Abstract
The distribution of certain permissive intermediate snail host species in freshwater is a crucial factor shaping transmission of intestinal schistosomiasis, a neglected tropical disease that causes much human suffering in Africa. To shed new light on southern Malawi, where cases of intestinal schistosomiasis have been found, repeated malacological surveys were conducted in Chikwawa and Nsanje Districts in the Lower Shire Valley, to detect and to characterize populations of Biomphalaria, the intermediate host for intestinal schistosomiasis. Sampling took place across a total of 45 freshwater sites, noting water conductivity, pH, temperature, total dissolved salts (TDS) and geographical elevation. The presence or absence of snails was predicted upon physiochemical and environmental conditions in Random Forest modelling. Water conductivity, TDS and geographical elevation were most important in predicting abundance of snails with water temperature and pH of slightly less important roles. This first report of B. pfeifferi in the Lower Shire Valley enhances understanding of the environmental factors that strongly associate and allow prediction of its local distribution. This represents a useful step towards developing appropriate intervention strategies to mitigate intestinal schistosomiasis transmission.
Similar content being viewed by others
Introduction
Several freshwater snail species within the genus Biomphalaria (Gastropoda: Planorbidae) serve as obligatory intermediate hosts of Schistosoma mansoni (Trematoda: Schistosomatidae), a parasitic blood fluke that causes intestinal schistosomiasis1. Among these snail species, Biomphalaria pfeifferi is most often regarded with greatest medical interest in sub-Saharan Africa1 for it is considered a major intermediate host, and often an invasive species, which facilitates disease transmission in many African countries2,3,4. In East and Central Africa, B. pfeifferi is commonly found predominately in riverine environments within Uganda5, Kenya3,6 and Tanzania6, while in Malawi its distribution is not fully understood7,8. Recently, this species has successfully colonized the Lake Malawi shoreline2,9. However, until our observations reported here, its occurrence in the Lower Shire Valley was suspected but not confirmed. Of note, a decade ago the prevalence of intestinal schistosomiasis in Chikwawa District, southern Malawi, as determined by urine-cathodic circulating antigen dipsticks, was 9.1% in pre-school-aged children and 24.9% in their mothers, with eggs of S. mansoni often confirmed in stool. However, concurrent malacological surveys failed to find Biomphalaria locally, despite extensive searching10. Prior to this, B. pfeifferi has only been confirmed in Dowa District, central Malawi7 and around Lake Malawi, specifically Mangochi District2, and in the past, in Karonga2, a much more northerly location and marginal along the lake.
Draining southwards, the Shire River exits Lake Malawi and flows towards Chikwawa and Nsanje Districts. Despite regulation of water flow for hydroelectricity, for example, seasonal flooding with local formation of potential freshwater snail habitats along both sides of the river may occur. To better understand the epidemiology of intestinal schistosomiasis transmission, repeated malacological surveys were conducted from May 2023 onwards to investigate and characterize the putative occurrence of Biomphalaria in the Lower Shire Valley: Chikwawa and Nsanje Districts. Determining the spatial presence of Biomphalaria sp. in microhabitats is necessary for identifying focal sources of intestinal schistosomiasis transmission and inform more effective mitigating disease control actions4,5,11. In Malawi, the National Schistosomiasis Control Programme provides praziquantel by regular mass drug administration (MDA)12,13 and to augment this foundational intervention, it is important to monitor and ascertain the geographical distribution of permissive intermediate snail hosts. Furthermore, better snail surveillance provides essential data on freshwater invertebrate biodiversity, and environmental management needs, against a background of wider climate change and anthropogenic impacts14,15,16,17.
Our aim was to identify habitats occupied by Biomphalaria in the Lower Shire Valley, determining the environmental factors influencing the distribution of these snails. We examined five abiotic factors that may affect the spatial extent of suitable habitats for these snails across the study area.
Materials and methods
Study area
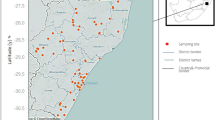
The study area covers Chikwawa (coordinates: 34.81265° E, 16.03223° S; land area: 4,878 km2) and Nsanje (coordinates: 35.26185° E, 16.92282° S; land area: 1,945 km2) districts, located along the lower flat basin of Shire River in southern Malawi, (Fig. 1). Most of Chikwawa and Nsanje have the lowest elevation in the country, ranging from ~ 42 − 1,584 m and ~ 31–965 m above mean sea level, respectively18,19. Both districts experience wet and dry tropical climates and are vulnerable to floods and droughts, attributed to climate change19,20. In 2018, Chikwawa and Nsanje districts had a population of 564,684 and 299,168 people, respectively21. Irrigated agriculture, fishing and subsistence farming in naturally occurring floodplains and riverine wetlands are currently dominant land- and water-use activities18. The prevalence of schistosomiasis from urine surveys conducted by the Ministry of Health in Chikwawa in 2008 and Nsanje in 2010 ranged from 14 to 56% and 44–62%, respectively12.
