Abstract
Drug-resistant epilepsy (DRE) presents significant challenges in treatment and management. While seizure-related alterations in peripheral immune players are increasingly recognized, the involvement of the immune complement system remains insufficiently explored in DRE. We studied complement components and their relationship to cytokine profiles in serum samples from 46 DRE patients and 45 matched healthy controls. We examined relationships between these molecules and clinical outcomes, including epilepsy duration, intelligence scores, and age. We identified DRE-associated complement decreases, including reduced levels of C1q, Factor H, C4, C4b, C3, and C3b/iC3b, as well as elevated bFGF. DRE females showed dysregulation of the classical complement pathway and lower TNFα and interleukin-8 compared to healthy females. DRE males exhibited dysregulation of the classical, lectin, and terminal complement pathways, with trends of increased CCL2 and CCL5 compared to healthy males. Specific complement and inflammatory markers (C2, IL-8, and IL-9) correlated with full-scale IQ scores in DRE patients. Our study reveals significantly lower levels of circulating complement components in DRE and sex-specific complement dysregulation and cytokine imbalances. These findings suggest an underlying peripheral immune system vulnerability that may be sex-dependent and warrants further investigation in DRE.
Similar content being viewed by others
Introduction
Epilepsy is a chronic neurological disorder characterized by spontaneous and recurrent seizures. One-third of epilepsy patients experience seizures that are resistant to standard anti-seizure medications (ASM), a condition known as drug-resistant epilepsy (DRE)1. Within the features of DRE, contemporary studies indicate the presence of immune system dysregulations with a complex interplay between brain and peripheral inflammation, possibly destabilizing the neurovascular interface and contributing to neurological defects2,3,4,5. Cellular and molecular immunity players have been examined in blood in association with seizures and epileptic conditions3,6,7,8,9,10, with indications of altered levels of pro-inflammatory cytokines such as Interleukin 6 (IL-6), IL-1β, and Tumor necrosis factor alpha (TNFα) suggesting a loss of homeostatic control of inflammation11,12,13,14,15,16,17,18.
This emerging research has, however, neglected the immune complement system, a key orchestrator of inflammation19. The complement system consists of over 30 small proteins produced in the liver and found in blood and tissue fluids19,20,21. Under healthy conditions, the complement system contributes to immune surveillance and homeostasis. Its activation occurs through three pathways: classical, lectin, and alternative (Fig. 1A), triggering proteolytic reactions that split complement proteins to create activation products. These proteins enhance the immune response by acting as anaphylatoxins, chemokines, opsonins, and cell-lysis complexes19,20,21. Activation of the complement system enables the release of inflammatory cytokines, which can, in turn, further activate the complement cascade, creating a feedback loop crucial for modulating immune responses19. Complement system dysregulations occur in infections, autoimmune disorders22,23,24, and neurological conditions25, including epilepsy26,27,28, potentially impacting symptoms and disease progression.
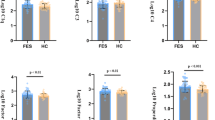
Serum complement component concentrations in patients with drug-resistant epilepsy (DRE) and healthy controls. (A) The diagram illustrates the convergence of the three complement pathways: alternative, classical, and lectin, leading to the cleavage of C3 and C5. The alternative pathway is activated through spontaneous hydrolysis of C3. The formation of C3b and Bb components (from Factor B) allows the cleavage of C3, being regulated by Factor H. All the pathways converge in the cleavage of C5 into C5b which helps assemble the membrane-attack complex (MAC) (terminal pathway). (B) Volcano plot illustrates the differential expression of complement proteins between DRE patients and healthy controls. The y-axis represents -log10(p-values) from t-tests and the x-axis represents log2 fold change (FC). (C-O) Serum concentrations of multiple complement molecules in DRE patients (blue) and healthy controls (gray): C1q, Mannose-binding Lectin (MBL), Factor B, Factor H, C2, C4, C4b, C3, C3a, C3b/iC3b, C5, C5a, and C5b-9. Violin plots show individual data points, median, and quartiles. Statistical analysis was performed using an unpaired t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Given the complement system’s role in immune regulation, investigating its activation in epilepsy is of significant interest, with possible diagnostic and therapeutic value. Studies on circulating complement levels in epilepsy patients have yielded mixed results26,27. One report found lower levels of complement component iC3b and higher levels of C4 in patients with uncontrolled focal seizures26, while another showed reduced C3 and C4 levels in patients with idiopathic generalized epilepsy27. Gene ontology enrichment and KEGG pathway analyses of serum from patients with DRE revealed upregulation in complement activation and coagulation cascades28. However, the extent of complement alterations and their relationship to the inflammatory response remains unclear. To address this knowledge gap, we measured the levels of multiple complement components and cytokines in the serum of individuals with DRE. We compared them to healthy age- and sex-matched controls. In this cohort, we assessed potential sex dimorphism in complement and cytokine serum concentrations and their relation to epilepsy duration, Full-Scale Intelligence Quotient (FSIQ) scores, and age.
