Abstract
Archerfish hunt by shooting a jet of water at aerial targets, a behavior used to study their visual processing by presenting a set of images on a screen above the water tank and observing the behavioral response. Building on this unique behavior, it was recently shown that archerfish can be trained to distinguish between different object categories by generalizing from examples. Analysis of the archerfish’s behavior revealed that the fish visual system relies on a small set of visual features for categorization and is more sensitive to object contours than to textures. To understand the neural basis of this object recognition, we investigated the neural representation of features and objects in the archerfish optic tectum using recording of single cells. We found that, although the optic tectum is an early stage of visual processing, a small population of neurons in this region contains information about the object category. This contrasts with the primate visual system, where the representation of objects emerges only at later stages of visual processing. These results suggest that early-stage feature extraction and object categorization in archerfish might represent a form of specialized visual processing. This contributes to a broader understanding of visual processing across taxa.
Similar content being viewed by others
Introduction
Object recognition is defined as the ability to identify or categorize an object appropriately despite substantial differences in the retinal representation of the object or category members1,2. Variations in object’s retinal image typically arise from different conditions under which the object may be viewed, such as illumination level, color of incident light, viewing distance, and angle, as well as other environmental variables. The ability of animal brains to recognize objects in an efficient and accurate manner depends on sophisticated neural computations that represent the features of an object in a way that enables classification and identification3,4.
Due to their importance, the mechanisms underlying object detection and recognition have been extensively investigated in mammals, predominantly primates, where these processes are associated with neural activity in the cortex1,5,6,7. In mammals, object recognition involves a hierarchical processing pathway in the ventral stream of the visual cortex. This visual stream is initiated when a signal is transferred from the retina to the inferior temporal cortex through several cortical areas. At each level, various features of the objects are processed depending on their complexity4,8,9.
Although non-mammalian species, which do not possess a neocortex, also demonstrate the ability to recognize and categorize objects10,11,12, the mechanisms involved are less well understood. The lack of extensive research on these species leaves a significant gap in our understanding of how different species process visual information. Addressing this gap is crucial for a comprehensive understanding of the evolution and diversity of visual processing across species.
Our study focuses on visual processing in teleost fish, specifically the archerfish, as a representative of non-mammalian vertebrates. We chose archerfish due to their evolutionary distance from mammals, potentially revealing unique visual mechanisms. Notably, archerfish exhibit distinctive hunting behaviors, using water jets to target aerial insects, a trait that allows conducting behavioral experiments in controlled laboratory conditions12,13,14,15,16,17,18,19,20,21.
Recent studies have shown that archerfish can distinguish between images of edible (animal) and images of non-edible (foliage) objects under various conditions, even categorizing objects they have never seen before. These abilities are primarily based on shape features, with the fish being more sensitive to object contours, such as convex hull and eccentricity, rather than texture22. The criticality of shape features was demonstrated by showing that the fish could continue to distinguish between edible and non-edible objects even when texture information was removed. In contrast, removing shape information while retaining texture resulted in a significant drop in recognition success.
In our study, we focused on the optic tectum, the primary visual processing unit in the archerfish brain, to investigate the neural basis of object recognition. We recorded and analyzed the activity of single neuronal cells within the superficial layers of the optic tectum in response to visual stimuli from two categories and schematic features. Our findings suggest that information about the object category can be extracted from a population of cells in the fish optic tectum, indicating that object recognition is achieved early in this animal.
Results
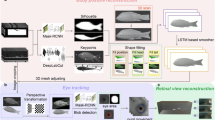
We explored the neural mechanisms that underlie the object recognition process in the archerfish brain, specifically in the optic tectum. We recorded the activity of 79 neuronal cells and measured their firing rate in response to various visual stimuli (Fig. 1). Overall, we used 100 images of objects from two categories: 50 images of edible (from the point of view the archerfish) objects – insects and spiders, and 50 images of the inedible objects – leaves and flowers. We also used three types of images with schematic features – shape compactness, shape eccentricity, and texture standard deviation - five levels each (Fig. 1b). We characterized the response of the individual cells and the population of cells.
Schematic view of the experimental setup and stimuli: (a) A single electrode is inserted into the optic tectum of the archerfish while a visual stimulus is presented on a computer screen within the receptive field of the recorded neuron. The obtained signal is then transferred through the amplifier and stored on the recording computer. (b) Objects used in the experiment as stimuli: the fish were presented with 100 different images of objects – 50 from each category of animals (insects and spiders) and non-animals (leaves and flowers), and 15 schematic features – 5 levels of shape compactness, shape eccentricity and texture standard deviation. (c) Stimulus timeline: a randomly selected image is presented within the receptive field of the cell for 200 msec. Time between the presentations is generated from the uniform distribution in 290–310 msec range. (d) Distribution of the normalized features extracted from 100 objects from two categories – animals (red) and non-animals (green): shape compactness, shape eccentricity, texture standard deviation, and average energy.
