Abstract
This study aimed to develop and validate a nomogram, which can effectively predict the risk of contralateral asymptomatic femoral head collapse in patients with bilateral osteonecrosis of the femoral head (ONFH), undergoing unilateral total hip arthroplasty (THA). We retrospectively analyzed the clinical data of patients who underwent unilateral THA for bilateral non-traumatic ONFH in our center from 2015 to 2018. A total of 103 patients participated in at least 5 years of follow-up. The patients were randomly divided into a training set (70%) and a validation set (30%). Univariate and multivariate Cox analyses were used to determine the independent risk factors for contralateral femoral head collapse. Based on these factors, a predictive nomogram model for 3, 4, and 5 years after THA was developed, and the model was evaluated using receiver operating characteristic (ROC) curve analysis, area under the curve (AUC), decision curve analysis (DCA), and calibration curves. Among the103 patients, 64 patients (62.1%) experienced contralateral femoral head collapse after surgery. Independent risk factors included Japanese investigation committee (JIC) types C1 and C2, lower limb length difference, CE angle, and Harris hip score (HHS) one month after the primary THA. The AUC, calibration curves, and DCA for the predictive model at 3, 4, and 5 years demonstrated good performance of the nomogram. The predictive nomogram model shows good accuracy and clinical utility. Using this tool, clinicians can accurately judge the collapse of the contralateral asymptomatic femoral head after unilateral THA in patients with bilateral ONFH, and they can formulate individualized treatment plans.
Similar content being viewed by others
Introduction
Osteonecrosis of the femoral head (ONFH) is a common refractory and disabling disease in orthopedics. The precise pathogenesis of ONFH remains unclear, and it is associated with various etiological factors, such as the administration of systemic corticosteroids, trauma, alcoholism, abnormal coagulation function, and so on1. These factors contribute to ischemic necrosis and subsequent repair processes in the bone tissue and bone marrow constituents2. The disease tends to occur in young and middle-aged people. In the United States, it accounts for an annual incidence of 100,000 to 200,000 new cases3, with a staggering bilateral occurrence rate of 63–70%4,5. ONFH happens pretty insidiously. It is characterized as a chronic and progressive disease that follows the pattern of necrosis and collapse, eventually leading to hip osteoarthritis6. A survey from the Association of Hip and Knee Surgeons, reported that total hip arthroplasty (THA) is the most common intervention for treating ONFH in the post-collapse stages7. Total hip arthroplasty (THA) is the only proven treatment option to alleviate pain and restore mobility, employed for elderly patients and those with advanced-stage arthritis, after extensive femoral head collapse3,8.
In recent years, clinicians have predominantly opted for simultaneous or staged THA for patients with bilateral ONFH. Among patients with unilateral femoral head collapse and an intact contralateral femoral head, approximately 22–35% of individuals will eventually undergo contralateral THA due to disease progression9,10. Considering that ONFH predominantly affects younger patients, both patients and orthopedic surgeons tend to prioritize preserving the native joint whenever possible11. Therefore, simultaneous THA is typically chosen for patients experiencing persistent bilateral hip pain caused by femoral head collapse. In contrast, unilateral THA is performed when symptoms are limited to one side or the patient prefers a two-stage surgical approach12. Furthermore, currently, most authors agree that simultaneous bilateral THA should only be performed under strict inclusion criteria, which generally include patients under the age of 70 to 80 years, without significant cardiopulmonary comorbidities, and with an American Society of Anesthesiologists (ASA) score of 1 or 213. Recent research has provided compelling evidence supporting the favorable outcomes associated with simultaneous bilateral THA14. This approach offers benefits such as reduced cumulative hospital stay, diminished overall treatment expenses, and a decreased risk of anesthesia-related complications within a single operative intervention15. Currently, some scholars propose that only a small fraction of asymptomatic ONFH cases progress to collapse, particularly in patients with small to moderate lesions, warranting close monitoring16. Moreover, some scholars believe it is optimal to preserve the hip joint by delaying or halting disease progression at the early stage of ONFH17. This can be achieved through core decompression combined with non-surgical treatments or vascularized bone grafting to restore the femoral head structure and mechanical function, promote the regeneration of arterial vascular networks and new bone tissue, and maintain the stability and functionality of the intraosseous microenvironment18. However, some patients may still experience collapse as the disease progresses, leaving the optimal treatment strategy a subject of ongoing debate19. Therefore, it is crucial to identify the factors influencing ONFH progression. To our knowledge, no study has explored a prediction model for contralateral asymptomatic femoral head collapse following unilateral THA in patients with bilateral ONFH. Nomograms are straightforward visual tools that integrate independent disease-related risk factors, transforming complex predictive models into quantitative measures20. In recent years, they have been widely used to assess tumor prognosis, predict the incidence of chronic orthopedic diseases, and estimate the risk of surgical complications, with an increasing number of studies confirming their ability to provide more accurate individualized diagnosis and prognosis21. For example, the Breast Cancer Risk Assessment Tool developed by the National Cancer Institute (http://www.cancer.gov/bcrisktool) employs statistical models to calculate an individual’s risk of invasive breast cancer. Hence, the objective of this study is to develop and validate a nomogram that predicts the risk of asymptomatic contralateral femoral head collapse after unilateral THA in patients with bilateral ONFH. This endeavor aims to provide clinicians with a comprehensive understanding of the factors influencing ONFH outcomes, serving as a significant stride toward the indispensable realm of precision medicine.
