Abstract
Breast cancer (BC) is a heterogeneous disease, traditionally classified by hormone receptor and HER2 (human epidermal growth factor receptor 2) status. HER2-low, characterized by low HER2 expression without gene amplification, recently gained attention. While new therapies are promising, its clinical significance remains unclear. We analysed 241,510 BC patients diagnosed between 2000 and 2020 in Germany using data from the German Cancer Registry Group. HER2 status was determined using immunohistochemistry and fluorescence in situ hybridization results, and patients were classified as HER2-positive, HER2-low or HER2-zero. Clinical features, chemosensitivity and long-term outcomes - metastasis-free survival (MFS), recurrence-free survival (RFS), and overall survival (OS) - were analysed using Cox models. HER2-low comprised 42% of female and 47% of male patients, predominantly hormone receptor positive. Metastatic patterns in HER2-low and HER2-zero were similar but differed from HER2-positive, which showed more liver metastasis and multiple metastatic sites. HER2-positive showed worse MFS, RFS, and OS than HER2-zero and HER2-low subtypes. Pathological complete response (pCR) rates after neoadjuvant chemotherapy were highest in HER2-positive. HER2-low has higher hormone receptor positivity, distinguishing it from HER2-zero. While metastatic behaviour, treatment and long-term response in HER2-low were comparable to HER2-zero, the hormone receptor status seems to play a critical role in these outcomes.
Similar content being viewed by others
Introduction
Breast cancer (BC) is a complex disease with significant pathological heterogeneity. Molecular and clinical studies addressing this heterogeneity have led to subtype classification based on hormone receptor and human epidermal growth factor 2 (HER2) status1,2. Over the years, therapeutic advances targeting HER2-positive BC have substantially improved treatment outcomes3. However, conventional anti-HER2 therapies have shown limited efficacy in HER2-zero tumours4,5, prompting a re-evaluation of the binary classification system.
A subset of BCs display low to moderate levels of HER2 expression, termed HER2-low, despite the absence of HER2 gene amplification6. Notably, early clinical trials have shown promising responses of HER2-low tumours to novel anti-HER2 therapies such as trastuzumab deruxtecan7,8,9, underscoring the potential therapeutic implications of HER2-low BC.
However, the classification and clinical significance of HER2-low BCs remain incompletely understood. While some studies have suggested molecular and clinical distinctions between HER2-low and other subtypes10,11, others have questioned the impact of such differences12. Recent updates from oncology guidelines advocate for further investigation into the molecular and phenotypic characteristics of HER2-low tumours and their implications for treatment.
HER2-status plays a crucial role in determining tumour aggressiveness and metastatic potential. HER2-positive breast cancers (BC) are associated with an increased risk of metastasis compared to HER2-zero counterparts, often leading to a more aggressive course of disease. The metastatic spread of HER2-positive BC frequently involves the brain, whereas HER2-negative BC showed an increased propensity for lung metastasis, reflecting the distinct subtype-specific mechanisms of metastatic spread and influencing treatment strategies13. Recent research has provided insights into the patterns of metastasis and outcomes in patients with different breast cancer subtypes, including HER2-positive and HER2-negative tumours14. While data on HER2-low BC metastatic patterns are still limited, emerging evidence suggests that HER2-low BC may have a lower metastatic predisposition compared to HER2-positive tumours. However, the specific metastatic patterns and factors predicting metastasis risk in HER2-low BC, along with their clinical implications, are still largely unknown. This knowledge gap underscores the urgent need to identify reliable prognostic markers and predictive biomarkers for metastasis in HER2-low tumours. Ongoing research efforts are focused on better characterizing the metastatic potential and patterns of HER2-low BC, which could ultimately inform treatment strategies and improve patient outcomes.
Regarding metastasis-free survival (MFS), patients with HER2-positive BC often experience shorter MFS compared to HER2-zero patients15,16. HER2-targeted therapy has significantly improved MFS rates in HER2-positive metastatic BC. However, studies investigating metastasis-free survival in HER2-low BC are scarce, and the impact of HER2-low status on MFS outcomes relative to HER2-zero and HER2-positive subtypes requires further investigation.
In this context, our study addresses two critical aspects. Firstly, we explore whether HER2-low represents a unique clinical entity with distinct metastatic patterns. Secondly, we investigate the response of early-stage HER2-low BC patients to neoadjuvant chemotherapy and assess the implications of HER2-low expression on treatment response and prognosis. For this purpose, we conducted a comprehensive retrospective cancer registry data analysis utilizing a large cohort of BC patients.
