sediment from the Buriganga River, Bangladesh: An ecological risk appraisals. Sci. Total Environ. 857 (2023).
Plastics Europe. Plastics – the fast facts 2023. Plastics Europe (2023). https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/
Sharma, S., Bhardwaj, A., Thakur, M. & Saini, A. Understanding Microplastic Pollution of Marine Ecosystem: A Review. Environmental Science and Pollution Research (Springer, 2023). https://doi.org/10.1007/s11356-023-28314-1
Khan, M. B. et al. Abundance, distribution and composition of microplastics in sediment and fish species from an Urban River of Bangladesh. Sci. Total Environ. 885, 163876 (2023).
Ferdous, S. R., Amin, A., Hasan, J., Alam, M. S. & Shahjahan, M. Prevalence of microplastics in commonly consumed fish species of the River Old Brahmaputra, Bangladesh. Environ. Sci. Pollut. Res. 30, 85639–85654 (2023).
Akter, A. et al. Microplastic pollution: A review of techniques to identify microplastics and their threats to the aquatic ecosystem. Environ. Monit. Assess. 1–26. https://doi.org/10.1007/s10661-024-12441-4 (2024).
Sajad, S., Allam, B. K., Mushtaq, Z. & Banerjee, S. Microplastic detection and analysis from water and sediment: A review. Macromol. Symp. 407, 1–10 (2023).
Prapanchan, V. N., Kumar, E., Subramani, T., Sathya, U. & Li, P. A. Global perspective on microplastic occurrence in sediments and water with a special focus on sources, analytical techniques, health risks, and remediation technologies. Water (Switzerland) 15 (2023).
Yardy, L. D., Al-Jaibachi, R. & Callaghan, A. Microplastics in Freshwater Ecosystems with Special Reference to Tropical Systems: Detection, Impact, and Management. Emerging Freshwater Pollutants: Analysis, Fate and Regulations (Elsevier Inc., 2022). https://doi.org/10.1016/B978-0-12-822850-0.00017-X
Nejat, N., Sattari, M., Mohsenpour, R., Shi, X. & Rasta, M. Microplastics abundance, distribution and composition in surface waters, sediments and fish species from Amir ˗ Kalayeh Wetland. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-024-32627-0 (2024).
Islam, M. S., Islam, Z. & Hasan, M. R. Pervasiveness and characteristics of microplastics in surface water and sediment of the Buriganga River, Bangladesh. Chemosphere 307, 135945 (2022).
Sipps, K. et al. Pervasive occurrence of microplastics in Hudson-Raritan estuary zooplankton. Sci. Total Environ. 817, 152812 (2022).
Botterell, Z. L. R. et al. Microplastic ingestion in zooplankton from the Fram Strait in the Arctic. Sci. Total Environ. 831, 154886 (2022).
Hitchcock, J. N. Microplastics can alter phytoplankton community composition. Sci. Total Environ. 819, 153074 (2022).
Khan, H. M. S. & Setu, S. Microplastic ingestion by fishes from Jamuna River, Bangladesh. Environ. Nat. Resour. J. 20, 157–167 (2022).
Haque, M. R. et al. Assessment of microplastics pollution in aquatic species (fish, crab, and snail), water, and sediment from the Buriganga River, Bangladesh: An ecological risk appraisals. Sci. Total Environ. 857, 159344 (2023).
Khedre, A. M., Ramadan, S. A., Ashry, A. & Alaraby, M. Ingestion and egestion of microplastic by aquatic insects in Egypt wastewater. Environ. Qual. Manag. 33, 135–145 (2023).
Adler, P. H. & Courtney, G. W. Ecological and societal services of aquatic diptera. Insects 10, 70 (2019).
Akindele, E. O., Ehlers, S. M. & Koop, J. H. E. Freshwater insects of different feeding guilds ingest microplastics in two Gulf of Guinea tributaries in Nigeria. https://doi.org/10.1007/s11356-020-08763-8/Published
Akindele, E. O., Ehlers, S. M. & Koop, J. H. E. Freshwater insects of different feeding guilds ingest microplastics in two Gulf of Guinea tributaries in Nigeria. Environ. Sci. Pollut. Res. 27, 33373–33379 (2020).