Materials and methods
Patients selection and recruitment
This retrospective study utilized data from patients diagnosed with bilateral nontraumatic ONFH who underwent unilateral THA at the Department of Orthopedics, Affiliated Hospital of Beihua University, between January 2015 and January 2018. The diagnosis followed the ONFH diagnostic criteria, which included a comprehensive clinical physical examination, as well as assessment by magnetic resonance imaging (MRI) and X-ray22. Routine follow-up evaluations are arranged at one month postoperatively, followed by assessments at three and six months thereafter. Subsequent evaluations are conducted every six months or promptly after the onset of pain episodes23.A follow-up period of 5 years or until the femoral head collapsed radiographically after surgery was conducted, whichever came first. A total of 103 individuals, aged 35–70, were randomly divided into a training set (70%, n = 72) and a validation set (30%, n = 31). The training set was used for model development, while the validation set was used to internally validate the predictive capability of the developed model. Informed consent was obtained from patients or their families. (The study did not involve human subjects or infringe upon personal privacy, hence the absence of informed consent requirements.)
Inclusion and exclusion criteria
The inclusion criteria were as follows
(1) Patients aged ≥ 18 years old, diagnosed with bilateral nontraumatic ONFH. (2) Patients undergoing unilateral THA for the first time, with asymptomatic contralateral ONFH at Association Research Circulation Osseous (ARCO) stage I-II without collapse, received temporary conservative treatment. (3) All primary THA procedures were performed by two experienced surgeons using the posterolateral approach. (4) Due to subjective neglect or economic constraints, no effective intervention was taken by the patient for the contralateral ONFH. (5) Complete clinical data of patients were available.
The exclusion criteria were as follows
(1) Impaired limb function is frequently observed in patients with underlying neurological and muscular diseases. (2) Patients with uneven lower limb lengths due to other preoperative conditions, such as knee and ankle. (3) The occurrence of serious complications such as infection, dislocation, periprosthetic fracture, and prosthetic loosening was noted following the initial THA. (4) Patients who required revision surgery during the follow-up period were excluded from the analysis. (5) Individuals who underwent hip preservation surgery on the non-affected side were excluded from the study.
Data collection
Clinical indices
The electronic medical record system was utilized to collect data from all eligible patients’ general information, including gender, age, body mass index (BMI), etiology, the side of the primary THA, the ARCO stage of the contralateral hip24, Japanese Investigation Committee(JIC) type25, and the Harris hip score (HHS) one month after the primary THA. The JIC classification comprises four types: A, B, C1, and C2. The occurrence of segmental collapse of the contralateral femoral head was a primary trial endpoint. Femoral head collapse was defined as a radiolucent subchondral fracture line visible on X-ray films, including the “crescent sign”, or a depression of the articular surface26. The HHS was recorded to assess the hip function of the primary THA side. A double-blind method was utilized by two experienced radiologists and orthopedic surgeons to identify the presence of subchondral collapse or necrosis cases in mid-to-advanced stages. A flow diagram of the study design is shown in (Fig. 1).
Imaging metrics
Following the primary THA, weight-bearing anteroposterior X-ray films of both hips were routinely taken. All plain radiographs were taken by radiology technologists using standardized techniques. The main measurement metrics: (1) Lower limb length difference27. (2) Sharp Angle28. (3) Center–edge (CE) Angle29. (4) Medial space ratio (MSR)30. (5) Acetabular depth ratio (ADR)31. (6) Leg length difference (Whether the length of the operated leg is greater than the contralateral leg). (Fig. 2).
Statistical analysis
Statistical analyses were performed using R programming (R 4.3.0, http://www.rproject.org) and IBM SPSS Statistics (IBM Corp, v26.0, Armonk, NY, USA). The distribution of patients between the training and validation sets was compared using the t-test or chi-square test. Univariate and multivariate Cox analysis was conducted on the training set to identify independent risk factors influencing femoral head collapse. The optimal cut-off values for independent imaging factors associated with contralateral femoral head collapse were determined using X-tile software (Yale University, United States). A prediction nomogram based on these independent risk factors was developed using the “rms” package in R programming. Based on the characteristics of femoral head collapse time points reported in previous literature32,33 and our center’s experience, we chose 3 years (36 months), 4 years (48 months), and 5 years (60 months) as the time points. The model’s performance was assessed by plotting a receiver operating characteristic (ROC) curve and calculating the area under the curve (AUC), and calibration curves and decision curve analysis (DCA) were generated to evaluate the predictive reliability of the model. The patients were categorized into three risk groups (high, medium, and low) using the established nomogram and X-tile software. Survival differences among the risk groups were compared using Kaplan-Meier survival curves and the Log-Rank test. Statistical significance results were set at P < 0.05.