Methods
Study population
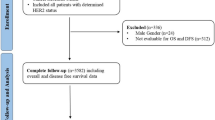
This study analysed BC patients diagnosed between January 2000 and December 2020, focusing on HER2-status characterisation. Data were retrieved from the database of the 10th nationwide oncological quality conference in Germany (as of July 2023), encompassing nearly 340 000 BC cases from 13 clinical cancer registries within the German Cancer Registry Group of the Association of German Tumour Centers (ADT). We used the data from those tumour registries, in which for at least 50% of cases a HER2 status was known, and in 30% of cases, a HER2 low expression was detected (suppl. Figure 1). The final study population comprised 241 510 patients. The anonymized unified dataset was gathered by data analysts of the ADT in accordance with this organization’s data protection standards. Data analyses were performed at the Clinical cancer registry Saxony-Anhalt in strict accordance with Good Epidemiological Practice, regional and federal data protection laws and reporting according to the STROBE Statement.
Clinical and pathological evaluation
Hormone receptor assessment followed the most recent ASCO/CAP guidelines, with positivity defined as ≥ 1% of invasive tumour cells staining positive by immunohistochemistry (IHC). The ASCO/CAP guidelines for HER2 assessment in breast cancer have evolved since their initial publication in 2007, with significant updates in 2013 and 2018. For this analysis, we used the HER2 definition from the most recent ASCO/ESMO guidelines17, with HER2 protein expression initially assessed by IHC and stratified as IHC0, IHC1+, IHC2+, and IHC3+. IHC2 + samples underwent additional fluorescence in situ hybridisation (FISH) to detect the HER2 gene amplification. Based on the IHC and FISH results, HER2 status was categorised as HER2-positive (IHC3+, or IHC 2 + and FISH-positive), HER2-low (IHC1+, or IHC2 + and FISH-negative), or HER2-zero (IHC0)18. To minimize inter-laboratory variability, registries have implemented standardized protocols for both IHC and FISH testing, ensuring alignment with ASCO/ESMO guidelines. External quality assurance programs and inter-laboratory proficiency testing were employed to maintain consistency in assay performance and interpretation. Each registry aggregated results from multiple laboratories, where adherence to guideline-based scoring criteria and cross-validation procedures helping to reduce potential variation in HER2 status determination.
Primary tumour staging (T1-T4), nodal status (N0-N3) and distant metastatic status (M0-M1) were defined according to the revised TNM classification system of the American Joint Committee on Cancer and International Association Against Cancer (UICC)19.
Outcomes
The median follow-up time was 8.9 years, with an interquartile range (IQR) of 4.8 to 12.7 years, facilitating reliable long-term survival analyses. Metastasis-free survival (MFS), recurrence-free survival (RFS), and overall survival (OS) were calculated.
MFS was defined as the time from diagnosis to first metastasis (at least 3 months after diagnosis) or death in patients with UICC stage I to III disease (122 750 patients). RFS was defined as the time from the tumour free diagnosis following operative therapy to the first local tumour recurrence or progression of disease, including ipsilateral, local/regional, or death in patients with UICC stages I to III disease (65 447 patients). OS was defined as the time from the date of diagnosis to death from any cause or last follow-up for censored patients (161 393 patients).
BC pathological complete response (pCR) was defined as the absence of invasive tumour in the breast following neoadjuvant therapy (ypT0/is).
Statistical analysis
Statistical analyses were performed using R-4.3.220. We examined the cohort clinical and pathological characteristics for each HER2 subtype. Variables associated with HER2 phenotypes were assessed using multivariable logistic regression. Kaplan–Meier curves were used to compare clinical outcomes (MFS, RFS and OS) according to primary HER2 status, and Cox-regression models were used to calculate crude and adjusted hazard ratios. Adjustments were made for age at diagnosis, hormone receptor status, tumour location, German Cancer Registry of the ADT and histology group. Directed acyclic graphs were used to define the confounder adjustment sets21. All analyses were sex-stratified.