Methods
Ethics statement
Serum samples were collected with patients’ informed consent under approved Institutional Review Board (IRB) protocol #1,011,004,282 (Development of a Biorepository for Methodist Research Institute; Indiana University Health Biorepository). All identifiable information was removed before conducting experiments and analyses were performed in accordance with relevant guidelines and regulations of IRB protocol #21–126 at Southern Methodist University.
Sample population
The study included a total of 91 subjects divided into two groups: patients with DRE and healthy controls (Table 1). The DRE group consisted of 46 patients (22 females and 24 males), while the healthy control group comprised 45 individuals (21 females and 24 males). The age distributions were similar across all subgroups, with DRE females ranging from 21 to 69 years (mean 38.54 ± 12.70), DRE males from 20 to 68 years (mean 38.25 ± 13.78), healthy females from 22 to 65 years (mean 36.86 ± 11.77), and healthy males from 19 to 69 years (mean 37.79 ± 14.15). Among the DRE patients, the mean duration of epilepsy was 20.85 ± 12.54 years for females and 18.56 ± 13.86 years for males. The DRE population selected for this study did not have a history of immune or inflammatory disorders. The DRE blood samples were collected as part of routine preoperative procedures. These patients subsequently underwent surgical resection to remove epileptogenic foci. Patients with DRE underwent neuropsychological assessment with a qualified neuropsychologist administering the Wechsler Abbreviated Scale of Intelligence, from which FSIQ scores were estimated. FSIQ scores were available for a subset of DRE patients, including 12 females (mean 86.92 ± 11.69) and 16 males (mean 89.50 ± 13.21). FSIQ scores were not assessed in the healthy control group. Our healthy cohort consisted of individuals undergoing routine outpatient procedures who had no history of immune system, inflammatory, or neurologic conditions. Following collection, serum samples were stored at -80 °C. Standardized protocols were followed for the collection, storage, and management of all serum samples within the biorepository facility.
Enzyme-linked immunoassay (ELISA)
Complement and cytokine levels were measured using various Milliplex and ELISA kits: Complement Panel 1 (HCMPEX1-19 K, Millipore Sigma): C2, C4b, C5, C5a, MBL; Complement Panel 2 (HCMP2MAG-19 K, Millipore Sigma): C1q, C3, C3b/iC3b, C4, Factor B, Factor H; Human C3a ELISA Kit (BMS2089, ThermoFisher Scientific); C5b-9 Singleplex Panel (EZS1M-140 K, Millipore Sigma); Bio-Rad Pro Human Cytokine Grp I panel 27-plex (M500KCSF0Y, BioRad). Kits were processed according to manufacturers’ instructions with serum dilutions ranging from 1:100 to 1:20000. Samples below the detection limit were excluded from analysis, including 12 cytokines from the 27-plex kit. The analysis of serum samples was conducted by laboratory personnel who were blinded to the group assignments.
Statistical analysis
Complement and cytokine comparisons between two groups (DRE and healthy controls) were performed using unpaired t-tests with Welch’s correction (Fig. 1 and Supplementary Fig. 5, Supplementary Table 1). For analysis of complement and cytokine blood levels across sex and health status (condition), a two-way analysis of variance (ANOVA) was used to assess the main effects of condition (healthy vs. DRE) and sex (male vs. female), as well as their interaction (Figs. 2 and 3; Supplementary Fig. 6; Supplementary Table 2). Šídák’s multiple comparisons test was applied for post-hoc pairwise comparisons between groups. The analyses were performed using GraphPad Prism, version 10.1.2, from GraphPad Software. Correlations were calculated using simple linear regression and Pearson correlation coefficient (r) analysis using R software (Version 4.4.0). The r value measures the strength and direction of the linear relationship between two variables, with values ranging from − 1 to 1 (Figs. 4 and 5). Correlation strength was interpreted as follows: Positive correlation: High (0.5 to 1.0), moderate (0.30 to 0.49), and small (0.10 to 0.29); Negative correlation: High (-1.0 to -0.5), moderate (-0.49 to -0.30), and small (-0.29 to -0.10). No correlation was defined as -0.1 < r < 0.1.
