Abstract
This multicenter, retrospective longitudinal study identified primary Sjögren’s syndrome (pSS) patients with clinically significant renal involvement, and analyzed factors associated with predisposition. To investigate clinical features and long-term prognosis of renal involvement, we compared the clinical outcomes for the entire cohort based on the presence or absence of renal involvement. Among 1306 pSS patients (mean age, 51 ± 12 years; 98% female), 36 (2.8%) had renal involvement; 17 patients had tubular involvement, 15 had glomerular involvement, one had both, and 3 were unclassified. The presence of anti-La antibodies was associated with renal involvement. Over the median 5-year follow-up period, the renal function did not change significantly; however, 44% of patients with renal involvement showed impaired renal function at the last visit. Impaired renal function at the last visit was inversely associated with baseline renal function and hemoglobin levels. Among the entire cohort, the prevalence of lymphoproliferative disease (LPD) was significantly higher in pSS patients with renal involvement than those without. Renal involvement is a rare manifestation of pSS; however, it is associated with impaired renal function and LPD. Therefore, screening for renal involvement is important for preserving renal function and early detection of LPD.
Similar content being viewed by others
Introduction
Primary Sjögren’s syndrome (pSS) is an autoimmune disease, characterized by impaired function of the exocrine glands due to lymphocyte infiltration1. The glandular epithelial cells are the primary target; thus, the histologic hallmarks include infiltration of the glandular epithelium by T cells, B cells, and plasma cells2.
Tubular interstitial nephritis (TIN) is the most common renal disease associated with pSS1. Histological findings reveal lymphoplasmacytic infiltration of the interstitium around the renal tubules, similar to that observed for the exocrine glands3. A recent study shows that lymphoplasmacytic infiltration in minor salivary gland biopsy is associated with renal lesions in patients with pSS4. Glomerulonephritis (GN) is the second most common renal manifestation5. The pathophysiological process of GN is deposition of secondary immune complexes1; in pSS, the membranoproliferative histological type is the main type of GN in pSS6.
The prevalence of renal involvement in pSS varies according to ethnicity and the definition of renal involvement. Prevalence is about 1% in large retrospective registries7,8, between 4 and 9% in most European studies1,5,9,10, and 30% or higher in Indian and Chinese studies11,12.
A limited number of studies report a heterogeneous long-term prognosis for pSS with renal involvement. One study reported that TIN in pSS does not seem to impact survival9, whereas another reported a lower 3-year survival rate for patients with GN than for those with TIN6. Regarding renal function in pSS with renal involvement, 10–30% of such patients present with chronically reduced renal function6,7,9,13.
Here, we investigated the prevalence, characteristics, treatment, and long-term prognosis of Korean patients with pSS with renal involvement. Because onset of TIN is usually insidious and without any apparent symptoms, it may remain undiagnosed1. We identified parameters suggestive of undiagnosed TIN, and subsequently defined this subgroup as having “persistent alkaline urine”. We tracked changes in renal function not only in pSS patients with significant renal involvement, but also in pSS patients with persistent alkaline urine despite the lack of clinically significant renal involvement, and those without renal involvement.
Methods
Patients
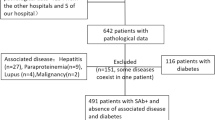
This multicenter retrospective longitudinal study enrolled patients with pSS treated at Seoul St. Mary’s Hospital, Bucheon St. Mary’s Hospital, and Uijeongbu St. Mary’s Hospital in Korea. All data were extracted from the clinical data warehouse (CDW) of The Catholic Medical Center. Based on the International Classification of Diseases (ICD) 10th revision, patients diagnosed with pSS from March 1, 2012, to February 28, 2022, were assigned code M35.0. Due to potential overlap syndromes, patients were excluded if they were positive for anti-centromere, anti-double strand DNA, anti-Smith, anti-Jo1, anti-ribonucleoprotein, or anti-citrullinated protein antibodies (Fig. 1A). The CDW provides anonymized access to electronic health records, imaging studies, and laboratory tests, therefore patients with pSS who fulfill the 2002 American-European Consensus Group classification criteria14 and/or the 2016 American College of Rheumatology/European League Against Rheumatism (EULAR) classification criteria15 were identified through an independent review of electronic health records by two board-certified rheumatologists.