To identify selective neurons, we compared the firing rates in response to the two categories and defined a cell as selective if its response to one category was at least 20% higher than its response to the other, with statistical significance (Figs. 2 and 3). We also investigated the relationship between the cells’ preference for schematic features and their category preference (Fig. 4). At the population level, we assessed the ability to decode the stimulus category by training classifiers on the cells’ activity patterns (Fig. 5).
Example cells with a preference for the non-animal (a–d) and the animal (e–h) categories. (a) Raster plot of the cellular response. First 1500 trails correspond to animal objects, the last 1500 trials correspond to non-animal. In the experiment, the trials were randomized, here they are sorted for presentation purposes. (b) Firing rate in response to the objects from two categories: average firing rate from the appearance of the stimuli until 100 milliseconds after its removal, in bins of 25 milliseconds, and firing rate for the whole period (inset). c Average firing rate of a neuron in response to each of the 100 stimulus images, sorted in descending order by firing rate. The x-axis (‘image rank’) represents the position of each image after sorting. The images are the six stimuli with the highest and the lowest response rate. (d) Firing rate in response to three schematic features with five levels each: shape compactness, shape eccentricity and texture standard deviation, and average image energy. The firing rate is higher for the more circular objects with smooth contours. (e–h) Response of the example cell with a preference for the animal category, elliptical objects, and objects with jagged contour.
Example cell with no preference for any category. (a) Raster plot of the cellular response. The first 1500 trails correspond to animal objects; the last 1500 trials correspond to non-animal. In the experiment, the trials were randomized, here they are sorted for presentation purposes. (b) Firing rate in response to the objects from two categories: average firing rate from the appearance of the stimuli until 100 milliseconds after its removal, in bins of 25 milliseconds, and firing rate for the whole period. (c) Average firing rate of a neuron in response to each of the 100 stimulus images, sorted in descending order by firing rate. The x-axis (‘image rank’) represents the position of each image after sorting. The images are the six stimuli with the highest and the lowest response rate. (d) Firing rate in response to three basic features with five levels each: shape compactness, shape eccentricity and texture standard deviation, and average image energy. (e) Flow diagram showing the distribution of neuronal selectivity and its transitions between two time windows. The left column represents the first time window (200 ms after stimulus onset), and the right column represents the second time window (100 ms after stimulus offset). Flows connecting the two windows indicate the extent of stability or change in selectivity.
Population of cellular tuning to the object category and basic visual features. (a) Shape compactness tuning score vs. category tuning score of each cell, Pearson correlation coefficient is 0.64. (b) Shape eccentricity tuning score vs. category tuning score of each cell, Pearson correlation coefficient is 0.78. (c) Texture standard deviation tuning score vs. category tuning score: Pearson correlation coefficient is 0.01. (d) Average image energy tuning score vs. category tuning score of each cell Pearson correlation coefficient is -0.01.
Decoding neural activity from a population of cells. (a) Out of 79 recorded cells, 11 showed preference for the non-animal category, 14 – for the animal category, 54 showed no significant preference. (b) The neural activity in response to images from two categories was analyzed using several classifiers as a decoder. The image was presented, cellular activity was recorded and fed into decoder. (c) Several classifiers’ success rate in predicting the objects’ true category given cells’ neural activity. Applying the decoders on the population responses results in saturation of success rates near 0.85 for the SVM classifier, above the behavioral success rate which is in the range of 0.65 to 0.8. (d) Success rates of SVM classifier based on 15 random selective cells, 15 random nonselective cells and raw images.
The motivation for this selection was the recent observation that archerfish can distinguish between edible and non-edible items based on a small set of features. These features were predominated by shape features, specifically, compactness, defined as the ratio between convex hull area to the area of the object, and eccentricity, defined as the ratio between the foci of the ellipse that surrounds the object and the length of its major axis. Features that characterize the texture of the object were of lesser importance22.
Part of the neuronal population in the optic tectum is selective to object categories and basic features
We tested the tuning of single cells for the two object classes and specific features. For this purpose, we used 100 images of objects from two categories, as well as three sets of images with artificial features—shape compactness, shape eccentricity, and texture standard deviation—with five different levels for each feature. Figures 2 and 3 present examples of cellular responses to images from different classes. The cells responded with a burst of activity at both the onset and offset of the object within the receptive field, with sustained firing in between.
We examined neuronal responses separately during the stimulus-on and post-stimulus periods to investigate how tuning evolved over time. While many cells exhibited selective responses in both time windows, others shifted their preferences between the two time periods (Fig. 3e). Some neurons that were selective during the stimulus presentation lost their selectivity after the stimulus disappeared, while others became selective only after stimulus offset. These dynamics highlight the temporal diversity in how neurons encode visual stimuli. However, for the following analysis of neuronal selectivity and population-level tuning, we used spike responses from the entire time window, combining both the stimulus-on and post-stimulus periods.