Chikwawa and Nsanje Districts in the Lower Shire of Malawi as presented with QGIS version 3.22.1 mapping software (https://qgis.org/). The inset map shows the location of Lower Shire Valley in the context of Malawi.
Malacological survey and cercarial shedding analysis
A prospective malacological survey was conducted from the 6th to 15th May 2023 to obtain data on the occurrence of Biomphalaria, estimate snail populations (numerical abundance) and measure environmental variables (conductivity, pH, salinity and temperature of water, and elevation) across 45 freshwater habitats in Chikwawa and Nsanje Districts. This study strategically sampled 45 potential freshwater snail habitats (i.e., aquatic microhabitats) in Chikwawa (n = 37) and Nsanje (n = 18) (Supplementary Table 1). The survey targeted aquatic habitats, such as lakes and marshlands, ponds and pools, rivers and canals, especially where human-water contact was known or observed to occur (including activities such as fishing, gardening, bathing, swimming, washing, etc.). The selection of sampling sites was based on accessibility and prior knowledge that molluscan intermediate hosts of schistosomes occur in lotic and lentic ecosystems and exhibit non-random distribution22,23. Thus, using stratified random sampling, we employed a direct method of surveying snails that involved passing a dip net, metal scoop and sieve (fine wire mesh kitchen strainer attached to a metal handle) through water, vegetation and mud to collect freshwater snails for 15 min24,25. In addition, the study investigated substrates, including plastic, sticks, stones, living and dead vegetation (e.g. water lily leaves) found in a water body. The morphology of shells was first used to distinguish Biomphalaria spp. from other genera of freshwater snails by a trained malacologist26,27. Dominant vegetation, water flow and presence of humans, animals and other freshwater snail genera such as Bulinus, Lymnaea, Melanoides and Lanistes were noted. However, given the focus on Biomphalaria here, it is beyond the scope of this study to examine these other data.
At each collection site, the study obtained geographical coordinates (Universal Transverse Mercator latitude and longitude) and elevation (m) using a hand-held Garmin Global Positioning Satellite (Garmin Montana 700 GPS, US), and water quality data. Water pH, water temperature (°C), conductivity (µS/cm) and total dissolved salts (TDS in ppm) were measured in situ using a combined pH, conductivity, TDS pocket tester: Hanna HI98129 (Hanna Instruments Ltd, UK). The pH, electroconductivity (EC), and TDS (ppm) readings are automatically temperature compensated to prevent temperature-related variations in the measurements. Of note, water samples taken for field measurement were collected immediately prior to collection of snails. This was to avoid disturbing sediments in the immediate sampling area. Additionally, when sampling in wadable waters, care was taken to avoid disturbing the stream bed or riverbed before collection of water in the sample container bottle. Elevation, temperature and water chemistry are generally important factors for the habitation of the Biomphalaria snails5,22,26. Natural factors (e.g., temperature) and physicochemical parameters of water constrain or expand the distribution of the freshwater snails of the genus Biomphalaria5,22,27. Against this background, a careful a priori selection of the environmental variables hypothesized to directly influence Biomphalaria distribution and abundance in freshwater ecosystems was made. Regarding the choice of the explanatory variables, we used five abiotic factors that are known to affect freshwater snail distribution, namely elevation, pH, temperature, conductivity and TDS27,28. To aid habitat reconnaissance, vertical and oblique aerial photographs (20MP camera sensor) and videos (5.1 K video recording) of the semi-aquatic habitat were captured by a small commercial-grade drone, Mavic 3 (DJI Shenzen, China), at an altitude of 40 m with the camera pointed at 90 and 45 degrees downwards and forwards, respectively.
Following malacological collections, all collected Biomphalaria were subjected to cercarial shedding analysis in attempt to identify any snails harboring patent infections with Schistosoma spp. trematodes according to a standard inspection protocol outlined previously29.