Serum complement component concentrations in male and female patients with drug-resistant epilepsy (DRE) and healthy controls. (A-C) Volcano plots illustrate the differential expression of complement proteins between male or female healthy and DRE groups. The y-axis represents -log10(p-values) from two-way ANOVA, and the x-axis represents log2 fold change (FC). (A) Healthy females and healthy males (B) DRE females and healthy females (C) DRE males and healthy males. (D-P) Serum concentrations of multiple complement molecules: C1q, Mannose-binding Lectin (MBL), Factor B, Factor H, C2, C4, C4b, C3, C3a, C3b/iC3b, C5, C5a, and C5b-9. DRE patients are represented in blue, and healthy controls in gray. Violin plots display individual data points, median, and quartiles. Statistical analysis was performed using two-way ANOVA with categorical variables: condition (DRE or healthy control) and sex (female and male). Significance levels: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Correlations between complement components. (A-D) Pearson correlation matrices of all complement components in (A) Healthy females, (B) DRE females, (C) Healthy males, and (D) DRE males. (E-H) Pearson correlations between C1q and C3b/iC3b in (E) Healthy females, (F) DRE females, (G) Healthy males, and (H) DRE males. I-L, Pearson correlations between MBL and C3b/iC3b in (I) Healthy females, (J) DRE females, (K) Healthy males, and (L) DRE males. (M-P) Pearson correlations between C5a and C3b/iC3b in (M) Healthy females, (N) DRE females, (O) Healthy males, and (P) DRE males. Gray areas in panels (E-P) indicate 95% confidence intervals. Top: Simplified diagram showing the classical (C1q) and lectin (MBL) pathways and downstream cleavage of C3 and C5. Red indicates correlations shown in panels (E-P). Supplementary Figs. 1–4 show all r and p values for all correlations by group.
Serum cytokine concentrations in male and female patients with drug-resistant epilepsy (DRE) and healthy controls. A-D, Volcano plots illustrate the differential expression of cytokines between male or female healthy and DRE groups. The y-axis represents -log10(p-values) from t-test or two-way ANOVA, and the x-axis represents log2 fold change (FC). (A) DRE patients and healthy controls (B) Healthy females and healthy males (C) DRE females and healthy females (D) DRE males and healthy males. E-I, Serum concentrations of TNFα, IL-8, CCL2, CCL5, and bFGF in male and female patients. DRE patients are represented in blue, and healthy controls in gray. Violin plots display individual data points, median, and quartiles. Statistical analysis was performed using two-way ANOVA with categorical variables: condition (DRE or healthy control) and sex (female and male). Significance levels: *p < 0.05, **p < 0.01, ***p < 0.001.
Correlations between serum concentrations of complement components and cytokines. A-D, Pearson correlation matrices between complement components and cytokines in (A) Healthy females, (B) DRE females, (C) Healthy males, and (D) DRE males. E-H, Pearson correlations between IL-17 and C3b/iC3b in (E) Healthy females, (F) DRE females, (G) Healthy males, and (H) DRE males. I-L, Pearson correlations between IL-8 and C4 in (I) Healthy females, (J) DRE females, (K) Healthy males, and (L) DRE males. M-P, Pearson correlations between CCL2 and C3b/iC3b in (M) Healthy females, (N) DRE females, (O) Healthy males, and (P) DRE males. Gray areas in panels E-P indicate 95% confidence intervals. Supplementary Figs. 1–4 show all r and p values for all correlations by group.
Results
Abnormal complement cascade components in DRE
The complement system consists of the classical (activated by C1q), lectin (activated by mannose-binding lectin; MBL, collectin, and ficolins), and alternative pathways which facilitate the cleavage of C3 into C3a and C3b (Fig. 1A). Subsequently, C3b facilitates C5 cleavage into C5a and C5b, with C5b initiating the formation of the membrane attack complex (MAC) (terminal pathway). C3a and C5a act as inflammatory signals, while C3b and C5b can also aid in phagocytosis. To assess the status of the complement system in DRE patients, we quantified the levels of these key components and compared them to those in healthy controls (Fig. 1; see Table 1 for details on patient cohorts). The overall profile of this complement cascade indicated a general decrease in the serum levels of several complement components in DRE patients compared to healthy individuals (Fig. 1B). Compared to healthy individuals, DRE patients showed evidence of lower abundance, by approximately 30%, in the components C1q (p < 0.0001), C4 (p < 0.001), C4b (p < 0.0001), C3 (p = 0.006), C3b/iC3b (p = 0.006), C5 (p = 0.038); and regulatory Factor B (p < 0.001) and Factor H (p < 0.0001) (Fig. 1C-O). In contrast, the levels of MBL, C2, C3a, C5a, and C5b-9 did not differ between the two groups (p > 0.05 and Supplementary Table 1). These findings suggest a decrease in complement levels in the serum of DRE patients compared to age-matched healthy individuals.
Next, we investigated whether sex-dependent differences existed (Fig. 2), as emerging evidence suggests that immune responses can differ between sexes29,30,31,32,33,34,35. The levels of complement components were similar between healthy males and females (p > 0.05, Fig. 2A) (Two-Way ANOVA shown in Supplementary Table 2). A decrease of approximately 30% in the levels of complement components C1q (p < 0.001), Factor B (p = 0.026), C4 (p = 0.015), C4b (p = 0.019), and Factor H (p = 0.001) was consistently observed in both female and male DRE groups compared to their healthy counterparts (Fig. 2B,C). However, DRE males had lower levels of C3b/iC3b compared to healthy males (p = 0.039), while DRE females showed no evidence of reduction (p = 0.372). The C3a, C5, C5a, and C5b-9 levels were similar between males and females in both groups (p > 0.05 and Supplementary Table 2). These findings suggest that both males and females with DRE have lower levels of circulating complement components.