We found three types of neuronal responses. First, there were cells that were selective for images of objects in the non-animal category (example cell in Fig. 2a–d). Out of 14 cells with a preference for the non-animal category, 12 were significantly tuned to shapes with smoother contours and a more circular form compared to ellipses or jagged circles. Second, we found cells with a preference for the animal category. They exhibited the opposite tuning to shape features: out of 13 cells selective to object from the animal category, 10 were significantly tuned to shapes with jagged edges and a more elliptical form. (example cell in Fig. 2e–h). Only 4 cells exhibited significant tuning to the texture standard deviation, but we found no relationship between cell preference for category and tuning to texture. Finally, there were cells that showed no preference for image categories; of these 52 cells, 29 showed no tuning to any of the schematic features’ (example cell in Fig. 3a–d).
To examine the connection between cell preference for image category and basic features, we analyzed the correlation between tuning to the category and tuning to the features. The correlation was high for shape compactness and shape eccentricity (Pearson correlation coefficients of 0.73 and 0.81, respectively) and low for texture standard deviation and image energy (Pearson correlation coefficients of -0.02 and − 0.09) (Fig. 4).
Overall, out of the 79 recorded cells, 14 cells had a significantly higher firing rate for images of objects in the non-animal category; 13 cells had a significantly higher firing rate in response to images of objects in the animal category; and 52 cells showed no preference for any specific category (Fig. 5a).
Information about object categories present in optic tectum on the network level
To address the amount of information present in the network of neuronal cells, we adopted an approach that uses a decoding algorithm to establish a lower bound on this information (Rieke et al., 1999). If a decoder can identify the object based on the cellular or population responses alone, then the information presented in this stage of visual processing must be substantial.
For this purpose, we used four classifiers as decoders to determine whether information about the category of the image stimulus was present in the population of neurons we recorded (Fig. 5b,c). We analyzed the activity of the neuron population in response to images of objects from the two categories.
We found that within the small population of 79 cells, there was information about the object category: the readout rate of the decoder reached 85%, i.e., the SVM classifier trained on the neural activity of the cells was able to correctly classify the stimulus images into their categories based on the neural responses to these images (Fig. 5c). This success rate is also higher than the level exhibited by the archerfish in behavioral experiments22. Other classifiers also reached rates above the chance level of 50%, though they remained within the range of behavioral success rates.
We also examined the contribution of category-selective cells compared to nonselective cells. We calculated the average success rate over 30 iterations of the SVM classifier trained on 15 random category-selective cells and then on 15 random nonselective cells. The classifier trained on the selective cells achieved a higher average success rate than the classifier trained on nonselective cells (75% vs. 63%, p = 2.8 * 10–29, Fig. 5d) or the classifier trained on raw images of objects, i.e. the pixel matrices of the stimuli used for direct classification without neural responses (p = 2*10–20). No significant difference was found between classifier success rate on non-selective cells and on raw images of objects. This indicates that the contribution of selective cells to the classification process is significantly greater than that of nonselective cells.
Interestingly, even the classifier trained on non-selective cells performed above chance level. This could indicate that some neurons carried weak but consistent category-related information that did not meet our statistical threshold for selectivity but still contributed to the classification. Additionally, some neurons may have been genuinely selective but failed to meet the criterion due to variability in firing rates or limited sample size. This highlights the potential for subtle, distributed coding of category information across the neural population.
Discussion
Our investigation into the neural representation of natural objects and schematic features in the archerfish optic tectum revealed several important findings regarding how object recognition occurs in a non-mammalian species. We identified various types of neuronal responses in the optic tectum, including those that displayed increased neural activity when exposed to images of objects from animal categories, those that exhibited a preference for non-animal category, and those that showed no preference. While the activity of individual neurons did not reliably discriminate between categories with high accuracy, the collective response patterns of a small population of neurons allowed us to classify images of objects into two categories with an 85% success rate. This success rate is comparable to those observed in behavioral discrimination tasks, suggesting that the optic tectum contains sufficient information for object categorization early in the visual processing pathway.
One of the key insights from our study is the role of feature selectivity in the neural representation of object identity. We observed that neurons in the optic tectum exhibit selectivity for specific visual features, particularly shape features such as convex hull and eccentricity. These features appear to play a central role in the archerfish’s ability to categorize objects, consistent with previous behavioral studies that highlighted the importance of shape over texture in object recognition22. The high correlation between neuronal tuning to object categories and these shape features suggests that cell selectivity to features may underlie the representation of object categories in the optic tectum.