Results
Table 1 shows the clinical and pathological characteristics of the 241 510 BC patients diagnosed between 2000 and 2020, 239 527 (99%) women and 1 983 (1%) men. Of these, 43% of the women and 42% of the men were classified in the HER2-zero group, 42% of women and 47% of men were in the HER2-low group and 15% of the women and 11% of the men were in the HER2-positive group. Women with HER2-positive tumours were younger, more likely to have hormone receptor negative tumours, higher T stage, positive lymph node metastases (N+) and distant metastases (M1), less differentiated tumours, and more frequent ductal tumours than women with HER2-zero tumours. On the other hand, women with HER2-low tumours were more likely to have hormone receptor-positive tumours compared with HER2-zero tumours. Men were more likely than women to have a positive hormone receptor status, higher T, positive lymph node metastases (N+) and distant metastases (M1) and higher grading, regardless of HER2 status. Most tumours in men were ductal. Between 2000 and 2020, the use of anti-HER2 therapies in breast cancer treatment has markedly increased, reflecting advancements in HER2-targeted treatment strategies (Supplementary Table 1). Among women, the proportion of HER2-low cases receiving anti-HER2 therapy rose from 0.3 to 7%, while for HER2-positive cases, this proportion increased dramatically from 6% to over 70%. Trastuzumab remains the most used anti-HER2 therapy, followed by pertuzumab, whereas the use of other anti-HER2 agents remains less well documented. Similar trends are observed among men with breast cancer, although the absolute numbers are significantly smaller (data not shown).
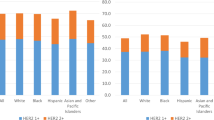
We also looked at the different metastatic patterns. Figure 1 shows the rates of different metastatic sites by diagnosis stratified by sex and HER2-status. Among the women, 5 522 with HER2-zero status (8%), 5 742 women with HER2-low status (8%) and 3 138 women with HER2-positive status (13%) had metastases at the time of diagnosis. This compared to 52 men with HER2-zero status (10%), 91 men with HER2-low status (14%) and 29 with HER2-positive status (20%). More patients with HER2-positive status had primary metastases. In women, the distribution of metastases was similar between HER2-low and HER2-zero status and quite different from HER2-positive. In women, most metastases (alone or in combination) occurred in bone 8 676 (3.6%), followed by lung 4 795 (2.0%), liver 3 613 (1.5%), and brain 420 (0.2%). HER2-positive was associated with more combinations of multiple metastases (liver and bone) and more liver metastases. There were no differences between the patterns for HER2-zero and HER2-low. In men the number of metastases in bone was 110 (5.5%), in lung 77 (3.9%), in liver 26 (1.3%), and in brain 4 (0.2%). Due to the very small numbers, it was not possible to make a meaningful comparison between the groups. However, there was a similar trend towards more liver metastases with positive HER2 status. Supplementary Fig. 2 shows that regardless of hormone receptor status, HER2-positive was more likely to be associated with all metastases, especially bone and liver. While hormone receptor-positive tumours were most associated with bone metastases, hormone receptor-negative tumours were most associated with liver metastases, followed by bone and lung.
Survival analyses were performed to assess 5-year MFS, RFS and OS. MFS: 122 750 patients with tumours at stages I - III were followed-up, 10 336 (8%) developed a metastasis and 17 013 (14%) died in the 5-years following diagnosis. In the population of women with BC diagnosed at stage I – III, univariate analysis showed that HER2 status was associated with MFS, with HER2-low patients having a superior MFS compared to HER2-zero patients (hazard ratio (HR) = 0.90, 95% CI 0.87–0.93) (Fig. 2). However, after adjustment for age at diagnosis, hormone receptor status, localisation, German Cancer Registry of the ADT and histology group in the multivariable analysis no difference was seen (HR = 0.98, 95% CI 0.94–1.01). HER2-positive patients had a worse MFS than HER2-zero patients in univariable and multivariable analysis (crude HR = 1.19, 95% CI 1.15–1.23, adjusted HR = 1.08, 95% CI 1.03–1.12). Thus, small differences in time to metastasis were seen between HER2-low and HER2-zero patients, but larger differences were seen with HER2-positive patients (Fig. 2). After full adjustment for age at diagnosis, hormone receptor status, location, German Cancer Registry of the ADT and histology group, HER2-low and HER2-zero were similar, but HER2-positive had worse MFS. No differences between the HER2-status and MFS were found for men (crude HR = 1.00, 95% CI 0.77–1.24, adjusted HR = 1.04, 95% CI 0.79–1.30 for HER2-low and crude HR = 1.08, 95% CI 0.71–1.45, adjusted HR = 1.04, 95% CI 0.65–1.43 for HER2-positive).