Dysregulation of complement pathways in DRE
We further analyzed these molecular differences to elucidate potential functional defects within the complement cascade (Fig. 3, Supplementary Figs. 1–4). The sequential activation of specific components regulates the complement system, necessitating precise coordination to generate active molecules such as C3a, C3b, C5a, and C5b (Fig. 1A)36. Correlation analyses revealed distinct sex-specific patterns in complement pathway dysregulation. In healthy individuals (Fig. 3A,C), we observed high positive correlations between classical (C1q to C3b/iC3b; females, r = 0.72, p < 0.001; males, r = 0.89, p < 0.001), lectin (MBL to C3b/iC3b; females, r = 0.82, p < 0.001; males, r = 0.71, p = 0.001), and late cleavage pathways (C3b/iC3b to C5a; females, r = 0.92, p < 0.001; males, r = 0.92, p < 0.001), suggesting that these pathways may be functioning in a coordinated manner to maintain a balanced immune response. However, DRE females showed no evidence of a linear association between levels of MBL and C3b/iC3b (Fig. 3J), suggesting selective disruption of lectin pathway coordination. In contrast, male DRE patients exhibited broader pathway disruption, with a loss of normal correlations between complement components in both classical (Fig. 3H) and lectin pathways (Fig. 3L). While positive correlations between C3b/iC3b and C5a remained intact across all experimental groups (Fig. 3M-P), only healthy males maintained a statistically discernible positive correlation between C3b/iC3b and terminal pathway activation (C5b-9) (r = 0.61, p = 0.01). This loss of coordinated complement cascade progression, particularly pronounced in male DRE patients, suggests more extensive complement dysregulation that may contribute to broader immune homeostasis disruption in this population.
Cytokine serum levels in health and DRE
We investigated the association between the concentrations of complement components and cytokines in serum (Figs. 4 and 5; Supplementary Figs. 1–5). Our findings revealed distinct cytokine profile patterns between healthy individuals and DRE patients (Fig. 4A), as well as between males and females in both healthy (Fig. 4B) and DRE groups (Fig. 4C,D) (Supplementary Tables 1 and 2). We observed that the DRE group exhibited lower serum IL-8 levels (p = 0.001) and higher CCL2 (p = 0.03) and CCL5 (p = 0.009) levels compared to the healthy group (Fig. 4A). Additionally, there was a trend toward a difference in TNFα levels between the DRE and healthy groups (p = 0.06). ANOVA analysis revealed sex-specific differences in cytokine levels, with DRE females showing lower TNFα (p < 0.001) and IL-8 (p = 0.007) compared to healthy female controls (Fig. 3E,F), indicating unique immunological changes in the DRE female population. In contrast, DRE males showed a trend towards elevated CCL2 (p = 0.125) and CCL5 (p = 0.066) compared to healthy males (Fig. 3G,H). This trend was not observed in female DRE vs. healthy groups (CCL2, p = 1; CCL5, p = 0.261). Notably, bFGF was detected only in the DRE groups, while levels within the healthy group were below the sensitivity limit of the assay kit. We acknowledge that this limitation in detection sensitivity could affect group comparisons.
Our correlation analysis revealed marked differences in complement and cytokine interactions. While healthy females (Fig. 5A) and males (Fig. 5C) demonstrated greater positive correlations, these relationships were changed in DRE females (Fig. 5B) and males (Fig. 5D). Sex-specific patterns emerged in complement-cytokine relationships. Female subjects, both healthy and DRE, displayed positive correlations between C3b/iC3b and IL-17 levels (Fig. 5E,H), while this relationship was absent in males. Conversely, male subjects uniquely exhibited negative correlations between C4 and IL-8 (Fig. 5I,L). Notably, both male and female DRE patients showed positive correlations between C3b/iC3b and CCL2 that were not present in their healthy counterparts (Fig. 5M,P). These altered correlation patterns suggest dysregulated immune homeostasis in DRE patients, with the emergence of new C3b/iC3b-CCL2 relationships potentially indicative of pathological immune responses.
Complement and cytokine correlations with age and clinical outcomes
Finally, we analyzed the associations between complement components and cytokine profiles with epilepsy duration, intelligence scores, and age (Supplementary Figs. 1–4). There was no evidence of a correlation between epilepsy duration and complement or cytokine levels in DRE patients of either sex (Supplementary Figs. 1–4). Lower FSIQ scores were correlated with higher levels of C2 (r = -0.63, p = 0.039) and IL-8 (r = -0.66, p = 0.02) in DRE females (Supplementary Fig. 2), while in DRE males, lower FSIQ scores were linked to higher levels of IL-9 (r = -0.65, p = 0.007) (Supplementary Fig. 4). Intriguingly, despite the observed complement reduction in male DRE patients compared to healthy males, we found evidence of positive correlations between serum levels of C1q, C3, and C5 with age (Supplementary Fig. 4), suggesting a potential progressive activation of the complement system with aging in the male DRE group.