Interestingly, the selective tuning of neurons to shape features, as opposed to texture, may reflect the ecological demands faced by archerfish in their natural habitat. As visual hunters that rely on accurately targeting aerial prey, archerfish must quickly and reliably identify objects based on their contours and overall shape, which are more stable and invariant across different lighting and environmental conditions compared to texture. This ecological specialization likely drives the neural circuitry in the optic tectum to prioritize shape information, facilitating efficient object recognition and successful hunting behavior.
Visual processing pathways in teleost and mammals
In comparing these findings to the object recognition process in mammals (Fig. 6a), it is important to consider the fundamental differences in brain organization between mammals and non-mammalian species like archerfish. In mammals, particularly primates, object recognition is primarily a cortical function, with the ventral stream of the visual cortex playing a central role in processing increasingly complex features of objects. The visual signal is transferred from the retina to the primary visual cortex V1, where basic features such as oriented lines are extracted23,24. Information is then transferred to V2 cortical areas which are selective to combinations of lines, gratings with different spatial frequencies, and color25. Different regions of visual area V4 are also responsive to orientation and spatial frequency but also to brightness and features of an object that define its shape, such as curvature26,27. Finally, the inferior temporal cortex receives input from other visual areas and processes complex object features. Areas of the inferior temporal cortex are tuned to specific concepts such as faces or body parts that are tolerant to image transformations including resizing and translation28,29. The cortical processing hierarchy allows for a sophisticated and flexible recognition system that can handle a wide range of visual challenges. However, in the absence of a cortex, archerfish rely on different neural structures, such as the optic tectum, to perform analogous functions.
Comparison of the archerfish and primate object recognition processing cascade. (a) In primate visual processing, the representation of object identity can be found only in the later stages of the visual pathway. Starting from the retina and passing through the lateral geniculate nucleus (LGN), visual information is transferred to the cortical areas V1, V2, V4, and the inferior temporal cortex (IT), with features of different complexity processed in each stage. (b) Schematic representation of the visual pathway and the visual feature processing in teleost fish: projections from retina target the optic tectum, pretectum, and prethalamus. The prethalamus is represented by the ventromedial (VM) and ventrolateral (VL) nuclei. The pretectum includes nuclei such as the dorsal pretectal nucleus (DP), periventricular pretectal nucleus (PPN), commissural pretectal nucleus (CPN), superficial pretectal nucleus (SPN), and others. Some of the nuclei project to optic tectum. Projections from the optic tectum extend to the nucleus isthmi (NI), preglomerular complex (PG) which relay information to the telencephalon. We found that a small group of neurons in the optic tectum can be decoded to identify objects. In the optic tectum there are neurons selective to basic features like bar orientation and moving frequency, and more complex stimuli like object identity.
Visual processing in teleost fish (Fig. 6b) originates in the retina, where photoreceptors convert light into neural signals30,31. These signals are transmitted by retinal ganglion cells through the optic nerve, which projects to three primary targets: the prethalamus, pretectum, and optic tectum32,33,34. The prethalamus, including the ventromedial (VM) and ventrolateral (VL) nuclei, plays a crucial role in sensory-motor integration and motor coordination. Signals from the prethalamus are directed to motor control areas, facilitating visually guided actions33,35. The pretectum, a key processing center for reflexive visual responses, includes multiple nuclei with distinct roles. For example, the dorsal pretectal nucleus (DP) and periventricular pretectal nucleus (PPN) are critical for brightness detection and pupillary reflexes36, while the superficial pretectal nuclei (SPN), and central pretectal nucleus (CPN) specialize in motion detection and visuomotor reflexes37. These pretectal regions project visual information to motor control centers and provide feedback to the optic tectum, enhancing visuomotor coordination38.
The optic tectum is the primary hub for retinal projections and visual signal processing in teleost fish32,33. Visual outputs from the optic tectum target the nucleus isthmi (NI), preglomerular complex (PGC), and telencephalon39,40. The NI provides reciprocal feedback to the tectum, enhancing the salience of behaviorally relevant visual stimuli through attentional modulation41. The PG functions as a thalamus-like sensory relay, transmitting visual information to the telencephalon for higher-order processing33,39.
The optic tectum serves as the central processing unit for visual signals in teleosts, functioning as an integrative hub for visual and other sensory information with its layered structure and extensive connectivity supporting diverse processing tasks30,32. A defining feature of tectal neurons is their mixed selectivity, where specific populations respond to combinations of visual features, enabling efficient recognition of ecologically relevant stimuli, such as prey and predators. Recent studies have revealed that distinct tectal neurons exhibit category-specific tuning, responding preferentially to objects based on size, orientation, contrast, and motion speed42,43,44,45. This suggests that aspects of object categorization occur at the tectal level, akin to feature processing observed in intermediate cortical areas of mammals, where features are combined for higher-order recognition tasks.