Flowchart of patients screening and classification (A) Application of selection criteria and patient disposition. (B) An overview of clinical data collection over time. For each patient with renal involvement, five patients without renal involvement and three patients with persistent alkaline urine (propensity score-matched) were selected.
The study protocol was approved by the institutional review board of the Catholic Central Medical Center (UC22WIDE0042). The requirement for informed consent was waived due to the retrospective nature of the study.
Definition of renal involvement
Clinically significant renal involvement was defined according to one or more of the following criteria9,16; identification was made in the context of pSS, with no findings suggestive of another etiology.
-
1.
Metabolic acidosis and alkaline urine (urine pH ≥ 6.5) for > 6 months.
-
2.
Proteinuria ≥ 0.5 g/day or spot urine protein-to-creatinine ratio ≥ 0.5 g/g for > 6 months.
-
3.
Active urine sediment (> 10 red blood cells per high-power field or red blood cell casts).
-
4.
Impaired renal function [estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2]
-
5.
Kidney biopsy demonstrating histologic features consistent with GN and/or TIN.
Patients with proteinuria, active urinary sediment, or kidney biopsy findings consistent with GN were classified as having glomerular involvement. In contrast, patients with renal tubular acidosis or biopsy findings consistent with TIN were classified as having tubular involvement.
Kidney function was evaluated by eGFR using the CKD-EPI equation17. The eGFR was checked at the time of renal involvement identification, at each follow-up year, and at the last visit. For patients without renal involvement, the eGFR measured at the time of pSS diagnosis was recorded.
To compare longitudinal changes in renal function among patients with and without renal involvement, a case-control study was performed. Considering the possibility of underdiagnosed TIN, the “persistent alkaline urine group” was established and used as an additional control group; this group comprised patients showing persistent alkaline urine for two consecutive years, without any documented significant renal involvement. None of the patients with renal involvement had diabetes mellitus, which may affect renal function, patients with diabetes mellitus were excluded from the “without renal involvement” and “persistent alkaline urine” groups. In addition, propensity score matching was used to minimize potential selection biases. Each patient was propensity scored, based on logistic regression model addressing age at pSS diagnosis, sex, and follow-up period. For each patient with renal involvement, five patients without renal involvement and three patients with persistent alkaline urine were selected as controls (Fig. 1B).
Changes in the eGFR from baseline to the last visit were calculated and compared between groups; a relevant change in renal function was defined as a change in eGFR of 20% or more from baseline to last follow-up.
Data collection
Demographic data included sex, date of birth, date of pSS diagnosis, date of identification of renal involvement, date of last follow-up, status at last follow-up (dead or alive), and comorbidities. Malignancies were diagnosed according to the World Health Organization classifications18,19.
Laboratory parameters recorded at diagnosis included antinuclear antibody, anti-Ro/SSA, anti-La/SSB, and rheumatoid factor levels, complete blood counts, creatinine levels, electrolytes, urine pH, and the spot urine protein-to-creatinine ratio. Data missing from the baseline laboratory study were assessed as ‘Missing Completely At Random’ (Little’s test, P = 0.256); thus, all analyses were carried out using observed data, with pairwise deletion of missing values.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, or as the median and interquartile range (IQR), and categorical variables are expressed as percentages. A t-test or the Mann-Whitney U test was used to compare groups of continuous variables, while the χ2 test and Fisher’s exact test were used to compare categorical variables. Changes in the eGFR of patients with renal involvement (and their respective controls) were compared using the Wilcoxon signed-rank test.
Cox proportional hazard models were used to identify risk factors for development of renal involvement and lymphoproliferative disease in pSS. Observation time was calculated as the time of pSS diagnosis to the appearance of renal involvement and lymphoproliferative disease, respectively, or until the last follow-up visit. Variables with a P value < 0.1 in univariate analysis were entered into multivariate models. The results of these analyses are presented as hazard ratios (HRs), along with 95% confidence intervals (CIs).