S, S., Kurup, B. S. & R, S. & Aquatic insects as bioindicators of water quality in the Achenkovil River, Kerala, India. Curr. World Environ. 18, 1192–1202 (2024).
Chowdhury, S. et al. Insects as bioindicator: A hidden gem for environmental monitoring. Front. Environ. Sci. 11, 1146052 (2023).
Khedre, A. M., Ramadan, S. A., Ashry, A. & Alaraby, M. Abundance and risk assessment of microplastics in water, sediment, and aquatic insects of the Nile River. Chemosphere 353, 141557 (2024).
Ribeiro-Brasil, D. R. G. et al. The impacts of plastics on aquatic insects. Sci. Total Environ. 813 (2022). https://doi.org/10.1016/j.scitotenv.2021.152436
Cormier, B. et al. Environmental microplastics disrupt swimming activity in acute exposure in Danio rerio larvae and reduce growth and reproduction success in chronic exposure in D. Rerio and Oryzias Melastigma. Environ. Pollut. 308, 119721 (2022).
Rafa, N. et al. Microplastics as carriers of toxic pollutants: Source, transport, and toxicological effects. Environ. Pollut. 343, 123190 (2024).
Nair, G. A., Morse, J. C. & Marshall, S. A. Aquatic insects and their societal benefits and risks. J. Entomol. Zool. Stud. 3, 171–177 (2015).
Starr, S. M. & Wallace, J. R. Ecology and Biology of aquatic insects. Insects 12, 51 (2021).
Suter, G. W. & Cormier, S. M. Why care about aquatic insects: Uses, benefits, and services. Integr. Environ. Assess. Manag. 11, 188–194 (2015).
Elango, K., Vijayalakshmi, G., Arunkumar, P., Sobhana, E. & Sujithra, P. Aquatic insect’s biodiversity: Importance and their conservation. Biol. Divers. Curr. Status Conserv. Policies 289–303. https://doi.org/10.26832/AESA-2021-BDCP-019 (2021).
Yap, N. I. R. et al. Aquatic insects as bio-indicators of Water Quality – A Study on Sungai Kawal, Johor National Park of Endau-Rompin, Peninsular Malaysia. IOP Conf. Ser. Earth Environ. Sci. 736, 012072 (2021).
Heckman, C. W. Encyclopedia of South American aquatic insects: Hemiptera - Heteroptera: Illustrated keys to known families, genera, and species in South America. Encyclopedia of South American Aquatic Insects: Hemiptera - Heteroptera: Illustrated Keys to Known Families, Genera, and Species in South America 1–679 (2011). https://doi.org/10.1007/978-94-007-0705-4
Fraser, F. C. Fauna of British India, Including Ceylon and Burma. vol. Odonata. Vol. III. (Taylor & Francis Group, 1936).
Fraser, F. C. Fauna of British India, Including Ceylon and Burma. vol. Odonata. Vol. II. (Taylor & Francis Group, 1934).
Fraser, F. C. Fauna of British India, Including Ceylon and Burma. vol. Odonata. Vol. I. (Taylor & Francis Group, 1933).
Chandra, K. & Jehamalar, E. E. Morphological differences in three species of the Genus < I > Diplonychus (Hemiptera: Belostomatidae) known from India. Rec Zool. Surv. India. 112, 91–99 (2012).
Nuelle, M. T., Dekiff, J. H., Remy, D. & Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 184, 161–169 (2014).
Ziajahromi, S., Neale, P. A., Rintoul, L. & Leusch, F. D. L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 112, 93–99 (2017).
Tagg, A. S., Sapp, M., Harrison, J. P. & Ojeda, J. J. Identification and quantification of microplastics in wastewater using focal plane array-based reflectance micro-FT-IR imaging. Anal. Chem. 87, 6032–6040 (2015).
Rana, M. M., Haque, M. R., Tasnim, S. S. & Rahman M. M. The potential contribution of microplastic pollution by organic fertilizers in agricultural soils of Bangladesh: Quantification, characterization, and risk appraisals. Front. Environ. Sci. 11, 1205603 (2023).