Another important aspect of our study is the comparison between the population-level analysis and the behavior of individual neurons. While individual neurons in the optic tectum may not always provide clear-cut category discrimination, the collective activity of a population of neurons offers a more robust representation of object identity. This population coding strategy is consistent with findings in other sensory systems, where information about stimuli is distributed across a network of neurons, allowing for more accurate and reliable perception46. In archerfish, this approach to coding object identity early in the visual processing pathway suggests a level of redundancy and resilience in the visual system, which may be particularly advantageous in the dynamic and complex environments these fish inhabit.
Moreover, our findings raise questions about the role of other brain regions, such as telencephalon, in visual processing in archerfish. Although the optic tectum appears to be a key player in early object recognition, telencephalon may also contribute to higher-order visual processing, similar to the role of the cortex in mammals. Recent evidence from zebrafish suggests that neurons in the dorso-central division of the telencephalon are selectively responsive to changes in visual numerosity, indicating a role in advanced cognitive processing47. Previous studies have also suggested that the telencephalon in teleosts is involved in various cognitive functions, including spatial processing and decision-making processing48,49,50,51. However, its specific role in object recognition remains largely unexplored. Further research is needed to determine how visual information processed in the optic tectum is integrated with inputs from the telencephalon and other brain regions, and how this integration supports complex behaviors such as prey capture and predator avoidance.
The anatomical and functional differences between the optic tectum in archerfish and the visual cortex in mammals highlight the diversity of solutions that evolution has produced for the problem of object recognition. While mammals have developed a highly specialized cortical system for visual processing, archerfish and other non-mammalian species have evolved different neural architectures that nonetheless achieve similar outcomes. This convergence of function, despite differences in structure, underscores the importance of studying a wide range of species to fully understand the principles of visual processing.
In conclusion, our study provides new insights into the early stages of visual processing in archerfish, demonstrating that object recognition can occur at a much earlier stage in the visual pathway than previously thought, at least in non-mammalian species. The optic tectum plays a central role in this process, encoding critical information about object identity through selective tuning to shape features. These findings not only contribute to our understanding of visual processing in archerfish but also have broader implications for the study of sensory systems across different taxa. By comparing the neural mechanisms of object recognition in archerfish and mammals, we can gain a deeper appreciation of the evolutionary diversity of visual systems and the adaptive strategies that different species have developed to navigate their environments.
Methods
Animals
The experiment was conducted on 19 archerfish Toxotes chatareus (6–14 cm; 10–18 gm). Adult fish were purchased from a local supplier and kept in 100-liter aquaria with brackish water with temperatures ranging from 25° to 29° C on a 16 − 8 light-dark cycle. Fish care and experimental procedures were approved by the Ben-Gurion University of the Negev Institutional Animal Care and Use Committee and were in accordance with the government regulations of the State of Israel. Additionally, all procedures were conducted in compliance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments).
Surgery and in vivo electrophysiology
The fish were anesthetized with a 0.1% solution of MS-222 (A-5040, Sigma-Aldrich, USA) and secured in a restraining device. Throughout the surgical procedure, the gills were continuously flushed with tank water containing a reduced concentration of MS-222 (0.05%) to both maintain the anesthesia and prevent respiratory distress. Lidocaine gel (Instillagel 2%, Farco-Pharma, Germany) was applied to the skin above the skull ten minutes prior to the incision. The skin and connective tissue were removed to expose the skull bone, followed by a reapplication of lidocaine to the perimeter of the incised area. To prevent muscle activity during the experiment, a 15 µl dose of gallamine triethiodide (1.7 gr per liter, G 8134, Sigma-Aldrich, USA) was administrated via injection near the spine of the lower body section. A craniotomy procedure was carried out over the right hemisphere of the optic tectum with a dental drill and fine-tip forceps. To minimize the motion-related artifacts in the electrophysiological recording, the fish’s head was securely immobilized.
Following the surgery, the restrained fish was placed in a small transparent tank filled with anesthetic-free water to a level just marginally higher than the fish’s eyes (Fig. 1a). The fish underwent a 30-minute period for the anesthetic to dissipate. Gill movement, a sign of active breathing, was used to confirm the fish’s return to wakefulness. A ground wire (silver, diameter 76.2 μm) was inserted into the brain tissue near the target area of the recording. A recording electrode (Platinum Iridium, impedance 2.7–3.2 MΩ, Alpha-Omega, Israel) was inserted into the outer layers of the right optic tectum (Karoubi et al., 2016) using a calibrated manipulator (Narishige, Japan) for precise control of the electrode position. The recorded signal was transferred to the amplifier (DAM 50, WPI, USA), and then sampled at 20,000 Hz using a custom LabVIEW program. The stimulus screen was attached to a monitor set mounted 15–25 cm away from the fish tank, adjustable for optimal location of the stimuli to the fish’s left eye.