65 447 patients with tumours at stages I - III and tumour free after primary tumour resection, were followed-up for up to 5 years, 19 431 (30%) developed a local recurrence and 29 112 (44%) died in the 5-years following diagnosis. Univariable analysis showed that HER2 status is an independent prognostic factor for RFS for women, and HER2-low patients had a similar RFS compared to HER2-zero patients in crude (HR = 0.97, 95% CI 0.94–0.99) and adjusted (adjusted for age at diagnosis, hormone receptor status, tumour location, German Cancer Registry of the ADT and histology group) analysis (HR = 1.01, 95% CI 0.98–1.03) (Fig. 3). HER2-positive patients had a worse RFS than HER2-zero patients in univariate and multivariate analysis (crude HR = 1.08, 95% CI 1.05–1.12, adjusted HR = 1.10, 95% CI 1.06–1.13). In women, there were no differences in RFS between HER2-low and HER2-zero patients, but there were differences with HER2-positive patients (Fig. 3). In men, no differences were seen between HER2-zero and HER2-low (crude HR = 0.98, 95%CI = 0.77–1.18 and fully adjusted HR = 0.95, 95%CI = 0.73–1.17) as well as between HER2-zero and HER2-positive (crude HR = 1.11, 95%CI = 0.80–1.42 and fully adjusted HR = 1.03, 95%CI = 0.71–1.35).
Finally, OS was analysed for patients with more than 1 day of follow-up. From the 161 393 patients, 1 535 were men (10%) and 42 675 (26%) died in the following 5 years. There were no differences between HER2-low and HER2-zero for OS, but differences with HER2-positive (Fig. 4) (HR = 0.98, 95%CI: 0.96-1.00). After full adjustment for age at diagnosis, hormone receptor status, location, German Cancer Registry of the ADT and histology group, the HRs for HER-zero were HR = 1.02, 95%CI: 0.99–1.04, whereas for HER-positive were crude HR = 1.13, 95%CI: 1.10–1.16, adjusted HR = 1.10, 95%CI: 1.07–1.13. After adjustment for hormone receptor status, most of the differences disappeared, suggesting that the effect was hormone receptor related. No differences were found in men: no differences between HER2-zero compared to HER2-low (crude HR = 1.06, 95%CI = 0.89–1.24 and adjusted HR = 1.10, 95%CI = 0.92–1.28) and no differences between HER2-zero and HER2-positive (crude HR = 1.11, 95%CI = 0.85–1.37 and adjusted HR = 1.18, 95%CI = 0.92–1.44).
Neoadjuvant therapy
We performed an analysis of patients with non-metastatic BC who received neoadjuvant therapy between 2000 and 2020 (including only those with completed treatment: 13 403 from 18 703; 72%). Parameters assessed were initial tumour size, hormone receptor status, HER2 status, type of neoadjuvant therapy and pathological complete response rates. Regarding neoadjuvant regimens, 10 082 (75%) of 13 403 patients received anthracycline- and/or paclitaxel-based neoadjuvant chemotherapy. 9% (373 of 4 386) of HER2-positive patients received neoadjuvant chemotherapy without anti-HER2 targeted therapy. After neoadjuvant chemotherapy, mastectomy was performed in 28% of cases, while breast conservation therapy was performed in 51% of cases, 7% did not undergo surgery and for the remaining 14% it was not known which surgery was performed. Few patients with hormone receptor positive tumours received no endocrine therapy (2% of HER2-zero; 3% of HER2-low and 2% of HER2-positive patients).
We observed an association between HER2 expression and the rate of pCR in patients treated with anthracycline/paclitaxel (suppl. Figure 3). The pCR rate was 39% in HER2-zero (1 271 from 3 235), 30% in HER2-low (943 from 3 164) and 55% in HER2-positive patients (1 733 from 3 130) (χ2 = 177, df = 2, p-value < 0.001). In addition, when considering each hormone receptor status, the association between pCR rates and HER2 expression was consistent throughout, with the pCR rate for hormone receptor-positive HER2 cases (23%, 19%, and 47% for HER2-zero, HER2-low and HER2-positive, respectively) being consistently lower than that for hormone receptor-negative cases (53%, 50% and 71% for HER2-zero, HER2-low and HER2-positive, respectively) (Fig. 5).
Discussion
In this analysis using data from the German Cancer Registry Group, we examined clinicopathological characteristics, metastatic patterns, long-term outcomes and pCR following neoadjuvant therapy according to HER2 status. While other studies have reported conflicting results regarding the distinctiveness of HER2-low as a separate entity, with this analysis we contribute to the literature and confirm previous findings that HER2-low primary BC shares minimal distinct clinicopathological features with HER2-zero BC and has substantial differences when compared to HER2-positive BC. In addition, we present new insights into the long-term behaviour of HER2-low. Furthermore, the metastatic pattern and response to treatment were comparable between the HER2-low and HER2-zero groups and differed from HER2-positive BC. Our data showed a pCR rate of 30% in HER2-low BC patients, which is comparable to the 39% seen in HER2-zero patients but lower than the 55% seen in HER2-positive patients. This finding is consistent with recent studies showing that HER2-positive BC tends to be more chemosensitive, as evidenced by a higher pCR rate, possibly due to a more aggressive biology and response to HER2-targeted therapies. Survival rates at 1-year and 5-years were similar in the HER2-low and HER2-zero groups (94% and 68%, for both respectively) and marginally better than those observed in the HER2-positive group (93% and 65%, respectively). These findings suggest that while HER2-low and HER2-zero BC have a comparable prognosis, HER2-positive BC may have a worse long-term outcome despite higher initial chemosensitivity, possibly due to the aggressive nature and higher metastatic potential of HER2-positive tumours.