Afrin, S., Uddin, M. K. & Rahman, M. M. Microplastics contamination in the soil from Urban Landfill site, Dhaka, Bangladesh. Heliyon 6 (2020).
Thompson, R. C. et al. Lost at sea: Where is all the plastic? Science 304, 838 (2004).
Parvin, F., Jannat, S. & Tareq, S. M. Abundance, characteristics and variation of microplastics in different freshwater fish species from Bangladesh. Sci. Total Environ. 784, (2021).
Hossain, M. B. et al. First evidence of microplastics and their characterization in bottled drinking water from a developing country. Front. Environ. Sci. 11, 1232931 (2023).
Tanaka, K. & Takada, H. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 6, 1–8 (2016).
Alam, M. J., Shammi, M. & Tareq, S. M. Distribution of microplastics in shoreline water and sediment of the Ganges River Basin to Meghna Estuary in Bangladesh. Ecotoxicol. Environ. Saf. 266, 115537 (2023).
Rana, M. M., Haque, M. R., Tasnim, S. S. & Rahman M. M. The potential contribution of microplastic pollution by organic fertilizers in agricultural soils of Bangladesh: Quantification, characterization, and risk appraisals. Front. Environ. Sci. 11, 1–17 (2023).
Wu, X., Zhong, C., Wang, T. & Zou, X. Assessment on the pollution level and risk of microplastics on bathing beaches: A case study of Liandao, China. Environ. Monit. Assess. 195 (2023).
Zhao, W. et al. Seasonal variations of microplastics in surface water and sediment in an inland river drinking water source in southern China. Sci. Total Environ. 908, 168241 (2024).
Rakib, M. R. J. et al. Spatial distribution and risk assessments due to the microplastics pollution in sediments of Karnaphuli River Estuary, Bangladesh. Sci. Rep. 12, 1–15 (2022).
Zhao, X. et al. Distribution of microplastic contamination in the major tributaries of the Yellow River on the Loess Plateau. Sci. Total Environ. 905, 167431 (2023).
Hossain, M. N. et al. Identification and quantification of microplastics in agricultural farmland soil and textile sludge in Bangladesh. Sci. Total Environ. 858, 160118 (2023).
Talukdar, A., Kundu, P., Bhattacharya, S. & Dutta, N. Microplastic contamination in wastewater: Sources, distribution, detection and remediation through physical and chemical-biological methods. Sci. Total Environ. 916, 170254 (2024).
Bashar, K. et al. Faunistic study of odonata (dragonfly & damselfly) in some selected regions of Bangladesh. J. Entomol. Zool. Stud. 2 (2014). https://www.researchgate.net/publication/263807688
Ferreras-Romero, M., Márquez-Rodríguez, J. & Ruiz-García, A. Implications of anthropogenic disturbance factors on the Odonata assemblage in a Mediterranean fluvial system. Int. J. Odonatol. 12, 413–428 (2009).
Nayak, A. K. & Roy, U. S. An observation on the Odonata fauna of the Asansol-Durgapur Industrial Area, Burdwan, West Bengal, India. J. Threat Taxa 8, 8503–8517 (2016).
Berliani, N., Kardiman, R. & Satria, R. Vauzia Species diversity of Odonata as a bioindicator of water pollution in the Batang Harau watershed, Tanah Datar District, West Sumatra. In IOP Conference Series: Earth and Environmental Science vol. 1346 (Institute of Physics, 2024).
Crocothemis servilia (Drury, 1770) (2024). https://indiabiodiversity.org/species/show/234361 (accessed 22.
Nummelin, M., Lodenius, M. & Tulisalo, E. Water striders (Heteroptera, Gerridae) as bioindicators of heavy metal pollution. Entomol. Fenn. 8, 185–191 (1997).
Maneechan, W. & On Prommi, T. Occurrence of microplastics in edible aquatic insect Pantala sp. (Odonata: Libellulidae) from rice fields. PeerJ 10, 1–13 (2022).
Maneechan, W. & On Prommi, T. Occurrence of microplastics in edible aquatic insect Pantala sp. (Odonata: Libellulidae) from rice fields. PeerJ 10 (2022).