To locate a cell, we inserted the electrode into the optic tectum and gradually adjusted its depth while presenting the fish with a full field flash stimulus. This process continued until a stable unit was detected. The boundaries of the receptive field were estimated by moving a white bar on a black background and noting the region in which the bar evoked a response from the neuron. The image stimuli were presented in the middle of the identified receptive field.
Spike sorting
Spike sorting was implemented using a custom MATLAB program. We applied a band-pass filter (0.3–5 kHz) to a signal. Potential spikes were identified as signal peaks that exceeded a threshold set at four times the standard deviation of the signal. Then the spikes were sorted into single units based on their features: width and amplitude of the peaks, PCA vectors, and the inter-spike interval.
Stimuli
We presented five types of visual stimuli to the fish: 50 different images of the objects from the category of insects and spiders, 50 images of the objects from the category of leaves and flowers, 5 schematic images that represent the feature of shape compactness at different levels, 5 schematic images that represent the feature of shape eccentricity at different levels, 5 schematic images that represent the feature of texture with a standard deviation at different levels (Fig. 1b–c). The images of the insects and the spiders were sourced from the BugwoodImages project website (insectimages.org). Images of flowers were obtained from the Oxford Flowers 102 dataset52, while leaf images were taken from the Flavia Plant Leaf Recognition project dataset53. Schematic images of the basic features were generated using Matlab. The images of the objects were normalized in their energy levels, with the energy levels for both categories ranging from 80 to 160. The objects’ shape compactness, eccentricity, and texture were preserved as in the original images (Fig. 1d). All images were presented in grayscale on a gray background. Each image was displayed 30 times in pseudo-random order for a duration of 200 ms. The time interval between image stimuli presentation ranged from 290 to 310 ms (Fig. 1c).
Data analysis
To characterize the activity of individual neurons, we measured the action potentials of the neurons in response to each type of stimulus and analyzed neuronal activity dynamics across different time periods. Specifically, we counted the number of spikes in three time windows: 0-200 ms (stimulus-on time), 200–300 (100 ms after stimulus disappearance), and the entire 0-300 ms period. Selectivity was assessed separately for each of these time windows. The final selectivity analysis and population-level analysis were conducted using spike counts from the 0-300 ms window.
To determine whether the neuron exhibited a category preference, we performed a permutation ANOVA with category (animal vs. non-animal) as a two-level factor. The permutation ANOVA was conducted using 10,000 permutations to assess the significance of the difference in neuronal responses between the two stimulus categories. If a significant effect of stimulus type was observed (p < 0.01), we performed a follow-up two-sample t-test to directly compare the mean firing rates for the two categories. This test was used to confirm the significance of the difference and identify the stimulus category eliciting the strongest neuronal response. To interpret meaningful differences, a minimum relative difference of 20% in firing rates between the categories was required to conclude a preference.
To determine whether cells exhibited a preference for schematic features, we analyzed their responses across five levels of each feature. For each cell, we fitted a linear model with feature level as the independent variable and firing rate as the dependent variable. The slope of the fitted model indicated the trend in neuronal responses across the feature levels, with a significant slope (p < 0.01) indicating a systematic preference for increasing or decreasing levels of the feature.
To assess the relationship between neuronal preferences for schematic features and stimulus categories on a population level, we calculated a tuning index for each cell’s response to both features and categories. We then computed the correlation between the tuning indices for each feature-category pair using Pearson’s correlation coefficient. This analysis was performed separately for each of the three schematic features, and also for the average energy, to determine whether feature tuning was associated with category selectivity.
The tuning index to category was calculated as the difference in response divided by the combined response to the two categories:
The tuning to the schematic features was defined as the ratio of the difference in firing rate in response to the strongest and the weakest levels of the schematic features, divided by the average firing rate in response to all levels:
The ratio was then normalized to the range between 0 and 1.
To analyze the activity of the population of neurons, we estimated the amount of information about the object’s category in the network of the recorded neurons. We assessed whether, given the activity of neural population in response to visual stimuli, we could predict the category of the object image that evoked this response. This analysis was conducted using four different classifiers as decoders: Support Vector Machine (SVM) with the Radial Basis Function kernel, Linear Discriminant Analysis (LDA), Naïve Bayes classifier, and k-Nearest Neighbors classifier. The classifiers were trained using a matrix of neuron activity as predictors and object image categories as class labels. The training set consisted of a random 75% of the images, while the remaining 25% were used as the test set. After 30 iterations, the average success rate of the classifier was calculated.
Data availability
The data and code used for analysis in this study are available upon request from Ronen Segev, ronensgv@bgu.ac.il.