Discussion
Our study presents evidence of decreases in the levels of complement components in the serum of DRE patients, suggesting potential implications for a seizure-related immune vulnerability that may be exacerbated in a sex-dependent manner. Within the DRE population, we observed a general reduction in the serum levels of multiple complement analytes (C1q, Factor B, C4, C4b, and Factor H) (Fig. 4) along with common increases in bFGF (Fig. 3). Additionally, we found sex-specific differences in both the levels and coordination between complement components and cytokines (Figs. 3, 4 and 5) in healthy individuals and DRE patients. Our analysis also revealed associations between specific complement and inflammatory markers (C2, IL-8, and IL-9) and FSIQ scores in DRE patients, suggesting their potential as biomarkers for cognitive disability.
Our findings demonstrate lower serum concentrations of complement components such as C1q, C3, and C4 in DRE cases (Figs. 1 and 2). This evidence aligns with previous research showing alterations in the levels of these molecules in different populations of epilepsy patients26,27. A study of 157 epilepsy patients found evidence of decreases in iC3b levels among patients with uncontrolled seizures compared to those with controlled seizures26. Another study of 37 patients with idiopathic generalized epilepsy reported reduced serum levels of C3 and C4 compared to healthy controls, with a more pronounced decrease in untreated patients27. Taken together, these consistent findings suggest the possibility of a weakened complement system in association with epilepsy.
A weakened complement system can lead to various health challenges, including compromised innate and adaptive immunity, increased susceptibility to infections, a higher risk of autoimmune diseases, and chronic inflammation22,23,24. Deficiencies in classical pathway components (C1q, C1, C2, C4) are linked to autoimmune disorders like systemic lupus erythematosus (SLE)23,37,38, while MBL, C3, and C5 deficiencies increase susceptibility to bacterial and viral infections22,24. We speculate that reduced levels of complement components in epilepsy patients may increase susceptibility to immune-related comorbidities. Although our literature review did not identify studies linking epilepsy with increased susceptibility to infections or prolonged inflammation, some autoimmune disorders can be comorbid with epilepsy39. Unprovoked seizures have been reported in patients with SLE23,40,41,42, and C1q knockout mice exhibit neuronal hyperexcitability and epilepsy43, suggesting a plausible mechanistic link. However, it is important to note that the complement cascade involves numerous components beyond those evaluated here. Thus, to clarify the clinical relevance of these findings, longitudinal studies examining a broader range of complement components are needed.
Additionally, our findings reveal sex-specific variations in the complement system of DRE patients. Females exhibit a targeted impairment of the lectin pathway, while males show a broader dysregulation of the classical, lectin, and terminal pathways. Our novel findings suggest a more severe complement system dysregulation in males with epilepsy (Fig. 3 and Supplementary Fig. 4). Despite the absence of sex-dependent differences in complement component levels within the healthy groups in this study, previous research has shown evidence of sex-based variations in both the levels and functional activity of serum complement among healthy individuals34,35. Research involving 120 healthy Caucasian individuals revealed lower basal serum concentrations of C3, MBL, and C5-C9 components in females compared to males35. Conversely, another study reported higher serum levels of C1q and C3b and enhanced antibody-mediated cell lysis in healthy females, while healthy males showed increased activation of early complement pathway components34. This discrepancy between the current findings and existing literature highlights the complexity of complement system regulation and suggests that factors like sample size, population demographics, or methodological variations (e.g. sensitivity of ELISA kits) may have an impact on the observed outcomes.
The complement system contributes to maintaining immune homeostasis through its reciprocal relationship with cytokines19,20,21. Our study revealed common alterations in all DRE cases, including lower complement levels and detectable bFGF (Fig. 4 and Supplementary Figs. 5–6). DRE females showed reduced TNFα and IL-8 levels, while DRE males exhibited a trend toward elevated CCL2 and CCL5 levels, indicating sex-specific immune signatures. Reduced TNFα may impair immune responses and inflammation resolution, as lower levels of TNFα are also linked to increases in IL-644. Reduced IL-8 could impact infection clearance and wound healing through decreased neutrophil recruitment45. The elevated CCL2 and CCL5 levels in DRE males suggest a possible change in inflammation and immune cell recruitment46. The detection of bFGF in DRE cases, but not in healthy controls, suggests a potential compensatory mechanism to enhance tissue repair and angiogenesis47. Although these findings provide insights into the immune system in DRE patients, more detailed research on immune signaling molecules is needed to fully understand sex-specific differences in epilepsy.