Results
Following the predefined inclusion and exclusion criteria, a total of 103 patients with asymptomatic non-operated hips, aged between 35 and 70, were enrolled in this study. The collapse rates of the contralateral femoral head following unilateral THA were as follows: at 3 years, 22.3% (23/103); at 4 years, 39.8% (41/103); and at 5 years, 62.1% (64/103). According to the JIC type, the collapse rates for types A, B, C1, and C2 at five years were 22.7% (5/22), 60.9% (14/23), 73.7% (28/38), and 85.0% (17/20). The patients were randomly divided into a training set (72 cases, 70%) and a validation set (31 cases, 30%) at a ratio of approximately 7:3. The baseline characteristics of all 103 patients, as shown in Table 1, did not exhibit any statistically significant differences between the two sets (P > 0.05). (Table 1).
Univariate and multivariate Cox analysis
In order to identify the risk factors associated with contralateral femoral head collapse after unilateral THA in bilateral ONFH patients, we conducted a univariate Cox regression analysis on 14 potential predictors. The analysis revealed that eight predictors showed a significant correlation with contralateral femoral head collapse, including mean BMI (p = 0.023), etiology (p = 0.049), ARCO stage of the contralateral femoral head (p = 0.038), JIC type (C1: p = 0.008, C2: p = 0.002), CE Angle (p = 0.004), HHS of the operated hip one month after surgery (p = 0.002), and leg length discrepancy (p = 0.036). Subsequently, we performed multivariate Cox regression analysis on these significant variables, resulting in the identification of four potential independent risk factors: JIC type (C1:HR 6.093,95% CI 1.700–21.836, p = 0.006; C2: HR 11.095, 95% CI 2.965–41.521, p < 0.001), lower limb length difference (HR 1.209, 95% CI 1.013–1.443, p = 0.035), CE Angle (HR 0.843, 95% CI 0.734–0.968, p = 0.016), and HHS of the operated hip joint one month after surgery (HR 0.894, 95% CI 0.825–0.968, p = 0.006) (Table 2).
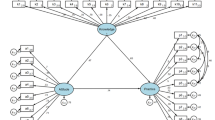
Nomogram creation and verification
Based on the results of multivariate Cox regression analysis, x-tile software was used to find the optimal cut-off value for the independent prognostic related factors, such as postoperative length difference of both lower limbs, CE angle of the contralateral femoral head and HHS3 one month after the surgical hip joint operation, and then they were grouped, and then the JIC type of the contralateral femoral head was included in the nomogram (Fig. 3).The results indicate that the JIC type emerged as the most influential risk factor, followed by the leg length discrepancy. Subsequently, ROC curves were constructed for the training set and the validation set, respectively, for the third, fourth, and fifth years, to evaluate the diagnostic efficacy of the nomogram model (Fig. 4). Furthermore, calibration curves and DCA were performed on the training set and the validation set, demonstrating the favorable consistency and clinical applicability of the nomogram in predicting the collapse rate of the contralateral femoral head in the third, fourth, and fifth years (Figs. 5 and 6).
The sample size corresponding to the variable is reflected by the rectangular area corresponding to the variable in the figure. For every patient in the nomogram, four lines are drawn upward to calculate the points received from the four predictors. The sum of the points is located on the “Total Points” axis. Then, a vertical line was drawn from the total points scale to the Pr axis to obtain the probability.
Calibration curve, and DCA curve analysis of the nomogram training set at 3–5 years. (A) 3-year survival prediction calibration curve; (B) 4-year survival prediction calibration curve; (C) 5-year survival prediction calibration curve; (D) 3-year survival prediction DCA; (E) 4-year survival prediction DCA; (F) 5-year survival prediction DCA.
Calibration curve, and DCA curve analysis of the nomogram validation set at 3–5 years. (A) 3-year survival prediction calibration curve; (B) 4-year survival prediction calibration curve; (C) 5-year survival prediction calibration curve; (D) 3-year survival prediction DCA; (E) 4-year survival prediction DCA; (F) 5-year survival prediction DCA.
Comparison of the ROC curve between the nomogram and independent prognostic factors
The “survival ROC” package in R software was utilized to construct ROC curves for the four independent prognostic factors, as well as the nomogram, at the 3rd, 4th, and 5th years. The results demonstrated that all four independent factors exhibited some degree of predictive capability, with an AUC greater than 0.5 (Fig. 7). Nevertheless, whether in the training set or the validation set, the AUC value of the nomogram consistently surpassed that of any individual influencing factor. Consequently, the nomogram, which integrates these four variables, demonstrates superior predictive power in comparison to each variable alone when forecasting the collapse of the contralateral femoral head.