Molecular characterization and molecular xenomonitoring of collected Biomphalaria
DNA was isolated from eight randomly selected ethanol preserved Biomphalaria specimens using the QIAGEN DNeasy Blood & Tissue Kit [QIAGEN, UK]. These snails originated from Site 17, located within the Nchalo Estate, and were collected from a water site considered to have had the greatest human water contact based upon observations on site at the time of snail collections. DNA extraction was conducted according to manufacturer’s instructions with minor revisions including using double volume of ATL buffer and Proteinase K during tissue lysis30.
Following DNA extraction, these eight Biomphalaria specimens were characterised to species level using end-point PCR and Sanger sequencing of a 700-bp region of the mitochondrial cox1 gene, as detailed previously9,30. In addition, a recently developed S. mansoni-specific molecular xenomonitoring assay was also used in an attempt to detect patent, but non-shedding, or prepatent S. mansoni infections within these eight Biomphalaria specimens29.
Environmental data analysis
The study employed a Random Forest (RF) machine learning framework to determine the environmental variables that are directly linked with Biomphalaria spatial presence and absence31. The RF is resistant to overfitting, does not require cross-validation and provides comparatively excellent accuracy31,32,33. First, the study derived a presence-absence (yes/no) categorical variable from the numerical abundance of the Biomphalaria snails at each site. Then a spatially-naive RF classification model was fitted, using the present/absent variable as the response variable and elevation, water pH, temperature, conductivity and TDS as the explanatory variables. In RF, both the predictors and responses are randomly arranged in all possible ways31,32. The model was implemented using random Forest package (version 4.7.1.1)34 and visualised using reptree package (version 0.6)35 in R Statistical Software (version 4.2.2; R Core Team 2022)36. Model performance was assessed using accuracy, precision, recall (sensitivity) and F1 metrics calculated from confusion matrices and out-of-bag (OOB) error estimates. The OOB error is an estimate of prediction error on test data37,38. The importance of each explanatory variable in predicting Biomphalaria absence/presence was measured using mean decrease Gini (impurity) and mean decrease accuracy (permutation importance), MDG and MDA hereinafter, respectively. In an RF, MDG measures how much an input variable reduces the impurity of the tree39. The MDA measures the reduction in accuracy of the model when a variable is randomly permuted40. As the MDG or MDA values of a variable increase, its importance in the model increases40.
In addition, the Wilcoxon test was employed to evaluate the environmental ranges associated with the presence or absence of Biomphalaria, based on the statistical significance of the differences represented in the boxplots. This non-parametric approach was chosen due to its ability to accommodate the occurrence data, as it does not presume a normal distribution. The interpretability of the data is improved by incorporating the results of this analysis, which include p-values, into the boxplots.
Results
Current occurrence and abundance of Biomphalaria determined in Lower Shire
From the malacological surveys, several extant Biomphalaria populations were found to occur in Chikwawa, observed for the first time (Fig. 2) and a seminal report of this genus in the Lower Shire Valley. Of the 45 sites sampled, 11 sites (23%) were found to contain these snails. A total of 144 Biomphalaria were collected from irrigation canals in Chikwawa (Supplementary Table 1). The highest abundance of Biomphalaria (n = 122) was found in site number 17 representing almost 85% of the observed snail population from the sampled irrigation canals. A total of 22 Biomphalaria were recorded in low abundances in other sites (n ≤ 8 in 10/28 sites). In Nsanje, there were no Biomphalaria recorded during the survey (Fig. 3). No collected Biomphalaria were found to be shedding Schistosoma spp. cercariae during cercarial shedding analysis.
A drone imagery of a semi-aquatic snail habitat (S16.17069, E34.8487) in Chikwawa which yielded a total of 122 Biomphalaria snails as presented with QGIS version 3.22.1 mapping software. The habitat is a narrow earth-lined irrigation canal/drain with deep, slow flowing water (mean pH = 8.2, mean temperature = 29.4 °C, mean conductivity = 1001 µS/cm, mean TDS = 501 ppm). Here, the snails were found floating on the surface or attached to the underside of water lilies (A) being collected in large numbers (B) with their shells shown in apical (C) and, apertural and umbilical (D) views.
Molecular characterization and molecular xenomonitoring of collected Biomphalaria
Genomic DNA (gDNA) was successfully isolated from all eight Biomphalaria specimens, all of which were identified as B. pfeifferi through cox1 analysis29. All eight B. pfeifferi cox1 sequences were submitted to the GenBank repository (accession numbers OR880274 - OR880281). No evidence of B. pfeifferi infection with S. mansoni (or any other species of Trematoda) was found using molecular xenomonitoring.
How is the occurrence of B. pfeifferi snails associated with variation in elevation and aquatic physicochemical conditions in lower Shire?
-
1.