A growing body of evidence in human and animal studies demonstrates sex-based disparities in cytokine production and immune responses in both health and disease conditions29,30,31,32,33, an aspect understudied in epilepsy. For example, studies of healthy human subjects revealed that males have higher plasma levels of TNFα, IL-1β, and IL-630 and a stronger monocyte-derived pro-inflammatory cytokine production in response to in vitro lipopolysaccharide challenge of blood samples than females32. In association with critical trauma events, males exhibit higher circulating concentrations of cytokines like TNFα and IFN-γ31. In the context of Alzheimer’s disease, evaluation of inflammatory profiles in peripheral blood leukocytes revealed higher levels of TNFα and IL-1β in males than females33. These findings collectively indicate sex-based differences in cytokine levels, with males generally displaying enhanced pro-inflammatory profiles compared to females in both healthy and injury or disease conditions. This pattern aligns with our findings, showing that DRE males exhibit elevated CCL2 and CCL5 levels. The alterations in these chemokine levels may be linked to the more extensive complement system dysregulation observed in DRE males. However, further research is necessary to confirm this potential connection.
Several limitations warrant consideration in future research. It remains unclear whether the observed decrease in complement levels and altered cytokine/chemokine profiles cause seizures, result from them, or have beneficial or harmful effects in epilepsy. Moreover, these alterations could also be attributed to the use of ASMs, as these medications can affect immune function48. Some ASMs, such as phenytoin, carbamazepine, and valproate, have immunosuppressive effects48, which may increase the risk of infection49. Additionally, immune system function can be influenced by factors such as sex hormones50 and age51. Our study also had some statistical limitations to consider. Our analysis used independent statistical tests to examine complement relationships, though the actual interactions are likely more complex and interdependent. Thus, the small sample size may have obscured some true relationships. Additionally, Pearson’s correlation only measures linear relationships between variables and is sensitive to outliers, potentially under- or over-estimating true correlations. Technical constraints must also be considered. The timing of blood sampling is critical, as interictal and ictal phases may show different blood biomarker profiles10,11,12,13,14,15,16, and our study was limited to a single-time collection. While sample storage could affect complement activation, we found no impact of storage duration on serum complement concentrations in either group (data not shown). Furthermore, conventional ELISA may lack sensitivity for low-abundance proteins. Future studies could enhance biomarker detection by employing single molecule array (SIMOA), which offers greater sensitivity52. These limitations highlight the need for controlled studies addressing ASMs, comorbidities, age, and seizure timing to clarify the complement system’s role in seizure generation and/or control.
Taken together, our findings of reduced complement components, along with sex-specific dysregulation of complement pathways and cytokine profiles suggest altered immune function in DRE. This idea is also supported by evidence of decreased CD4 + T cell populations in temporal lobe and focal epilepsies12,13 as well as increased CD4 + T cells in DRE14. However, it is important to note that the complex interplay between immune components, epilepsy outcomes, and potential immune vulnerability likely involves numerous factors that may be sex-specific and warrant further investigation.
Data availability
This study presents a comprehensive dataset sufficient for a thorough evaluation of the experimental outcomes. The data supporting these findings are available in the main article and accompanying supplementary material, with additional materials available from the corresponding author upon written request.
Change history
14 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-97486-2
Abbreviations
- DRE:
-
Drug-resistant epilepsy
- FSIQ:
-
Full-scale intelligence quotient
- ASM:
-
Standard anti-seizure medications
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- IL-1β:
-
Interleukin 1 beta
- IL-17:
-
Interleukin 17
- IFN-γ:
-
Interferon gamma
- CCL2:
-
C-C motif chemokine ligand 2
- CCL5:
-
C-C motif chemokine ligand 5
- TNFα:
-
Tumor necrosis factor-alpha
- WASI:
-
Wechsler abbreviate scale of intelligence
- MBL:
-
Mannose-binding lectin
- MAC:
-
Membrane attack complex
References
Asadi-Pooya, A. A., Stewart, G. R., Abrams, D. J. & Sharan, A. Prevalence and incidence of drug-resistant mesial temporal lobe epilepsy in the United States. World Neurosurg. 99, 662–666 (2017).
van Vliet, E. A. & Marchi, N. Neurovascular unit dysfunction as a mechanism of seizures and epilepsy during aging. Epilepsia 63(6), 1297–1313 (2022).
Hanin, A. et al. Cytokines in new-onset refractory status epilepticus predict outcomes. Ann. Neurol. 94(1), 75–90 (2023).
Villasana-Salazar, B. & Vezzani, A. Neuroinflammation microenvironment sharpens seizure circuit. Neurobiol. Dis. 178, 106027 (2023).
Janigro, D. et al. Peripheral blood and salivary biomarkers of blood-brain barrier permeability and neuronal damage: Clinical and applied concepts. Front. Neurol. 11, 577312 (2020).
Hosseini, S. et al. Neutrophil to lymphocyte ratio in epilepsy: A systematic review. Mediators Inflamm. 2022, 4973996 (2022).
Lim, H. K. et al. Seizure-induced neutrophil adhesion in brain capillaries leads to a decrease in postictal cerebral blood flow. iScience 26(5), 106655 (2023).
Stredny, C., Rotenberg, A., Leviton, A. & Loddenkemper, T. Systemic inflammation as a biomarker of seizure propensity and a target for treatment to reduce seizure propensity. Epilepsia Open 8(1), 221–234 (2023).