The prognostic accuracy of the nomogram was estimated by the independent influencing factors of the training and validation sets 3–5 and the ROC curve and AUC of the nomogram. (A) ROC curve of 3-year training set; (B) ROC curve of 4-year training set; (C) ROC curve of 5-year training set; (D) ROC curve of 3-year validation set; (E) ROC curve of 4-year validation set; (F) ROC curve of 5-year validation set.
Nomogram-based risk stratification system
By employing a nomogram, individual patients were assigned a risk score, and X-tile software was employed to determine two cut-off values (168 points and 219 points). Consequently, patients in the training set and the validation set were classified as low risk (total score < 168), medium risk (168 ≤ total score < 219), and high risk (total score ≥ 219). Within the training set, the distribution of patients across high, medium, and low-risk groups was respectively as follows: 9, 29, and 34. Survival curve analysis revealed significant differences in prognosis among the three groups (P < 0.0001) (Fig. 8A). The same stratification method was applied to patients in the validation set, resulting in 6, 10, and 15 patients categorized as high, medium, and low risk, respectively. Survival curve analysis revealed significant differences in prognosis among the three groups (P = 0.00016) (Fig. 8B). Thus, the nomogram-based risk stratification system effectively discerns the risk of contralateral femoral head collapse.
Discussion
Understanding the natural progression of ONFH is crucial for guiding early treatment decisions. In patients with bilateral ONFH, concerns often arise post-unilateral THA regarding the potential collapse of asymptomatic contralateral ONFH and the necessity for subsequent contralateral THA. Previous studies have investigated factors influencing the natural course of ONFH, but most of these studies have focused primarily on unilateral ONFH32,34. For patients with bilateral ONFH at different stages, there is relatively limited research data on the natural history of asymptomatic hip joints on the contralateral side following unilateral replacement surgery. Based on the above issues, this study utilized both univariate and multivariate Cox regression models to identify four independent risk factors. These factors include two unmodifiable variables during treatment: JIC classification and measured CE angle, as well as two intraoperative variables controllable and manageable by clinicians: postoperative leg length discrepancy and HHS one month after the primary THA. Based on these risk factors, we developed and validated a visualized nomogram to assess the risk of contralateral femoral head collapse in the 3rd, 4th, and 5th year following unilateral THA. The analysis using ROC curve, calibration curve, and clinical decision curve demonstrated that the nomogram based on the four aforementioned common clinical indicators exhibited robust predictive performance and good prognostic accuracy in both the training and validation cohorts. Finally, based on the risk stratification of the nomogram, it provides valuable guidance for determining the optimal treatment strategy, for the contralateral femoral head in patients with bilateral femoral head necrosis undergoing unilateral replacement. For patients with a low risk of contralateral femoral head collapse during follow-up after unilateral THA, adjunctive treatments such as hyperbaric oxygen therapy or bisphosphonates are recommended to alleviate pain and reduce the risk of collapse, with hip-preserving surgery reserved for necessary cases35,36. In contrast, patients at moderate risk should undergo hip-preserving treatments, such as femoral head core decompression or vascularized bone grafting37. For high-risk patients, in addition to traditional hip-preserving treatments, more aggressive approaches, such as cell-based regenerative therapies following core decompression or proximal femoral osteotomy, may be considered38,39.
Although there are many staging and typing methods for ONFH, such as Arco staging and Ficat staging. But now more and more scholars prefer to use JIC type, on the one hand, because the reproducibility, reliability and accuracy of JIC type are higher than other types, on the other hand, because the location of the necrotic area in the femoral head is an important factor affeting the prognosis of ONFH40. JIC type can ensure easy judgment and effectively evaluate the prognosis of diseases with different necrotic locations through four types A, B, C1 and C241. In a prospective study of 81 ONFH patients with asymptomatic hips and at least 5 years of follow-up by Min et al., the risk of collapse was higher in C2 lesions (86%) compared with C1 lesions (14%), suggesting that C2 necrosis is an independent risk factor for femoral head collapse33. Similarly, a retrospective analysis by Kuroda et al., involving 505 ONFH patients, revealed a collapse rate of 0% in type A hips, 7.9% in type B hips, 36.6% in type C1 hips, and 84.8% in type C2 hips42. In our study, the C2 type emerged as the most influential risk factor. Kaplan-Meier analysis of contralateral hip JIC type yielded similar results, showing a progressive decrease in the average survival time of the contralateral femoral head from JIC B to C2. Although the collapse trend from type A to C2 hips is consistent with previous research findings, our study reported collapse rates of 22.7% for type A hips, 60.9% for type B hips, 73.7% for type C1 hips, and 85.0% for type C2 hips. The reasons for the observed differences in our analysis are as follows: Firstly, our study included patients with bilateral ONFH who all underwent unilateral THA, which differs from previous research in terms of inclusion criteria. Current studies have also confirmed that the disease progression of the collapsed side of bilateral ONFH can affect the natural history of the femoral head43. Secondly, a study by Kim et al. found that post-THA, having a length discrepancy of ≥ 3 mm between the bilateral lower limbs was a significant accelerating factor for contralateral femoral head collapse. Patients with a postoperative limb length difference of ≥ 3 mm had a collapse rate of up to 68% at 5 years44. However, in the study by Kuroda et al., all patients did not undergo unilateral THA surgery, thus there was no occurrence of postoperative limb length discrepancy. Lastly, an early increase in weight-bearing on the contralateral limb postoperatively may also contribute to the acceleration of collapse45. This could also explain why the overall collapse rate in our study at 5 years, which was 62.1%, was higher than that reported in studies by Nishii et al. (52%) and Mont et al. (49%)32,46.