Our study contributes to the ongoing debate about whether HER2-low BC represents a distinct biological entity compared to HER2-zero tumours. Consistent with the existing literature, we found that HER2-positive tumours are more likely to present with aggressive disease features (younger age at diagnosis, higher T stage, positive lymph node metastases, distant metastases, and poor differentiation). These tumours are often hormone receptor-negative and more often NST histology, further supporting the link between HER2-positive BC and a poorer prognosis21,22. Conversely, HER2-low tumours are more likely to be hormone receptor-positive, which is generally associated with a better prognosis and response to hormone therapy23. This distinction has been supported by other studies, such as those by Tarantino et al.18, Denkert et al.24, and Schettini et al.11, which also reported higher hormone receptor-positivity in HER2-low tumours. These findings support the hypothesis that HER2-low tumours may indeed represent a unique subset within HER2- negative BC, particularly when hormone receptor expression is considered as a differentiating factor.
The confounding effect of hormone receptor status on the comparison between HER2-low and HER2-zero breast cancer subtypes is significant and has important implications for treatment stratification. HER2-low tumours are more likely to be hormone receptor-positive than HER2-zero tumours, which may account for their apparent better prognosis in some studies18. A meta-analysis by Atallah et al.25 found that HER2-low breast cancers were associated with better disease-free survival and overall survival compared to HER2-zero, regardless of HR status. However, Pascual et al.26 reported that most genomic differences between HER2-low and HER2-zero tumours were no longer present after stratification by hormone receptor status. The interplay between hormone receptor status and HER2 expression can mask or exaggerate differences between these subtypes, affecting treatment decisions. These findings underscore the need for careful consideration of both hormone receptor status and HER2 expression in developing personalized treatment strategies and designing clinical trials for breast cancer patients.
One of the novel aspects of our study is the examination of HER2 status in male BC. We found that lobular histotype is very rare (4%) in male BC compared to female BC (16%). The most common subtype in men was HER2-zero, hormone receptor-positive (80%), whereas HER2-zero and hormone receptor negative and HER2-positive subtypes were less common (2% and 10%, respectively). Men with BC, regardless of HER2 status, are more likely to have hormone receptor-positive tumours, higher T stage, positive lymph node involvement, distant metastases, and higher tumour grade. This is consistent with previous studies24,25,26, suggesting that male BC, although rare, tends to present at a more advanced stage and with more aggressive features than in women27,28. This finding highlights the need for sex-specific strategies in the diagnosis, treatment, and management of BC.
Our analysis showed that HER2-positive patients had a higher incidence of primary metastases at diagnosis compared to HER2-low and HER2-zero patients, a trend observed in both male and female cohorts. HER2-positive tumours were associated with a higher frequency of liver metastases and a greater tendency for multiple metastatic sites, consistent with the literature suggesting a more aggressive metastatic profile for HER2-positive BC29,30,31,32,33. In contrast, HER2-low and HER2-zero tumours showed similar patterns of metastasis, suggesting that HER2 expression level alone is not a significant determinant of metastatic behaviour. These findings were similar in male and female patients with BC. This highlights the potential influence of other factors, such as hormone receptor status, on metastatic patterns.
While early studies reported improved survival for HER2-low patients compared to HER2-zero patients regardless of hormone receptor status24, more recent large studies and meta-analyses have shown only small survival advantages for HER2-low BC when stratified by hormone receptor status34,35,36. HER2-positive BC showed worse MFS, RFS, and OS compared to HER2-zero and HER2-low subtypes. Importantly, our results show that HER2-zero disease is associated with an early decrease in mortality risk, which eventually falls below the risk for HER2-low patients.
Furthermore, we found no significant differences in pCR rates between HER2-low and HER2-zero tumours when analysed stratified by hormone receptor status, confirming recent reports23,32 that within the HER2-zero cohort, hormone receptor status is an important determinant of chemosensitivity. This suggests that the distinction between HER2-low and HER2-zero BC in terms of treatment response may be less important than previously thought, emphasising the role of hormone receptor positivity as a confounder in these analyses. Unfortunately, subgroup sizes for analysing other treatments were insufficient for robust analysis.