Variable association
Upon the molecular identification and confirmation of the B. pfeifferi, Fig. 4 below indicates the ranges associated with snail’s presence or absence across the surveyed area. From the plot, generally B. pfeifferi presence is probable if elevation is around > 150 m, pH is < 8.5, temperature is between > 25 to < 31 °C. In addition, conductivity of around 1000 µS/cm and TDS of around 500 ppm are associated with B. pfeifferi presence.
-
2.
Variable importance
Figure 5 provides the most and least important variables in explaining the spatial presence and absence of B. pfeifferi in the study area. From the plot, conductivity contributed significantly to the prediction accuracy of the RF model, followed by TDS, then elevation, temperature and lastly, pH. Based on the MDA, conductivity (MDA = 12.78), TDS (MDA = 11.98) and elevation (MDA = 10.03) rank highly. From the fitted model, the prediction accuracy decreased significantly when the TDS and conductivity variables are removed from the model. With an MDA of 5.65, temperature ranked moderately, whereas pH ranked lowest, with an MDA of -0.45. Figure 6 shows that elevation, conductivity and TDS had a higher MDG of 3.24, 2.98 and 2.93, respectively. This indicates that these variables were more important for the model’s predictive power. While temperature had a moderate MDG of 2.08, in contrast, pH had low MDG of 0.81, indicating less impact of the former on the accuracy of the B. pfeifferi prediction model.
Variable importance plot ranking the explanatory variables (y-axis) based on their predictive power and contribution to the accuracy of the predicted B. pfeifferi presence and absence in the study area by the RF model. A higher MDG value indicates that the variable was significant in distinguishing between classes in the model. A variable with a higher MDA contributed more to the predictive accuracy of the model, as its absence (or randomization) lead to a significant drop in model performance.
Figure 6 below shows the relative importance of the explanatory variables in predicting B. pfeifferi based on the depth at which the variables appeared in any of the decision trees when constructing the forest. From Fig. 7, it can be observed that elevation, TDS, conductivity and temperature had relatively low minimal depth. This observation differs from that of pH.
The minimal depth plot indicates the impact of the explanatory variables on the predictive ability of the RF model. Lower values indicate the most important variables in determining the decision boundary relative to the number of trees. Elevation, TDS, conductivity, temperature and pH contributed to the decision trees when 150, 100, 110, 100, and 10 trees were split in the ensemble, respectively. Noticeably, from >200 trees onwards, pH and temperature were not selected for splitting in the classification trees. Note that the mean of the distribution is marked by a vertical bar with a value label on it (the scale for it is different than for the rest of the plot), and the scale of the X axis goes from zero to the maximum number of trees in which any variable was used for splitting.
-
3.
RF model prediction
The RF classification tree for B. pfeifferi presence-absence in the Lower Shire is set out in Fig. 7. From the tree, conductivity is the root, indicating that it was measured as the most important variable for the prediction of B. pfeifferi occurrence. From the RF prediction, B. pfeifferi presence is probable if conductivity > 931 µS/cm, TDS < 509 ppm and elevation > 146 m. This is consistent with the in situ aquatic physicochemical and topographic conditions observed at the sites where B. pfeifferi occurred (Supplementary Table 1). Collectively, conductivity, TDS and elevation were important for the snail’s presence or absence in the study area.
Model performance
RF model performance was evaluated using accuracy, precision, recall (sensitivity) and F1 metrics calculated from the confusion matrix (Table 3). The accuracy of the model on testing data was 0.8 (80%) with 95% CI (0.4439, 0.9748). The precision, recall and F1 scores on the ‘positive’ class (absent) were 0.7778 (~ 78%), 1 (100%) and 0.8750 (~ 88%), respectively. Together, these high values indicate that the model performed well for the given presence/absence prediction task (Supplementary Fig. 3 and Fig. 4, Supplementary Table 4). Consider, for example, the F1 score. A high F1 score suggests that the model balanced precision and recall, and vice-versa41.
Discussion
Using a combination of malacology surveys, geospatial techniques and molecular analyses, our study has detected and identified several B. pfeifferi populations for the first time in Chikwawa District. To our knowledge, this constitutes the first formal report of this species in the Lower Shire Valley, an important finding given its well-known role in intestinal schistosomiasis transmission elsewhere (Supplementary Fig. 1, Fig. 2 and Fig. 5). We posit that across the Lower Shire Valley, this species is difficult to find because the population size and density are low, and the Shire River landscape is complex with many ephemeral water habitats that may be colonized by snails outside of the dry season. The RF model results indicate that B. pfeifferi occurrence varies across fine-scale water chemistry and physical environmental gradients and is related with conductivity, TDS and elevation (Supplementary Fig. 3 and Fig. 4). Conversely, the results show that while temperature and pH are related with the spatial presence and absence of B. pfeifferi, the association of the snails to these variables, however, is weak. This suggests that across the study area, water pH and temperature moderately influence B. pfeifferi occurrence. Because the predictive power of elevation and water conductivity and TDS was high, the RF model suggests that these three variables are the most important predictors of B. pfeifferi spatial presence/absence in lower Shire. This finding supports evidence from previous observations5,23,42.