HHS is widely used to assess improvement in joint function and quality of life after THA47, showing commendable reliability and validity48. Studies have demonstrated that unilateral THA significantly improves the range of motion in patients with hip osteoarthritis on the contralateral side. Yusuke et al. suggested that pain in the collapsed hip accelerates the progression of contralateral hip osteoarthritis43. Early consideration of THA might alleviate the burden on the contralateral hip and impede collapse. Yoshii et al. reported a 20–25% improvement in the flexion range of the non-operated side during the last follow-up after unilateral THA49. Takegami et al. followed 195 patients with bilateral hip osteoarthritis and found that daily activities and flexion capacity of the contralateral hip joint improved one year after unilateral THA50. These studies demonstrate that the function of one hip joint can influence the function of the contralateral hip joint. However, there is limited literature quantifying hip joint function after THA to assess the risk of contralateral femoral head collapse. This study revealed that HHS is an important factor affecting contralateral femoral head collapse one month after replacement, with patients scoring below 70 on the HHS being at a higher risk. This association may be attributed to increased reliance on the contralateral limb during early postoperative rehabilitation, resulting in a shift in the body’s center of gravity and subsequently increased weight bearing on the contralateral limb. Such increased weight bearing is likely to accelerate the progression of femoral head collapse45. While most patients are capable of full weight bearing after one month51, the trend of contralateral femoral head collapse is irreversible. As the function of the operated limb and hip joint is fully restored, the patient’s static center of gravity may exceed the preoperative equilibrium point and shift towards the operated side, partially delaying the progression of the contralateral side52. Other studies have demonstrated the safety of immediate full weight bearing after THA and the efficacy of early supervised maximal strength training (MST) within one week of THA to improve muscle strength in the operated lower limb51. Therefore, we recommend early initiation of rehabilitation after THA to facilitate early improvement in HHS and partially delay the progression of contralateral femoral head collapse, thus optimizing patient outcomes.
Lower limb length difference is a common complication after THA and can result in symptoms such as waist pain, groin pain, abnormal gait, and limb numbness53,54 Therefore, it is recommended to minimize the difference in leg length during THA procedures. Kim et al. conducted a retrospective analysis on 121 patients with bilateral nontraumatic ONFH, and found that a length difference of ≥ 3 mm between the bilateral lower limbs and having a shorter contralateral limb were more likely to lead to contralateral femoral head collapse44. Swaminathan et al. found that it has been observed that when both lower limbs are of equal length, the left limb bears 54% of the body load. When the left lower limb is longer than the right lower limb, the load borne by the left limb decreases to 39%. Conversely, when the left lower limb is shorter than the right lower limb, the load borne by the left limb increases to 65%55. Consequently, it is crucial to avoid excessive lengthening of the surgical side’s lower limb to prevent or slow the progression of contralateral femoral head disease resulting from increased load. In our study, when the difference in lower limb length was ≥ 4.3 mm, the collapse rate of the contralateral femoral head significantly increased. we recommend avoiding reducing the difference in the length of the lower limbs by more than 4.3 mm during THA to prevent or accelerate the progression of the contralateral femoral head condition due to increased load. However, in the multivariate analysis, we did not find that having a shorter contralateral limb than the replacement side was a risk factor for contralateral femoral head collapse, which differs from the findings of previous studies. Accurately measuring leg length discrepancies can be challenging. While computed tomography (CT) is the most reliable method56, it is costly and has limited clinical application. Currently, full-length X-ray films of the lower limbs are commonly used, but it can be difficult to ensure that every patient receives a full-length film. In our study, we employed the indirect method described by Woolston et al.27 to measure the length of both lower limbs. The discrepancy in our analysis results may be attributed to the measurement error associated with this indirect method.