The biological rationale and clinical behaviour of HER2-low breast cancer remain subjects of ongoing debate and investigation. While some studies have identified molecular distinctions supporting HER2-low as a unique entity, including differences in gene expression profiles, ERBB2 copy number, and mutation patterns in pathways such as PI3K-Akt signaling11,37, others challenge this classification. Pascual et al.38noted overall genomic similarity between HER2-low and HER2-zero tumours, with the exception of ERBB2 copy number variations. The importance of hormone receptor status in determining tumour biology has also been emphasized, potentially overshadowing the significance of low HER2 expression39. Despite these conflicting findings, subtle molecular differences suggest a “luminal-like” pattern for HER2-low BC18,24,36, which may explain its intermediate prognosis between HER2-zero and HER2-positive BC. Furthermore, HER2-driven tumour cell dormancy may contribute to the long-term behaviour of HER2-low BC34,35, particularly in the absence of prolonged adjuvant HER2-targeted therapy that could maintain dormancy or eradicate minimal residual disease18,40. The observation that ERBB2 mRNA levels in HER2-low tumours more closely resemble those in HER2-negative tumours35 further complicates our understanding of this subtype’s biological distinctiveness. These findings underscore the need for further research to definitively establish whether HER2-low represents a truly distinct biological entity or if it exists on a continuum within the broader spectrum of HER2-negative breast cancers. Future studies should focus on elucidating the unique biology of HER2-low BC and exploring the potential for tailored therapeutic approaches that consider both HER2 expression levels and hormone receptor status.
The strengths of our study include the use of large cancer registry datasets, which provide extensive sample sizes and increase the generalizability of our findings across different subpopulations. In addition, cancer registries often cover diverse populations in different regions, which increases the representativeness of the study. Cancer registries also adhere to standardized data collection protocols and classification systems, ensuring consistency and comparability of data across studies and regions. Importantly, registry data reflect real-world clinical practice and patient outcomes, providing valuable insights into the effectiveness of treatments outside the controlled environment of clinical trials. However, several limitations must be acknowledged. The retrospective nature of our analysis introduces potential biases and data inaccuracies. A major limitation is the variability in HER2 testing protocols between institutions and over time, which may lead to misclassification of HER2 status, particularly in the HER2-low category. In addition, there are studies suggesting that there is a low-level of agreement between pathologists in categorising HER2-low cases41. This variability may affect the accuracy of our findings and their generalizability. Missing data on endocrine therapy or targeted therapies is another major limitation, as these treatments can substantially influence patient outcomes. The lack of this information may confound our analysis of survival differences between HER2-low and HER2-zero patients. Other missing information, such as androgen receptor status, could also confound our analysis. Androgen receptor signalling has been suggested to play a role in modulating the presentation patterns and clinical behaviour of HER2-low and HER2-zero breast cancers. Recent evidence suggests that androgen receptor expression may influence the molecular characteristics and therapeutic response in these subtypes, particularly in luminal and triple-negative breast cancers with low HER2 protein expression25. Furthermore, the interplay of androgen receptor with oestrogen receptor signalling in HER2-negative tumours has been highlighted as a potential modifier of disease progression and treatment outcomes, emphasizing the need for further exploration in this context42. Our study may also be affected by unmeasured confounders, such as socioeconomic factors or access to care, which can significantly affect patient outcomes but are not typically captured in cancer registry data. These factors may bias our results and limit the interpretation of observed differences between HER2 subgroups. In addition, there may be biases related to different modalities of follow-up or radiological monitoring for metastatic disease. As highlighted by Chagpar et al.43, there is considerable variation in the metastatic workup of patients with invasive breast cancer, which may lead to differences in the detection and timing of diagnosis of metastatic disease. In addition, Bonotto et al.44 demonstrated controversies in the monitoring of metastatic breast cancer during systemic treatment, which could affect the accuracy of progression-free survival estimates in our study. Future prospective studies with standardized testing protocols, comprehensive treatment data collection, and consistent follow-up procedures are needed to validate our findings and to further explore the biological and clinical significance of the HER2-low phenotype.
In conclusion, our study highlights important differences in clinicopathological features and response to therapy between HER2-low and HER2-zero BC, with hormone receptor status emerging as a key differentiator. While metastatic patterns and pCR rates did not differ significantly between HER2-low and HER2-zero tumours, the higher hormone receptor positivity in HER2-low tumours suggests a distinct biological subset. These findings, together with the limitations of our retrospective study, highlight the need for prospective research to refine our understanding of HER2-low BC and guide future therapeutic strategies.