In the case-control study conducted to compare renal function, the impact of clinical factors associated with impaired renal function (eGFR < 60 ml/min/1.73 m2) at the last visit was assessed using logistic regression analyses. Model assumptions were tested using residual analysis. To mitigate multicollinearity among variables in the multivariate regression model, the Variance Inflation Factor was assessed. Selection of the most appropriate regression model was based on the minimization of the Akaike Information Criterion. The results of these analyses are presented as odds ratios (ORs), along with 95% CIs.
All P values were two-sided, with P < 0.05 considered statistically significant. R 4.3.2 (R Foundation, Vienna, Austria) was used for data analysis, and graphs were drawn using GraphPad Prism 10.2 (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics
We identified 1306 patients with pSS. The mean age at the time of pSS diagnosis was 51 ± 12 years and 1274 patients (98%) were female. Among 346 patients who underwent minor salivary gland biopsy, 86% had a focus score of 1 or more.
During the median 6.2 years (IQR 3.2–10.6) follow-up, 2.8% (n = 36) of patients with pSS developed significant renal involvement. Renal disease preceded a diagnosis of pSS in six patients (17%). Nineteen patients (53%) were diagnosed with renal involvement at the time of pSS diagnosis, while eleven (30%) developed renal involvement at a median 8.7 years (IQR 4.7–14.6) post-pSS diagnosis. Bicarbonate was not measured in most cases without renal involvement. Among patients without renal involvement, 14% (n = 180) had persistent alkaline urine (pH ≥ 6.5) without evidence of metabolic acidosis.
Patients with renal involvement were diagnosed with pSS at a younger age, and had a higher prevalence of positive anti-Ro and anti-La autoantibodies than those without renal involvement (Table 1). Anemia, thrombocytopenia, and hypergammaglobulinemia were more common in pSS patients with renal involvement, than in those without (Table 1). A history of lymphoproliferative disease was more common in patients with renal involvement. EULAR Sjogren’s Syndrome Disease Activity Index (ESSDAI) data were available for 452 patients (34.6%); the data revealed that disease activity was higher in patients with renal involvement than in those without (Table 1). Multivariate Cox proportional hazard model found that the presence of anti-La antibodies increases the risk of renal involvement (Table 2).
Renal involvement in pSS
Of the 36 patients with renal involvement, 17 (47%) had tubular involvement, 15 (42%) had glomerular involvement, and one had both TIN and GN. Regarding the latter, the initial diagnosis was TIN, as determined by renal biopsy; however, 4 years later, this patient underwent a second biopsy due to a sudden decline in eGFR. This biopsy revealed coexistence of TIN and focal segmental glomerulosclerosis (FSGS) (Table 3, patient 11). The other three patients (8%) showed decreased eGFR, however, they were not classified as tubular or glomerular involvement (Table 3, patients 34–36).
The mean age at the time of diagnosis of renal involvement was 49 ± 14 years; this was similar for patients with tubular or glomerular involvement. Most patients with glomerular involvement underwent renal biopsy (94%), whereas most cases of tubular involvement were diagnosed clinically (89%).
Most patients with tubular involvement (89%) presented with distal renal tubular acidosis (RTA), although seven (44%) were asymptomatic. Nephrocalcinosis was found in nine patients, and three patients had renal colic pain. Two patients presented with hypokalemic paralysis as the first symptom of tubular involvement. The mean baseline eGFR at the time of tubular involvement diagnosis was 71.5 ± 25.5 ml/min/1.73 m2, and the mean urine pH was 7.0 ± 0.6.
Among patients with glomerular involvement, membranous glomerulonephritis (MGN) was the most prevalent (seven cases; 44%), followed by FSGS (six cases; 38%); the other two cases were proliferative GN. A case with diffuse proliferative GN had cryoglobulinemia (Table 3, patient 20). The mean baseline eGFR for patients with GN was 81.0 ± 31.3 ml/min/1.73 m2, and the median spot urine protein-to-creatinine ratio was 0.8 g/g (IQR 0.5–3.2).