Whilst MRI play a key role in preoperative therapeutic guidance, their utility is tempered by protracted scheduling and fiscal demands associated appointment scheduling. Consequently, radiographic assessment employing X-ray modality is employed to ascertain the relative angular disposition of the contralateral hip joint. The center-edge angle indicates the angle between a line drawn through the center of the femoral head and a line drawn from the center of the femoral head to the outer edge of the acetabulum. This angle is related to the contact area between the acetabulum and the femoral head57. When the necrotic area remains unchanged, a lower acetabular coverage (smaller CE angle) results in a more unfavorable JIC type. Taketa et al. conducted a retrospective study involving 97 patients with hip dysplasia, revealing that acetabular dysplasia represents a risk factor for ONFH, with a higher incidence in patients with smaller CE angles58. Additionally, certain studies have demonstrated that reduced acetabular coverage can increase pressure within the hip joint capsule and accelerate the progression of ONFH59,60. Swarup et al. identified that a smaller CE angle reduces the weight-bearing area of the femoral head, leading to cartilage wear61. Additionally, a retrospective study by Roush et al. indicated that nontraumatic osteonecrosis of the femoral head (ONFH) patients with CE angles less than 30° continued to exhibit a high rate of femoral head collapse following free vascularized fibular grafting surgery62. They suggest that patients with less acetabular coverage experience higher pressure on the femoral head. This is similar to our study findings; patients with a contralateral hip CE angle < 29.5° had a higher risk of femoral head collapse. Therefore, we believe that following nontraumatic ONFH, a smaller CE angle may lead to reduced acetabular coverage, increased pressure on the femoral head region, and dynamic instability. These unfavorable morphological factors may contribute to the progression of osteonecrosis.
Conclusions
This study developed and validated a nomogram for predicting contralateral asymptomatic femoral head collapse in patients who underwent unilateral THA for bilateral ONFH. The JIC classification is a strong predictor for contralateral femoral head collapse. However, the nomogram, which includes JIC type, CE angle, lower limb length difference, and Harris hip score one month post-surgery, offers improved accuracy, clinical utility, and precise prognostic capabilities. It serves as a convenient and reliable tool for forecasting the natural progression of contralateral femoral head collapse. This assists clinicians in identifying high-risk patients and potentially reducing the incidence of contralateral femoral head collapse.
Limitations
This study has several limitations that should be acknowledged. Firstly, as a retrospective study, it is susceptible to selection bias since the majority of patients sought medical attention only after experiencing clinical symptoms. Conducting a prospective study could yield more objective results. Secondly, the measurement of leg length discrepancy using anteroposterior plain radiographs may not provide as precise results as those obtained through CT scans or standing leg length measurements. Thirdly, the sample size is limited, the follow-up period is relatively short, and the findings may primarily reflect the characteristics of patients in our specific hospital setting. To enhance the reliability of the nomogram, it is recommended to validate it using data from multiple centers.
Data availability
The data for the current study used for statistical analysis are available from the corresponding author upon reasonable justification.
References
Larson, E., Jones, L. C., Goodman, S. B., Koo, K. H. & Cui, Q. Early-stage osteonecrosis of the femoral head: where are we and where are we going in year 2018? Int. Orthop. 42, 1723–1728 (2018).
Petek, D., Hannouche, D. & Suva, D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open. Rev. 4, 85–97 (2019).
Moya-Angeler, J., Gianakos, A. L., Villa, J. C., Ni, A. & Lane, J. M. Current concepts on osteonecrosis of the femoral head. World J. Orthop. 6, 590–601 (2015).
Hauzeur, J. P., Malaise, M. & de Maertelaer, V. A prospective cohort study of the clinical presentation of non-traumatic osteonecrosis of the femoral head: spine and knee symptoms as clinical presentation of hip osteonecrosis. Int. Orthop. 40, 1347–1351 (2016).
Osawa, Y. et al. Do femoral head collapse and the contralateral condition affect patient-reported quality of life and referral pain in patients with osteonecrosis of the femoral head? Int. Orthop. 42, 1463–1468 (2018).
Wang, P. et al. The role of structural deterioration and biomechanical changes of the necrotic lesion in collapse mechanism of osteonecrosis of the femoral head. Orthop. Surg. 14, 831–839 (2022).
McGrory, B. J. et al. Current practices of aahks members in the treatment of adult osteonecrosis of the femoral head. J. Bone Joint Surg. Am. 89, 1194–1204 (2007).
Park, J. W. et al. Trends in surgical treatment of femoral head osteonecrosis in South Korea: an analysis using nationwide claims database. Clin. Orthop. Surg. 14, 500–506 (2022).
Sayeed, S. A., Trousdale, R. T., Barnes, S. A., Kaufman, K. R. & Pagnano, M. W. Joint arthroplasty within 10 years after primary charnley total hip arthroplasty. Am. J. Orthop. 38, E141–E143 (2009).
Sanders, T. L., Maradit, K. H., Schleck, C. D., Larson, D. R. & Berry, D. J. Subsequent total joint arthroplasty after primary total knee or hip arthroplasty: a 40-year population-based study. J. Bone Joint Surg. Am. 99, 396–401 (2017).
Atilla, B., Bakircioglu, S., Shope, A. J. & Parvizi, J. Joint-preserving procedures for osteonecrosis of the femoral head. EFORT Open. Rev. 4, 647–658 (2019).
Kim, S. C. et al. Surgical accuracy, function, and quality of life of simultaneous versus staged bilateral total hip arthroplasty in patients with osteonecrosis of the femoral head. BMC Musculoskelet. Disord. 18, 266 (2017).