References
DiCarlo, J. J., Zoccolan, D. & Rust, N. C. How does the brain solve visual object recognition? Neuron 73, 415–434 (2012).
Logothetis, N. K. & Sheinberg, D. L. Visual object recognition. Annu. Rev. Neurosci. 19, 577–621 (1996).
Biederman, I. & Bar, M. One-shot viewpoint invariance in matching novel objects. Vision. Res. 39, 2885–2899 (1999).
Afraz, A., Yamins, D. L. K. & DiCarlo, J. J. Neural mechanisms underlying visual object recognition. Cold Spring Harb Symp. Quant. Biol. 79, 99–107 (2014).
Santos, L. R., Hauser, M. D. & Spelke, E. S. Recognition and categorization of biologically significant objects by rhesus monkeys (Macaca mulatta): The domain of food. Cognition 82, 127–155 (2001).
Lamme, V. & Roelfsema, P. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23, 571–579 (2000).
Bougou, V. et al. Neuronal tuning and population representations of shape and category in human visual cortex. Nat. Commun. 15, 4608 (2024).
Bracci, S., Ritchie, J. B. & de Beeck, H. O. On the partnership between neural representations of object categories and visual features in the ventral visual pathway. Neuropsychologia 105, 153–164 (2017).
Grill-Spector, K., Kourtzi, Z. & Kanwisher, N. The lateral occipital complex and its role in object recognition. Vis. Res. 41, 1409–1422 (2001).
Watanabe, A., Fujimoto, M., Hirai, K. & Ushitani, T. Pigeons discriminate shapes based on topological features. Vis. Res. 158, 120–125 (2019).
Werner, A., Stürzl, W. & Zanker, J. Object recognition in flight: how do bees distinguish between 3D shapes? PloS One 11, e0147106 (2016).
Newport, C., Wallis, G. & Siebeck, U. E. Object recognition in fish: Accurate discrimination across novel views of an unfamiliar object category (human faces). Anim. Behav. 145, 39–49 (2018).
Ben-Simon, A., Ben-Shahar, O., Vasserman, G., Ben-Tov, M. & Segev, R. Visual acuity in the archerfish: Behavior, anatomy, and neurophysiology. J. Vis. 12, 18–18 (2012).
Ben-Tov, M., Donchin, O., Ben-Shahar, O. & Segev, R. Pop-out in visual search of moving targets in the archer fish. Nat. Commun. 6, 6476 (2015).
Mokeichev, A., Segev, R. & Ben-Shahar, O. Orientation saliency without visual cortex and target selection in archer fish. Proc. Natl. Acad. Sci. U.S.A. 107, 16726–16731.
Reichenthal, A., Ben-Tov, M., Ben-Shahar, O. & Segev, R. What pops out for you pops out for fish: four common visual features. J. Vis. 19, 1 (2019).
Vasserman, G., Shamir, M., Simon, A. B. & Segev, R. Coding what and when in the archer fish retina. PLoS Comput. Biol. 6, e1000977 (2010).
Parker, A. N., Wallis, G. M., Obergrussberger, R. & Siebeck, U. E. Categorical face perception in fish: how a fish brain warps reality to dissociate same from different. J. Comp. Neurol. 528, 2919–2928 (2020).
Potrich, D., Zanon, M. & Vallortigara, G. Archerfish number discrimination. eLife 11, e74057 (2022).
Volotsky, S., Donchin, O. & Segev, R. The archerfish uses motor adaptation in shooting to correct for changing physical conditions. eLife 12, RP92909 (2024).
Newport, C. Abstract concept learning in fish. Curr. Opin. Behav. Sci. 37, 56–62 (2021).
Volotsky, S., Ben-Shahar, O., Donchin, O. & Segev, R. Recognition of natural objects in the archerfish. J. Exp. Biol. 225, jeb243237 (2022).
Felleman, D. J. & Van Essen, D. C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 .
Rust, N. C., Schwartz, O., Movshon, J. A. & Simoncelli, E. P. Spatiotemporal elements of macaque v1 receptive fields. Neuron 46, 945–956 (2005).
Hegde, J. & Van Essen, D. C. Selectivity for complex shapes in primate visual area V2. J. Neurosci. 20, RC61 .
Gallant, J. L., Connor, C. E., Rakshit, S., Lewis, J. W. & Van Essen, D. C. Neural responses to polar, hyperbolic, and cartesian gratings in area V4 of the macaque monkey. J. Neurophysiol. 76, 2718–2739 .
Cadieu, C. et al. A model of V4 shape selectivity and invariance. J. Neurophysiol. 98, 1733–1750 (2007).
Lehky, S. R. & Tanaka, K. Neural representation for object recognition in inferotemporal cortex. Curr. Opin. Neurobiol. 37, 23–35 (2016).