Anti-La antibodies were more common in patients with tubular involvement than in those with glomerular involvement (82.4% vs. 40%, respectively, P = 0.018). There were no significant differences in hematological and biological laboratory findings between patients with tubular and glomerular involvement.
Treatment for renal involvement
Seven patients with tubular involvement were prescribed glucocorticoids; one patient received high-dose glucocorticoids (≥ 30 mg of prednisolone equivalent dose). This patient (Table 3, patient 2) showed a ≥ 20% reduction in the eGFR from baseline to the first-year follow-up. Three patients with RTA (Table 3, patients 3, 6, and 14) showed a > 20% increase in the eGFR from baseline to the first-year follow-up.
Eleven patients with glomerular involvement received glucocorticoids, with six patients receiving a high-dose. Mycophenolate mofetil (MMF) and cyclophosphamide in combination with low-dose glucocorticoids were administered to two and one patient with GN, respectively. Patients treated with MMF showed a > 20% increase in the eGFR from baseline to the first-year follow-up.
Renal outcomes
For the case-control study, 108 patients with persistent alkaline urine and 180 patients without renal involvement were selected as propensity score-matched controls. The baseline characteristics between the three groups are summarized in Supplemental Table S1. During the median follow-up period of 5 (IQR 3–9) years, there was no significant change in the median eGFR of patients with and without renal involvement (Fig. 2A). The median eGFR was lower in patients with renal involvement than in those without. Of the patients with renal involvement, 33% (n = 12) showed impaired renal function (eGFR < 60 ml/min/1.73 m2) at the time that renal involvement was diagnosed, and 44% (n = 16) showed impaired renal function at the last visit (Fig. 2B). Moreover, 25% of patients with renal involvement (n = 9) experienced a significant decline in renal function (a > 20% decline in the eGFR from baseline). By contrast, only 4% of patients without renal involvement (n = 7) and 5% of patients with persistent alkaline urine (n = 5) experienced a significant decline in renal function (P < 0.001) (Fig. 2C). In addition, the eGFR of these patients did not decrease below 60 ml/min/1.73 m2. The renal function of most patients without renal involvement and of those with persistent alkaline urine did not change significantly (Fig. 2A,C). No patients with renal involvement underwent renal replacement therapy.
The long-term renal outcomes of pSS with renal involvement were grouped into three categories: patients with renal involvement, patients with “persistent alkaline urine” for two consecutive years, without any documented significant renal involvement, and patients without renal involvement. Annual changes in the eGFR according to the groups (A). The proportion of patients with chronic kidney disease (eGFR < 60 ml/min/1.73 m2) among those with renal involvement at baseline visit and the last follow-up. P values from the McNemar test are shown above the bars (B). Proportions of patients with a significant change in renal function (> 20% from baseline) during the observation period (C). In the multivariate logistic regression analysis, adjusted for the observational period and baseline eGFR, renal involvement was identified as an independent predictor for a significant decline in renal function (D).
The impaired renal function at the last visit was negatively associated with hemoglobin (OR 0.471; 95% CI 0.264–0.769) and eGFR (OR 0.895; 95% CI 0.844–0.932) levels at the time of diagnosis of renal involvement or pSS (Fig. 2D, Supplemental Table S2). Among patients with renal involvement, RTA, nephrocalcinosis, treatment with immunosuppressants or high-dose glucocorticoids was not associated with impaired renal function.
Clinical outcomes
During the follow-up period, lymphoproliferative diseases common in patients with renal involvement than in those without (5.6% vs. 1.0%, respectively, P = 0.085). Among two patients with renal involvement, one underwent surgery to remove a mass (confirmed as extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALToma) in the right parotid gland). The other patient underwent surgery to remove a brain mass (confirmed as lymphomatoid granulomatosis).
Considering cases of lymphoproliferative disease occurring before and after a diagnosis of pSS with renal involvement, the data showed that lymphoproliferative disease was more prevalent in patients with renal involvement than in those without (11.1% vs. 1.4%, respectively, P < 0.001). The Cox proportional hazard model identified newly diagnosed lymphoproliferative disease after pSS diagnosis as being associated with renal involvement (HR 4.89; 95% CI 1.043–22.936) (Table 2).