Serino, J. R. et al. Contralateral total hip arthroplasty staged within six weeks increases the risk of adverse events compared to unilateral surgery. J. Arthroplast. 38, S314–S318 (2023).
Rasouli, M. R. et al. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J. Arthroplast. 29, 142–148 (2014).
Ramezani, A. et al. Simultaneous versus staged bilateral total hip arthroplasty: a systematic review and meta-analysis. J. Orthop. Surg. Res. 17, 392 (2022).
Nam, K. W. et al. Fate of untreated asymptomatic osteonecrosis of the femoral head. J. Bone Joint Surg. Am. 90, 477–484 (2008).
Mont, M. A., Salem, H. S., Piuzzi, N. S., Goodman, S. B. & Jones, L. C. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A 5-year update. J. Bone Joint Surg. Am. 102, 1084–1099 (2020).
Quan, H. et al. Application of biomaterials in treating early osteonecrosis of the femoral head: Research progress and future perspectives. Acta Biomater. 164, 15–73 (2023).
Kuroda, Y., Matsuda, S. & Akiyama, H. Joint-preserving regenerative therapy for patients with early-stage osteonecrosis of the femoral head. Inflamm. Regen. 36, 4 (2016).
Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: more than meets the eye. Lancet Oncol. 16, e173–e180 (2015).
Wang, X. et al. From past to future: bibliometric analysis of global research productivity on nomogram (2000–2021). Front. Public Health 10, 997713 (2022).
Zhao, D. et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J. Orthop. Translat. 21, 100–110 (2020).
Hernigou, P., Poignard, A., Nogier, A. & Manicom, O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J. Bone Joint Surg. Am. 86, 2589–2593 (2004).
Yoon, B. H. et al. The 2019 revised version of association research circulation osseous staging system of osteonecrosis of the femoral head. J. Arthroplast. 35, 933–940 (2020).
Sugano, N. et al. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J. Orthop. Sci. 7, 601–605 (2002).
Huang, Z. et al. Chinese herbal Huo-Gu formula for the treatment of steroid-associated osteonecrosis of femoral head: a 14-year follow-up of convalescent SARS patients. J. Orthop. Translat. 23, 122–131 (2020).
Woolson, S. T. Leg length equalization during total hip replacement. Orthopedics 13, 17–21 (1990).
Heimer, C. et al. Rotational abnormalities in dysplastic hips and how to predict acetabular torsion. Eur. Radiol. 32, 8350–8363 (2022).
Jiang, Y. et al. Computer-aided system application value for assessing hip development. Front. Physiol. 11, 587161 (2020).
Lin, T. et al. Relationship between hip joint medial space ratio and collapse of femoral head in non-traumatic osteonecrosis: a retrospective study. J. Hip Preserv. Surg. 8, 311–317 (2021).
Huang, K. et al. Borderline developmental dysplasia of the hip: a risk factor predicting the development and poor prognosis after core decompression for idiopathic osteonecrosis of the femoral head. Orthop. Surg. 14, 2427–2435 (2022).
Mont, M. A., Zywiel, M. G., Marker, D. R., McGrath, M. S. & Delanois, R. E. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J. Bone Joint Surg. Am. 92, 2165–2170 (2010).
Min, B. W., Song, K. S., Cho, C. H., Lee, S. M. & Lee, K. J. Untreated asymptomatic hips in patients with osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 466, 1087–1092 (2008).
Osawa, Y. et al. Extension of the antero-posterior necrotic regions associated with collapse cessation in osteonecrosis of the femoral head. J. Arthroplast. 39, 387–392 (2024).
Bosco, G. et al. Hyperbaric oxygen therapy ameliorates osteonecrosis in patients by modulating inflammation and oxidative stress. J. Enzyme Inhib. Med. Chem. 33, 1501–1505 (2018).
Li, D., Yang, Z., Wei, Z. & Kang, P. Efficacy of bisphosphonates in the treatment of femoral head osteonecrosis: a prisma-compliant meta-analysis of animal studies and clinical trials. Sci. Rep. 8, 1450 (2018).
Petek, D., Hannouche, D. & Suva, D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open Rev. 4, 85–97 (2019).
Quaranta, M., Miranda, L., Oliva, F. & Aletto, C. Maffulli, N. Osteotomies for avascular necrosis of the femoral head. Br. Med. Bull. 137, 98–111 (2021).
Piuzzi, N. S. et al. Analysis of cell therapies used in clinical trials for the treatment of osteonecrosis of the femoral head: a systematic review of the literature. J. Arthroplast. 32, 2612–2618 (2017).
Takashima, K., Sakai, T., Hamada, H., Takao, M. & Sugano, N. Which classification system is most useful for classifying osteonecrosis of the femoral head? Clin. Orthop. Relat. Res. 476, 1240–1249 (2018).
Sultan, A. A. et al. Classification systems of hip osteonecrosis: an updated review. Int. Orthop. 43, 1089–1095 (2019).
Kuroda, Y. et al. Classification of osteonecrosis of the femoral head: who should have surgery? Bone Joint Res. 8, 451–458 (2019).