Sivagnaname, N. Selective and frequency dependent predation of aquatic mosquito predator Diplonychus indicus Venkatesan & Rao (Hemiptera: Belostomatidae) on immature stages of three mosquito species. Entomol. Res. 39, 356–363 (2009).
Aditya, G., Bhattacharyya, S., Kundu, N., Saha, G. K. & Raut, S. K. Predatory efficiency of the water bug Sphaerodema annulatum on mosquito larvae (Culex quinquefasciatus) and its effect on the adult emergence. Bioresour. Technol. 95, 169–172 (2004).
Ranjini, S., Kakkassery, F. K., Predatory potential of water bugs against the filarial vector Culex quinquefasciatus say. Indian J. Entomol. 81, 40–43 (2019).
Brahma, S. et al. Influence of density on intraguild predation of aquatic Hemiptera (Heteroptera): Implications in biological control of mosquito. J. Entomol. Acarol. Res. 46, 6 (2014).
Singh, R., Dhiman, R. & Singh, S. Laboratory studies on the predatory potential of dragon-fly nymphs on mosquito larvae. J. Commun. Dis. (2003).
Nasiruddin, M. & Banik, M. Food and feeding of the nymphs of Brachythemis contaminata Fabricius (Odonata: Anisoptera) (1989). https://www.researchgate.net/publication/343267697_FOOD_AND_FEEDING_OF_THE_NYMPHS_OF_BRACHYTHEMIS_CONTAMINATA_FABRICIUS_ODONATA_ANISOPTERA Accessed 11 Dec 2024.
Bhusnar, A. R. & Sathe, T. V. Biology of a dragaonfly Crocothemis Servilia servilia drury (Odonata: Libellulidae), a predator of paddy pests in Kolhapur. IOSR J. Pharm. Biol. Sci. 12, 18–20 (2017).
Rahman, K. M. Z. et al. Predatory efficiency of dragonfly nymphs, Crocothemis servilia and Rhyothemis variegata against the mosquito, Culex quinquefasciatus say. Asian-Aust. J. Biosci. Biotechnol. 7, 82–89 (2022).
Gabriel, A. D. & Bacosa, H. P. Microplastic abundance and distribution in the sediment of Cagayan De Oro River, Philippines. Soil. Sediment. Contam. 00, 1–17 (2024).
Baensch-Baltruschat, B., Kocher, B., Stock, F. & Reifferscheid, G. Tyre and road wear particles (TRWP) - a review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 733, 137823 (2020).
Ding, R. et al. Occurrence and distribution of microplastics in the adjacent environment of Yellow River Delta, China. Mar. Pollut. Bull. 199 (2024).
Akdogan, Z., Guven, B. & Kideys, A. E. Microplastic distribution in the surface water and sediment of the Ergene River. Environ. Res. 234, 116500 (2023).
Kumar, M. et al. Sediment-associated microplastics in Chilika Lake, India: Highlighting their prevalence, polymer types, possible sources, and ecological risks. Sci. Total Environ. 914, 169707 (2024).
Gabriel, A. D. & Bacosa, H. P. Microplastic abundance and distribution in the sediment of Cagayan De Oro River, Philippines. Soil. Sediment. Contam. https://doi.org/10.1080/15320383.2023.2301495 (2024).
Lei, L. et al. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 619–620, 1–8 (2018).
Au, S. Y., Bruce, T. F., Bridges, W. C. & Klaine, S. J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 34, 2564–2572 (2015).
Imhof, H. K. & Laforsch, C. Hazardous or not – are adult and juvenile individuals of Potamopyrgus antipodarum affected by non-buoyant microplastic particles? Environ. Pollut. 218, 383–391 (2016).
Berglund, E., Fogelberg, V., Nilsson, P. A. & Hollander, J. Microplastics in a freshwater mussel (Anodonta anatina) in Northern Europe. Sci. Total Environ. 697, 134192 (2019).
Magni, S. et al. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ. 631–632, 778–788 (2018).