In addition, one patient with tubular involvement (Table 3, patient 10) died due to aspiration pneumonia. The mortality rate did not differ according to the renal involvement (P = 0.851).
Discussion
Renal involvement in pSS is a rare manifestation, occurring in only 2.8% of patients in this cohort. Although more than half of patients with renal involvement were asymptomatic, 33% demonstrated impaired renal function at the time of diagnosis, and 25% experienced a significant decline in renal function during the follow-up period. In addition, renal involvement in patients with pSS was associated with lymphoproliferative disease.
In this cohort, the overall outcome of renal involvement in pSS was favorable, as no patients developed end-stage renal disease (ESRD), none required renal replacement therapy, and none died due to renal failure. A previous study reported that ESRD occurred in pSS patients with TIN, as well as those with GN9. Although this cohort showed great heterogeneity regarding both the type of treatment and the dosage, 19% of patients with renal involvement responded well to management, with a > 20% increase in the eGFR from baseline. Even though a few patients in this study were treated with immunosuppressants, those receiving MMF showed a good response. Studies show that cyclophosphamide is effective for pSS with renal involvement9,20.
In this study, the prevalence of renal involvement was lower than that reported in previous studies that used a similar definition of renal involvement9,10. One possible explanation for this is the lower prevalence of cryoglobulinemia in Asian groups than in other ethnicities21. Cryoglobulinemia is associated with GN in pSS patients, and is reported in 5–30% of pSS patients with biopsy-proven renal disease7,9. In this study, only 4% of patients with pSS had cryoglobulinemia, and among them, one patient had renal involvement.
Renal involvement seems to be prevalent in young onset pSS. In line with previous studies10,22,23, we found that patients with renal involvement were diagnosed with pSS at an earlier age than those without. Interstitial lung disease, another pathology associated with pSS, is more common in elderly patients10,24, suggesting that the prevalence of extraglandular manifestations varies according to age. In addition, more patients with renal involvement than those without were anti-La positive. Patients positive for anti-Ro and anti-La antibodies exhibit greater organ involvement and more active disease25,26; moreover, anti-La-positive pSS patients are younger at the time of disease diagnosis26,27.
Patients with pSS have an increased risk for lymphoma28,29. Interestingly, we found that B-cell lymphoid proliferative disease and lymphomas seemed to be associated with renal involvement in patients with pSS. The frequency of lymphoproliferative diseases in our cohort was small, however, it was much higher in patients with renal involvement. This result is consistent with those of previous studies that analyzed pSS patients with renal involvement6,7,9,30,31,32 (Table 4). Lymphoproliferative diseases and renal involvement in pSS may share immunopathogenic mechanisms. We found a case of salivary gland MALToma in a patient with RTA. In pSS, the histological findings of lymphocytic infiltration between salivary ductal epithelia and renal tubules are similar1, suggesting that these two organs may share epithelial antigens and autoreactive B cells, which are implicated in development of lymphoma33. The other three cases of lymphoproliferative disease developed in patients with GN, and all were B cell lymphoid neoplasms and plasma cell disease. Patients with MGN or membranoproliferative GN present with extra-membranous polyclonal deposits of comprising immunoglobulins and complement; these immune complexes stimulate B cells, and trigger development of monoclonal gammopathy and lymphoma1. Therefore, we suggest an association between renal involvement and lymphoproliferative diseases in pSS.
This study has several limitations. First, the number of cases with renal involvement is small. We may have underestimated the number of patients with renal involvement, as we did not measure bicarbonate or cryoglobulins in everyone. These test results are important for distinguishing renal involvement in pSS; however, they are not often included in routine tests in daily practice. Urinary acidification defects can be an early indicator of tubular dysfunction; however, urine pH does not provide information about distal urine acidification, since it measures the activity of free hydrogen ions, which comprise less than 1% of the total amount of excreted protons34. We identified some patients suspected of having a defect in urine acidification and analyzed their long-term prognosis with respect to renal function. The results were similar to those for patients without renal involvement. Taken together, the data indicate that these tests should be considered for patients with pSS when abnormal urinalysis findings are observed. Second, the number of lymphoproliferative diseases is small due to the low prevalence; therefore, to support the prevalence of lymphoproliferative diseases in pSS patients with renal involvement, we reviewed other studies (Table 4).