Osawa, Y. et al. Collapse progression or cessation affects the natural history of contralateral osteonecrosis of the femoral head. J. Arthroplast. 36, 3839–3844 (2021).
Kim, S. C. et al. Effect of leg-length discrepancy following total hip arthroplasty on collapse of the contralateral hip in bilateral non-traumatic osteonecrosis of the femoral head. Bone Joint J. 101-B, 303–310 (2019).
Wen, P. et al. The effect of the necrotic area on the biomechanics of the femoral head—A finite element study. BMC Musculoskelet. Disord. 21, 211 (2020).
Nishii, T. et al. Progression and cessation of collapse in osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 149–157 (2002).
Riddle, D. L., Stratford, P. W., Singh, J. A. & Strand, C. V. Variation in outcome measures in hip and knee arthroplasty clinical trials: a proposed approach to achieving consensus. J. Rheumatol. 36, 2050–2056 (2009).
Soderman, P. & Malchau, H. Is the harris hip score system useful to study the outcome of total hip replacement? Clin. Orthop. Relat. Res. 189–197 (2001).
Yoshii, T. et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J. Orthop. Sci. 14, 161–166 (2009).
Takegami, Y. et al. Does unilateral total hip arthroplasty improve pain and function in the non-operative hip joint? Eur. J. Orthop. Surg. Traumatol. 30, 1411–1416 (2020).
Winther, S. B. et al. A Randomized controlled trial on maximal strength training in 60 patients undergoing total hip arthroplasty. Acta Orthop. 89, 295–301 (2018).
Tayierjiang, J., Mieralimu, M., Wang, L., Zhao, W. & Yuan, H. Correlation between shifting of gravity center position and functional recovery after total hip arthroplasty in patients who suffered from avascular necrosis of the femoral head. Zhonghua Yi Xue Za Zhi 96, 879–882 (2016).
Halai, M. et al. The exeter technique can lead to a lower incidence of leg-length discrepancy after total hip arthroplasty. Bone Joint J. 97-B, 154–159 (2015).
Hofmann, A. A. & Skrzynski, M. C. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics 23, 943–944 (2000).
Swaminathan, V., Cartwright-Terry, M., Moorehead, J. D., Bowey, A. & Scott, S. J. The effect of leg length discrepancy upon load distribution in the static phase (standing). Gait Post. 40, 561–563 (2014).
Guggenberger, R., Pfirrmann, C. W., Koch, P. P. & Buck, F. M. Assessment of lower limb length and alignment by biplanar linear radiography: comparison with supine ct and upright full-length radiography. AJR Am. J. Roentgenol. 202, W161–W167 (2014).
Hsu, J. Y. et al. Radiographic outcomes of ganz versus modified triple osteotomies in femoral head medialization and coverage in acetabular dysplasia. J. Clin. Med. 11, (2022).
Taketa, M. et al. Correlation between center-edge angle and acetabulum-head index in developmental dysplasia of the hip with avascular necrosis of the femoral head. J. Pediatr. Orthop. B 12, 215–218 (2003).
Xie, J., Naito, M. & Maeyama, A. Intracapsular pressure and interleukin-1beta cytokine in hips with acetabular dysplasia. Acta Orthop. 81, 189–192 (2010).
Wingstrand, H. Intracapsular pressure in congenital dislocation of the hip. J. Pediatr. Orthop. B 6, 245–247 (1997).
Swarup, I., Zaltz, I., Robustelli, S. & Sink, E. Outcomes of periacetabular osteotomy for borderline hip dysplasia in adolescent patients. J. Hip Preserv. Surg. 7, 249–255 (2020).
Roush, T. F., Olson, S. A., Pietrobon, R., Braga, L. & Urbaniak, J. R. Influence of acetabular coverage on hip survival after free vascularized fibular grafting for femoral head osteonecrosis. J. Bone Joint Surg. Am. 88, 2152–2158 (2006).
Acknowledgements
Our research team would like to thank Dr. Zongtai Liu for helpful discussion.
Funding
This work was supported by the 13th Five-Year Plan Science and Technology Research Project of the Jilin Province Department of Education (Grant Nos. JJKH20200066KJ, JJKH20180358KJ), the Jilin Province Higher Education Teaching Reform Research Special Project (Grant No. SJYB20250012), and the Jilin City Key Medical and Health Innovation Special Fund (Grant No. 20190403193).
Author information
Authors and Affiliations
Contributions
Data collection and analysis were performed by JZ, PT, HW. JZ, HW contributed to the data analysis and manuscript revision. The first draft of the manuscript was written by JZ. LZ and XS conceived and designed the study, and all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Hospital of Beihua University (Approval No. 20230007).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, J., Sun, X., Zhang, L. et al. A nomogram for predicting contralateral femoral head collapse after unilateral replacement of bilateral femoral head necrosis. Sci Rep 15, 5983 (2025). https://doi.org/10.1038/s41598-025-88057-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88057-6