Redondo-Hasselerharm, P. E., Falahudin, D., Peeters, E. T. H. M. & Koelmans, A. A. Microplastic effect thresholds for freshwater benthic macroinvertebrates. Environ. Sci. Technol. 52, 2278–2286 (2018).
Colomer, J., Müller, M. F., Barcelona, A. & Serra, T. Mediated food and hydrodynamics on the ingestion of microplastics by Daphnia magna. Environ. Pollut. 251, 434–441 (2019).
Blarer, P. & Burkhardt-Holm, P. Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus Fossarum. Environ. Sci. Pollut. Res. 23, 23522–23532 (2016).
Wang, J., Wang, M., Ru, S. & Liu, X. High levels of microplastic pollution in the sediments and benthic organisms of the South Yellow Sea, China. Sci. Total Environ. 651, 1661–1669 (2019).
Han, W., Gao, G., Geng, J., Li, Y. & Wang, Y. Ecological and health risks assessment and spatial distribution of residual heavy metals in the soil of an e-waste circular economy park in Tianjin, China. Chemosphere 197, 325–335 (2018).
Lithner, D., Larsson, A. & Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 409, 3309–3324 (2011).
Bellasi, A. et al. Microplastic Contamination in Freshwater environments: A review, focusing on interactions with sediments and benthic organisms. Environ. 2020. 7, 30 (2020).
López-Rojo, N., Pérez, J., Alonso, A., Correa-Araneda, F. & Boyero, L. Microplastics have lethal and sublethal effects on stream invertebrates and affect stream ecosystem functioning. Environ. Pollut. 259, (2020).
Fudlosid, S. et al. Ingestion of microplastic fibres, but not microplastic beads, impacts growth rates in the tropical house cricket Gryllodes sigillatus. Front. Physiol. 13 (2022).
Zhao, L., Qu, M., Wong, G. & Wang, D. Transgenerational toxicity of nanopolystyrene particles in the range of µg L – 1 in the nematode Caenorhabditis elegans. Environ. Sci. Nano. 4, 2356–2366 (2017).
Ziajahromi, S., Kumar, A., Neale, P. A. & Leusch, F. D. L. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut. 236, 425–431 (2018).
Ramsperger, A. F. R. M. et al. Nano- and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 29, 100441 (2023).
Smith, M., Love, D. C., Rochman, C. M. & Neff, R. A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 5, 375–386 (2018).
Mowla, Q. A. & Islam, M. S. Natural Drainage System and Water Logging in Dhaka: Measures to Address the Problems. vol. 6 (2013).
Taleb, A. & Taleb, M. A. Comparative study of urban area extension and flood risk in Dhaka City of Bangladesh. Global J. Hum. Soc. Sci. 12 (2012). https://www.researchgate.net/publication/321035965
Arifuzzaman, M., Abdul Hannan, M., Redwanur Rahman, M. & Atiqur Rahman, M. Laws regulating water pollution in Bangladesh. J. Sociol. Anthropol. 3, 15–24 (2019).
Plastic, polypropylene bags banned in super shops from October 1 (2024). https://www.dhakatribune.com/bangladesh/bangladesh-environment/357807/plastic-polypropylene-bags-banned-in-super-shops (Accessed 22 oct 2024).
Polythene bag ban takes effect today: What it means for users. The Business Standard. https://www.tbsnews.net/bangladesh/environment/polythene-bag-ban-takes-effect-today-what-it-means-users-981606 (Accessed 22 Oct 2024).
Author information
Authors and Affiliations
Contributions
Credit authorship contribution statementMRH: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Roles/Writing - original draft, review, and editing.WA: Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Roles/Writing - original draft; review and editing.MAR: Sample Collection; Resources; Validation.KMZR: Conceptualization; Supervision; Validation; Visualization; Writing - review and editing.MMR: Conceptualization; Supervision; Resources; Software; Funding; Validation; Visualization; Roles/Writing – original draft; and Writing - review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Haque, M.R., Ahmed, W., Rahman, M.A. et al. Aquatic insects as mediator for microplastics pollution in a river ecosystem of Bangladesh. Sci Rep 15, 15635 (2025). https://doi.org/10.1038/s41598-025-88024-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88024-1