Data availability
Data were obtained from the German Cancer Registry Group of the Society of German Tumour Centres. German cancer patient data are not stored in a single national registry, but in many different registries depending on the patient’s state of residence. By law, each cancer registry manages its data independently. A research team must apply to each registry for data access and negotiate a separate contract with each registry for nationwide data analysis (https://www.adt-netzwerk.de/Vertrauensstelle/ADT_-_Vertrauensstelle/).
References
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752. https://doi.org/10.1038/35021093 (2000).
Goldhirsch, A. et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 24, 2206–2223. https://doi.org/10.1093/annonc/mdt303 (2013).
Loibl, S. & Gianni, L. HER2-positive breast cancer. Lancet Lond. Engl. 389, 2415–2429. https://doi.org/10.1016/S0140-6736(16)32417-5 (2017).
Fehrenbacher, L. et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1 + or 2. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 38, 444–453. https://doi.org/10.1200/JCO.19.01455 (2020).
Gianni, L. et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 28, 1131–1137. https://doi.org/10.1200/JCO.2009.24.1661 (2010).
Tarantino, P. et al. HER2-low breast cancer: Pathological and clinical landscape. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 38, 1951–1962. https://doi.org/10.1200/JCO.19.02488 (2020).
Banerji, U. et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20, 1124–1135. https://doi.org/10.1016/S1470-2045(19)30328-6 (2019).
Tamura, K. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet Oncol. 20, 816–826. https://doi.org/10.1016/S1470-2045(19)30097-X (2019).
Modi, S. et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in patients with HER2-Low-expressing advanced breast Cancer: Results from a phase Ib study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 38, 1887–1896. https://doi.org/10.1200/JCO.19.02318 (2020).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20. https://doi.org/10.1056/NEJMoa2203690 (2022).
Schettini, F. et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 7, 1. https://doi.org/10.1038/s41523-020-00208-2 (2021).
de Moura Leite, L. et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res. Treat. 190, 155–163. https://doi.org/10.1007/s10549-021-06365-7 (2021).
Gerratana, L. et al. Pattern of metastasis and outcome in patients with breast cancer. Clin. Exp. Metastasis 32, 125–133. https://doi.org/10.1007/s10585-015-9697-2 (2015).
Grinda, T. et al. Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008–2017 ESME cohort. ESMO Open 6, 100114. https://doi.org/10.1016/j.esmoop.2021.100114 (2021).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792. https://doi.org/10.1056/NEJM200103153441101 (2001).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372, 724–734. https://doi.org/10.1056/NEJMoa1413513 (2015).
Tarantino, P. et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 34, 645–659. https://doi.org/10.1016/j.annonc.2023.05.008 (2023).
Tarantino, P. et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur. J. Cancer Oxf. Engl. 1990. 163, 35–43. https://doi.org/10.1016/j.ejca.2021.12.022 (2022).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17, 1471–1474. https://doi.org/10.1245/s10434-010-0985-4 (2010).
R Core Team. A language and environment for statistical computing (2021).
Pearl, J. An introduction to causal inference. Int. J. Biostat. 6. https://doi.org/10.2202/1557-4679.1203 (2010).
Senkus, E. et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24, vi7–vi23. https://doi.org/10.1093/annonc/mdt284 (2013).
Pegram, M., Jackisch, C. & Johnston, S. R. D. Estrogen/HER2 receptor crosstalk in breast cancer: Combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. NPJ Breast Cancer 9, 1–19. https://doi.org/10.1038/s41523-023-00533-2 (2023).
Denkert, C. et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 22, 1151–1161. https://doi.org/10.1016/S1470-2045(21)00301-6 (2021).
Atallah, N. M. et al. Characterisation of luminal and triple-negative breast cancer with HER2 low protein expression. Eur. J. Cancer 195. https://doi.org/10.1016/j.ejca.2023.113371 (2023).
Pascual, T. et al. Ribociclib (RIB) vs. palbociclib (PAL) in patients (pts) with hormone receptor-positive/HER2-negative/HER2-enriched (HR+/HER2-/HER2-E) advanced breast cancer (ABC): A head-to-head phase III study—HARMONIA SOLTI-2101/AFT-58. J. Clin. Oncol. 41, TPS1125–TPS1125. https://doi.org/10.1200/JCO.2023.41.16_suppl.TPS1125 (2023).
Giordano, S. H., Buzdar, A. U. & Hortobagyi, G. N. Breast cancer in men. Ann. Intern. Med. 137, 678–687. https://doi.org/10.7326/0003-4819-137-8-200210150-00013 (2002).