Marchi, N., Granata, T. & Janigro, D. Inflammatory pathways of seizure disorders. Trends Neurosci. 37(2), 55–65 (2014).
Cresto, N., Givalois, L., Badaut, J., Janvier, A., Genin, A., Audinat, E., et al. Bursts of brain erosion: Seizures and age-dependent neurological vulnerability. Trends Mol Med. (2024).
Shin, H. R. et al. Neuropsychiatric symptoms and seizure related with serum cytokine in epilepsy patients. Sci. Rep. 12(1), 7138 (2022).
Bauer, S. et al. NK and CD4+ T cell changes in blood after seizures in temporal lobe epilepsy. Exp. Neurol. 211(2), 370–377 (2008).
Sanli, E. et al. Peripheral blood regulatory B and T cells are decreased in patients with focal epilepsy. J. Neuroimmunol. 387, 578287 (2024).
Ouedraogo, O. et al. Increased frequency of proinflammatory CD4 T cells and pathological levels of serum neurofilament light chain in adult drug-resistant epilepsy. Epilepsia 62(1), 176–189 (2021).
Wang, J. et al. Th1/Th2 imbalance in peripheral blood echoes microglia state dynamics in CNS During TLE progression. Adv. Sci. (Weinh) 11, e2405346 (2024).
Gao, F. et al. Alteration of plasma cytokines in patients with active epilepsy. Acta Neurol. Scand. 135(6), 663–669 (2017).
Aguilar-Castillo, M. J. et al. A systematic review of the predictive and diagnostic uses of neuroinflammation biomarkers for epileptogenesis. Int. J. Mol. Sci. 25(12), 6488 (2024).
Costagliola, G. et al. Targeting inflammatory mediators in epilepsy: A systematic review of its molecular basis and clinical applications. Front. Neurol. 13, 741244 (2022).
Lo, M. W. & Woodruff, T. M. Complement: Bridging the innate and adaptive immune systems in sterile inflammation. J. Leukoc. Biol. 108(1), 339–351 (2020).
Mastellos, D. C., Hajishengallis, G. & Lambris, J. D. A guide to complement biology, pathology and therapeutic opportunity. Nat. Rev. Immunol. 24(2), 118–141 (2024).
Schartz, N. D. & Tenner, A. J. The good, the bad, and the opportunities of the complement system in neurodegenerative disease. J. Neuroinflammation 17(1), 354 (2020).
Schroder-Braunstein, J. & Kirschfink, M. Complement deficiencies and dysregulation: Pathophysiological consequences, modern analysis, and clinical management. Mol Immunol. 114, 299–311 (2019).
van Schaarenburg, R. A. et al. C1q deficiency and neuropsychiatric systemic lupus erythematosus. Front. Immunol. 7, 647 (2016).
Bernacchia, A., Ginaca, A., Rotondo, S., Tejada, M. P. & Di Giovanni, D. Case report: C3 deficiency in two siblings. Front. Pediatr. 12, 1424380 (2024).
Petrisko, T. J., Gomez-Arboledas, A. & Tenner, A. J. Complement as a powerful “influencer” in the brain during development, adulthood and neurological disorders. Adv. Immunol. 152, 157–222 (2021).
Kopczynska, M. et al. Complement system biomarkers in epilepsy. Seizure 60, 1–7 (2018).
Liguori, C. et al. Complement system dysregulation in patients affected by Idiopathic Generalized Epilepsy and the effect of antiepileptic treatment. Epilepsy Res. 137, 107–111 (2017).
Ma, M. et al. Serum biomarkers in patients with drug-resistant epilepsy: A proteomics-based analysis. Front. Neurol. 15, 1383023 (2024).
Osborne, B. F., Turano, A. & Schwarz, J. M. Sex differences in the neuroimmune system. Curr. Opin. Behav. Sci. 23, 118–123 (2018).
Bernardi, S. et al. Sex differences in proatherogenic cytokine levels. Int. J. Mol. Sci. 21(11), 6861 (2020).
Guidry, C. A. et al. Sex- and diagnosis-dependent differences in mortality and admission cytokine levels among patients admitted for intensive care. Crit. Care Med. 42(5), 1110–1120 (2014).
Beenakker, K. G. M. et al. Men have a stronger monocyte-derived cytokine production response upon stimulation with the gram-negative stimulus lipopolysaccharide than women: A pooled analysis including 15 study populations. J. Innate. Immun. 12(2), 142–153 (2020).
Sochocka, M., Ochnik, M., Sobczynski, M., Orzechowska, B. & Leszek, J. Sex differences in innate immune response of peripheral blood leukocytes of Alzheimer’s disease patients. Arch. Immunol. Ther. Exp. (Warsz) 70(1), 16 (2022).
Kelkar, N. S. et al. Sex- and species-associated differences in complement-mediated immunity in humans and rhesus macaques. iBio 15(3), e0028224 (2024).