We believe that this study provides extensive follow-up data about pSS patients with renal involvement, who were identified in a non-experimental clinical setting. Furthermore, we provide evidence of a relationship between lymphoproliferative disease and renal involvement in pSS.
To summarize, renal involvement is rare in pSS, accounting for 2.8% of cases in this study. Because onset of renal involvement in pSS is insidious and asymptomatic, regular monitoring of serum and urine electrolytes together with urine pH, sediment, and protein levels is important for early detection. In addition, renal involvement is a predisposing factor for lymphoproliferative disease in patients with pSS.
Data availability
The data underlying this article are available in the article and supplementary materials.
References
François, H. & Mariette, X. Renal involvement in primary Sjögren syndrome. Nat. Rev. Nephrol. 12, 82–93 (2016).
Verstappen, G. M., Pringle, S., Bootsma, H. & Kroese, F. G. M. Epithelial–immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 17, 333–348 (2021).
Bossini, N. et al. Clinical and morphological features of kidney involvement in primary Sjögren’s syndrome. Nephrol. Dial. Transplant. 16, 2328–2336 (2001).
Luo, J. et al. Clinical features and potential relevant factors of renal involvement in primary Sjogren’s syndrome. Int. J. Rheum. Dis. 22, 182–190 (2019).
Ramos-Casals, M. et al. Systemic involvement in primary Sjogren’s syndrome evaluated by the EULAR-SS disease activity index: Analysis of 921 Spanish patients (GEAS-SS registry). Rheumatology (Oxford) 53, 321–331 (2014).
Kidder, D. et al. Kidney biopsy findings in primary Sjogren syndrome. Nephrol. Dial. Transplant. 30, 1363–1369 (2015).
Maripuri, S. et al. Renal involvement in primary Sjögren’s syndrome: A clinicopathologic study. Clin. J. Am. Soc. Nephrol. 4, 1423–1431 (2009).
Malladi, A. S. et al. Primary Sjögren’s syndrome as a systemic disease: A study of participants enrolled in an international Sjögren’s syndrome registry. Arthritis Care Res. (Hoboken) 64, 911–918 (2012).
Goules, A. V., Tatouli, I. P., Moutsopoulos, H. M. & Tzioufas, A. G. Clinically significant renal involvement in primary Sjogren’s syndrome: Clinical presentation and outcome. Arthritis Rheum. 65, 2945–2953 (2013).
Ramos-Casals, M. et al. Characterization of systemic disease in primary Sjogren’s syndrome: EULAR-SS task force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 54, 2230–2238 (2015).
Ramos-Casals, M. et al. EULAR recommendations for the management of Sjogren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 79, 3–18 (2020).
Lin, D. F. et al. Clinical and prognostic characteristics of 573 cases of primary Sjögren’s syndrome. Chin. Med. J. (Engl.) 123, 3252–3257 (2010).
Aiyegbusi, O. et al. Renal disease in primary Sjögren’s syndrome. Rheumatol. Ther. 8, 63–80 (2021).
Vitali, C. et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 61, 554–558 (2002).
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League against rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 69, 35–45 (2017).
Seror, R. et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): A user guide. RMD Open 1, e000022 (2015).
Miller, W. G. et al. National Kidney Foundation Laboratory Engagement Working Group recommendations for implementing the CKD-EPI 2021 race-free equations for estimated glomerular filtration rate: Practical guidance for clinical laboratories. Clin. Chem. 68, 511–520 (2022).
Khoury, J. D. et al. The 5th edition the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 36, 1703–1719 (2022).
Alaggio, R. et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 36, 1720–1748 (2022).
Shen, Y. et al. Combination cyclophosphamide/glucocorticoids provide better tolerability and outcomes versus glucocorticoids alone in patients with Sjogren’s associated chronic interstitial nephritis. Am. J. Nephrol. 46, 473–480 (2017).