Cardoso, F. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 31, 1623–1649. https://doi.org/10.1016/j.annonc.2020.09.010 (2020).
Eggemann, H. et al. Survival benefit of tamoxifen in male breast cancer: Prospective cohort analysis. Br. J. Cancer 123, 33–37. https://doi.org/10.1038/s41416-020-0857-z (2020).
Eggemann, H., Altmann, U., Costa, S-D. & Ignatov, A. Survival benefit of tamoxifen and aromatase inhibitor in male and female breast cancer. J. Cancer Res. Clin. Oncol. 144, 337–341. https://doi.org/10.1007/s00432-017-2539-7 (2018).
Cheng, X. A comprehensive review of HER2 in cancer biology and therapeutics. Genes 15, 903. https://doi.org/10.3390/genes15070903 (2024).
Boman, C. et al. A population-based study on trajectories of HER2 status during neoadjuvant chemotherapy for early breast cancer and metastatic progression. Br. J. Cancer. 131, 718–728. https://doi.org/10.1038/s41416-024-02777-6 (2024).
Geukens, T. et al. Intra-patient and inter-metastasis heterogeneity of HER2-low status in metastatic breast cancer. Eur. J. Cancer 188, 152–160. https://doi.org/10.1016/j.ejca.2023.04.026 (2023).
Molinelli, C. et al. Prognostic value of HER2-low status in breast cancer: A systematic review and meta-analysis. ESMO Open 8, 101592. https://doi.org/10.1016/j.esmoop.2023.101592 (2023).
Tarantino, P. et al. Prognostic and biologic significance of ERBB2-Low expression in early-stage breast cancer. JAMA Oncol. 8, 1177–1183. https://doi.org/10.1001/jamaoncol.2022.2286 (2022).
Peiffer, D. S. et al. Clinicopathologic characteristics and prognosis of ERBB2-low breast cancer among patients in the National Cancer Database. JAMA Oncol. 9, 500–510. https://doi.org/10.1001/jamaoncol.2022.7476 (2023).
Fernandez, A. I. et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 8, 607–610. https://doi.org/10.1001/jamaoncol.2021.7239 (2022).
Pascual, J. et al. Baseline mutations and ctDNA dynamics as prognostic and predictive factors in ER-positive/HER2-negative metastatic breast cancer patients. Clin. Cancer Res. 29, 4166–4177. https://doi.org/10.1158/1078-0432.CCR-23-0956 (2023).
Agostinetto, E. et al. PREDICT underestimates survival of patients with HER2-positive early-stage breast cancer. NPJ Breast Cancer. 8, 87. https://doi.org/10.1038/s41523-022-00452-8 (2022).
Ruth, J. R. et al. Cellular dormancy in minimal residual disease following targeted therapy. Breast Cancer Res. BCR 23, 63. https://doi.org/10.1186/s13058-021-01416-9 (2021).
Zaakouk, M. et al. Concordance of HER2-low scoring in breast carcinoma among expert pathologists in the United Kingdom and the Republic of Ireland—on behalf of the UK national coordinating committee for breast pathology. Breast 70, 82–91. https://doi.org/10.1016/j.breast.2023.06.005 (2023).
Basile, D. et al. Androgen receptor in estrogen receptor positive breast cancer: Beyond expression. Cancer Treat. Rev. 61, 15–22. https://doi.org/10.1016/j.ctrv.2017.09.006 (2017).
Chagpar, A. et al. Variation in metastatic workup for patients with invasive breast cancer. Am. J. Surg. 210, 1147–1154e2. https://doi.org/10.1016/j.amjsurg.2015.06.032 (2015).
Bonotto, M. et al. Controversies in monitoring metastatic breast cancer during systemic treatment. Results of a GIM (Gruppo Italiano Mammella) survey. Breast 40, 45–52. https://doi.org/10.1016/j.breast.2018.04.011 (2018).
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
AI conceptualised the study; MEL conducted the analyses and wrote the first draft of the manuscript; ST, ASP, IW, TE, FR, SRZ and KW acquired the data for the study; MKS, SRZ and BF administered the project; MEL, ST, ASP, IW and AI contributed to the design and interpretation of the analyses and the drafting of the final manuscript. All authors approved the final version and agreed to be accountable for the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
This is a retrospective study based on pseudonymized routine data from cancer registries. A separate informed consent is not required in the study according to German law on cancer registration.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lacruz, M.E., Thies, S., Schmidt-Pokrzywniak, A. et al. Clinical characteristics, metastasis patterns, and treatment outcomes of HER2-low breast cancer. Sci Rep 15, 4584 (2025). https://doi.org/10.1038/s41598-025-88394-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88394-6