Tanaka, K. Columns for complex visual object features in the inferotemporal cortex: clustering of cells with similar but slightly different stimulus selectivities. Cereb. Cortex 13, 90–99 (2003).
Robles, E. & Baier, H. Assembly of synaptic laminae by axon guidance molecules. Curr. Opin. Neurobiol. 22, 799–804 (2012).
Schmitt, E. A. & Dowling, J. E. Early retinal development in the zebrafish, Danio rerio: Light and electron microscopic analyses. J. Comp. Neurol. 404, 515–536 (1999).
Northmore, D. Optic tectum. Encyclopedia of Fish Physiology: From Genome to Environment 131–142 (Elsevier, 2011).
Mueller, T. What is the thalamus in zebrafish? Front. NeuroSci. 6, (2012).
Baier, H. & Wullimann, M. F. Anatomy and function of retinorecipient arborization fields in zebrafish. J. Comp. Neurol. 529, 3454–3476 (2021).
Mueller, T. & Wullimann, M. F. An evolutionary interpretation of teleostean forebrain anatomy. Brain Behav. Evol. 74, 30–42 (2009).
Wullimann, M. F. The neuromeric/prosomeric model in teleost fish neurobiology. Brain Behav. Evol. 97, 336–360 (2022).
Folgueira, M., Anadón, R. & Yáñez, J. The organization of the pretectal nuclei in the trout: A revision based on experimental holodogical studies. Brain Res. Bull. 75, 251–255 (2008).
Yáñez, J., Suárez, T., Quelle, A., Folgueira, M. & Anadón, R. Neural connections of the pretectum in zebrafish (Danio rerio). J. Comp. Neurol. 526, 1017–1040 (2018).
Hanako, H. Naoyuki, Y. Ascending visual pathways to the telencephalon in teleosts with special focus on forebrain visual centers, associated neural circuitries, and evolution. Zoolog. Sci. 40, 105–118 (2023).
Wullimann, M. F. & Mueller, T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J. Comp. Neurol. 475, 143–162 (2004).
Northmore, D. & Gallagher, S. Functional relationship between nucleus isthmi and tectum in teleosts: Synchrony but no topography. Vis. Neurosci. 20, 335–348 (2003).
Baier, H. & Scott, E. K. The visual systems of zebrafish. Annu. Rev. Neurosci. 47, 255–276 (2024).
Reichenthal, A., Ben-Tov, M. & Segev, R. Coding schemes in the archerfish optic tectum. Front. Neural Circuits 12, 18 (2018).
Bianco, I. H. & Engert, F. Visuomotor transformations underlying hunting behavior in zebrafish. Curr. Biol. 25, 831–846 (2015).
Preuss, T., Osei-Bonsu, P. E., Weiss, S. A., Wang, C. & Faber, D. S. Neural representation of object approach in a decision-making motor circuit. J. Neurosci. 26, 3454 (2006).
Averbeck, B. B., Latham, P. E. & Pouget, A. Neural correlations, population coding and computation. Nat. Rev. Neurosci. 7, 358–366 (2006).
Messina, A. et al. Neurons in the dorso-central division of zebrafish pallium respond to change in visual numerosity. Cereb. Cortex 32, 418–428 (2022).
Cohen, L., Vinepinsky, E., Donchin, O. & Segev, R. Boundary vector cells in the goldfish central telencephalon encode spatial information. PLoS Biol. 21, e3001747 (2023).
Vinepinsky, E. et al. Representation of edges, head direction, and swimming kinematics in the brain of freely navigating fish. Sci. Rep. 10, 14762 (2020).
Givon, S., Altsuler-Nagar, R., Oring, N., Vinepinsky, E. & Segev, R. Lateral and medial telencephalic pallium lesions impair spatial memory in goldfish. Brain Res. Bull. 204, 110802 (2023).
Calvo, R. & Schluessel, V. Neural substrates involved in the cognitive information processing in teleost fish. Anim. Cogn. 24, 923–946 (2021).
Nilsback, M. E. & Zisserman, A. In Automated Flower Classification over a Large Number of Classes 722–729 (IEEE, 2008).
Wu, S. G. et al. A leaf Recognition Algorithm for Plant Classification Using Probabilistic Neural Network 11–16 (IEEE, 2007).
Acknowledgements
We gratefully acknowledge financial support from The Israel Science Foundation (grant no. 824/21), and The Human Frontiers Science Foundation grant RGP0016/2019, and the Israel Scholarship Education Foundation (ISEF) foundation for SV.
Author information
Authors and Affiliations
Contributions
S.V. and R.S. designed the study. S.V. performed experiments. S.V. and R.S. analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Volotsky, S., Segev, R. Object identity representation occurs early in the archerfish visual system. Sci Rep 15, 4102 (2025). https://doi.org/10.1038/s41598-025-88660-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88660-7