Brito-Zerón, P. et al. Influence of geolocation and ethnicity on the phenotypic expression of primary Sjögren’s syndrome at diagnosis in 8310 patients: A cross-sectional study from the big data Sjögren project consortium. Ann. Rheum. Dis. 76, 1042–1050 (2017).
Chatterjee, R. et al. Renal involvement in Sjogren’s syndrome: Predictors and impact on patient outcomes. Rheumatol. Int. 43, 1297–1306 (2023).
Anquetil, C. et al. Is early-onset primary Sjögren’s syndrome a worse prognosis form of the disease?. Rheumatology (Oxford) 58, 1163–1167 (2019).
Lee, K. A., Nam, B. D., Hwang, J. H. & Kim, H. S. Clinical course and risk factors for development and progression of interstitial lung disease in primary Sjogren’s syndrome. Sci. Rep. 13, 9189 (2023).
Hernandez-Molina, G., Leal-Alegre, G. & Michel-Peregrina, M. The meaning of anti-Ro and anti-La antibodies in primary Sjogren’s syndrome. Autoimmun. Rev. 10, 123–125 (2011).
Cafaro, G. et al. Significance of anti-La/SSB antibodies in primary Sjogren’s syndrome patients with combined positivity for anti-Ro/SSA and salivary gland biopsy. Clin. Exp. Rheumatol. 38(Suppl 126), 53–56 (2020).
Danda, D. et al. Anti-La positive, anti-Ro negative subset of primary Sjogren’s syndrome: Anti-La is a reality but is the disease?. Clin. Exp. Rheumatol. 35, 438–444 (2017).
Kang, J. et al. Risk of malignancy in Korean patients with primary Sjogren’s syndrome. Int. J. Rheum. Dis. 23, 1240–1247 (2020).
Liang, Y., Yang, Z., Qin, B. & Zhong, R. Primary Sjogren’s syndrome and malignancy risk: A systematic review and meta-analysis. Ann. Rheum. Dis. 73, 1151–1156 (2014).
Goules, A. et al. Clinically significant and biopsy-documented renal involvement in primary Sjogren syndrome. Medicine 79, 241–249 (2000).
Jasiek, M. et al. A multicentre study of 95 biopsy-proven cases of renal disease in primary Sjogren’s syndrome. Rheumatology (Oxford) 56, 362–370 (2017).
Narvaez, J. et al. Clinically significant renal involvement in primary Sjogren’s syndrome is associated with important morbidity: Data from the Spanish Sjogrenser cohort. Clin. Exp. Rheumatol. 38(Suppl 126), 116–124 (2020).
Routsias, J. G. et al. Malignant lymphoma in primary Sjögren’s syndrome: An update on the pathogenesis and treatment. Semin. Arthritis Rheum. 43, 178–186 (2013).
Forni Ogna, V. et al. Signification of distal urinary acidification defects in hypocitraturic patients. PLoS ONE 12, e0177329 (2017).
Acknowledgements
We thank the Information Service Team of Catholic Medical Center.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00246642).
Author information
Authors and Affiliations
Contributions
J.H.K., H.J., S-K. K., W-U.K., and S-H. P. conceived of the presented idea. H.J., Y.S, Y.P., J.L., and J.H.K. contributed to data acquisition. J.H.K., H.J., Y.S., Y.P., and J.L. performed the computations. S-K.K., W-U.K, and S-H.P verified the analytical methods and supervised the findings of this work. J.H.K., H.J., Y.P., J.L., S-K.K, W-U.K., and S-H.P. contributed to interpreting the results. H.J., J.H.K. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and reviewed manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional review board of the Catholic Central Medical Center (UC22WIDE0042). The requirement for informed consent was waived due to the retrospective nature of the study, by the institutional review board of the Catholic Central Medical Center.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jeon, H., Park, Y., Lee, J. et al. The prevalence, clinical features, and long-term outcome of patients with primary Sjögren’s syndrome with renal involvement. Sci Rep 15, 4211 (2025). https://doi.org/10.1038/s41598-025-88368-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88368-8