Gaya da Costa, M. et al. Age and sex-associated changes of complement activity and complement levels in a healthy Caucasian population. Front. Immunol. 9, 2664 (2018).
Barnum, S. R. Complement: A primer for the coming therapeutic revolution. Pharmacol. Ther. 172, 63–72 (2017).
Kleer, J. S. et al. Complement C1s deficiency in a male Caucasian patient with systemic lupus erythematosus: A case report. Front. Immunol. 14, 1257525 (2023).
Schejbel, L. et al. Molecular basis of hereditary C1q deficiency–revisited: Identification of several novel disease-causing mutations. Genes. Immun. 12(8), 626–634 (2011).
Steriade, C., Titulaer, M. J., Vezzani, A., Sander, J. W. & Thijs, R. D. The association between systemic autoimmune disorders and epilepsy and its clinical implications. Brain 144(2), 372–390 (2021).
Mehta, P. et al. SLE with C1q deficiency treated with fresh frozen plasma: A 10-year experience. Rheumatology (Oxford) 49(4), 823–824 (2010).
Hannema, A. J. et al. SLE like syndrome and functional deficiency of C1q in members of a large family. Clin. Exp. Immunol. 55(1), 106–114 (1984).
Rodriguez-Hernandez, A. et al. Seizures in systemic lupus erythematosus: A scoping review. Seizure 86, 161–167 (2021).
Chu, Y. et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl. Acad. Sci. USA 107(17), 7975–7980 (2010).
Tuazon Kels, M. J. et al. TNF deficiency dysregulates inflammatory cytokine production, leading to lung pathology and death during respiratory poxvirus infection. Proc. Natl. Acad. Sci. USA 117(27), 15935–15946 (2020).
Matsushima, K., Yang, D. & Oppenheim, J. J. Interleukin-8: An evolving chemokine. Cytokine 153, 155828 (2022).
Gschwandtner, M., Derler, R. & Midwood, K. S. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 10, 2759 (2019).
Xie, Y. et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target Ther. 5(1), 181 (2020).
Beghi, E. & Shorvon, S. Antiepileptic drugs and the immune system. Epilepsia 52(Suppl 3), 40–44 (2011).
Zaccara, G. et al. Do antiepileptic drugs increase the risk of infectious diseases? A meta-analysis of placebo-controlled studies. Br. J. Clin. Pharmacol. 83(9), 1873–1879 (2017).
Sciarra, F., Campolo, F., Franceschini, E., Carlomagno, F. & Venneri, M. A. Gender-specific impact of sex hormones on the immune system. Int. J. Mol. Sci. 24(7), 6302 (2023).
Lewis, E. D., Wu, D. & Meydani, S. N. Age-associated alterations in immune function and inflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 118, 110576 (2022).
Yeung, D. et al. Evaluation of highly sensitive immunoassay technologies for quantitative measurements of sub-pg/mL levels of cytokines in human serum. J. Immunol. Methods 437, 53–63 (2016).
Acknowledgements
We thank IU Health ECRO Biorepository for providing us with all the serum samples utilized in this study.
Funding
This research was supported by the National Institute of Neurological Disorders and Stroke, Grant/Award Number, NS096234 (ALB); Children’s Brain Diseases Foundation (ALB), Dedman College Dean’s Research Council Grant and Laboratory startup funds SMU (ALB). ANR EpiNeurAge and EpiCatcher (NM). We thank IU Health ECRO Biorepository for providing us with all the serum samples utilized in this study.
Author information
Authors and Affiliations
Contributions
NPH: Conceptualization, Investigation, Methodology, Validation, Data curation, Formal analysis, Writing – original Draft, Writing – review and editing. YL: Investigation, Methodology, Data curation, Formal analysis, Writing – review and editing. MM: Formal analysis, Writing – review and editing. NPP: Writing – review and editing. NM: Writing – review and editing. AB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Serum samples were collected with patients’ informed consent under approved Institutional Review Board (IRB) protocol #1011004282 (Development of a Biorepository for Methodist Research Institute; Indiana University Health Biorepository). All identifiable information was removed before conducting experiments and analyses under IRB protocol #21–126 (Southern Methodist University).
Additional information
The original online version of this Article was revised: In the original version of this Article, Figures 3 and 4 were switched. Full information regarding the correction made can be found in the correction for this Article.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pinzon-Hoyos, N., Li, Y., McGee, M. et al. Drug-resistant epilepsy associated with peripheral complement decreases and sex-specific cytokine imbalances: a pilot study. Sci Rep 15, 5096 (2025). https://doi.org/10.1038/s41598-025-88654-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88654-5
Keywords
This article is cited by
-
Explainable machine learning identifies immune-inflammatory biomarkers and therapeutic candidates in drug-resistant epilepsy
Scientific Reports (2025)
-
Vascular Cell Adhesion Molecule-1 and Complement C3 involvement in Febrile Seizures in Children
Journal of Molecular Neuroscience (2025)