The study found that, generally, elevations from 160 to 180 m, aquatic TDS between 400 and 600 ppm (mg/L) and aquatic conductivity ranging from 800 to 1000 µS/cm are positively associated with B. pfeifferi presence across the lower Shire (see Figs. 4, 5, 6 and 7). Of note, water conductivity and TDS are correlated41, and conductivity is an indicator of salinity43. Collectively, it seems possible that B. pfeifferi is sensitive to aquatic salinity and can only tolerate a specific salinity range. This implies that salinity change, for example, due to human disturbances such as farming44,45, evaporation, run-off and flooding can affect the presence of the B. pfeifferi across the study area. Considering that the water physicochemical conditions across the study area exhibited spatial variation along an elevation gradient, it naturally follows that the distribution of B. pfeifferi is disjunct and non-uniform. Thus, this study: (1) revealed that B. pfeifferi is present in Chikwawa, (2) showed that B. pfeifferi was abundant at sites with slightly alkaline water, (3) asserts reports of B. pfeifferi snails having a narrow tolerance range for water conductivity and alkalinity26, and (4) supports the idea that hydrogen ion concentration (pH) is a weak limiting factor for the species distribution22. Overall, the observed B. pfeifferi occurrence and abundance appears to be influenced by the compound interactions among these aquatic physicochemical and environmental factors. For example, it is well known that the water temperature affects conductivity readings46,47 and TDS and conductivity are correlated42,47,48. In the variable importance plot, the close ranking of TDS and conductivity is hardly surprising because they both essentially measure salinity levels49. Taken together, it emerges that saltiness of water provides the best prediction of the snail’s occurrence.
The confusion matrix indicates that the accurate prediction of B. pfeifferi presence by the RF model proved difficult. The RF model performed extremely well in predicting the absence of the B. pfeifferi. Since misclassification errors were reasonably high for presence outcome, the model was at its worst when predicting presence (Supplementary Table 2). A possible explanation for this might be that the snails have low occurrence in “suitable habitat”. Very few presences were recorded in suitable habitat (Supplementary Table 1). Another reason may be the lack of adequate data of presence and absence records. There would therefore seem to be a definite need to explore methods of improving spatially-naive RF models for predicting B. pfeifferi, and for more records of B. pfeifferi presence/absence across lower Shire. Overall, the RF results are acceptable, given that 80% of the influence of the aquatic chemistry and elevation on B. pfeifferi occurrence across lower Shire can be explained by the fitted RF model. One notable strength of the RF algorithm is its ability to discover and explore non-linear relationships in complex, environmental data with high accuracy, even with limited sample size31,32,33. Another strength is its ability to determine variable importance and find an optimal solution while averting over-fitting and collinearity between variables, issues generally associated with traditional parametric models31,32. In important papers on RFs, Breiman31,37 provides empirical evidence for this assertion.
Finally, to augment our geospatial findings, a wider phylogenetic analysis of our cox1 sequences was recently reported by Archer et al.29, revealing strong affiliations with Mangochi District (Malawi) and Zimbabwe populations. It is reasonable to assume a relatively recent downstream dispersal of B. pfeifferi from Mangochi to Chikwawa, notably haplotype 2 as originally identified by Al-Harbi et al.9. Whilst Archer et al.29 detected a single B. pfeifferi shedding cercariae of S. mansoni in their shoreline surveys, interpretation of our molecular xenomonitoring findings presented here is more equivocal. The most recent epidemiological survey for intestinal schistosomiasis, conducted in September 2023 by Chiepa et al.50, is noteworthy. Using urine-circulating cathodic antigen dipsticks, the study sampled 1,134 school-aged children from 21 government-owned primary schools, identifying the highest local prevalence of 18.5% at Tomali Primary School. Of note, Tomali Primary School is located closest to sampling site 17, less than 5 km away, but to unequivocally prove autochthonous transmission, the search for locally shedding snails continues across the Chikwawa floodplain continues.
Conclusions
Our study presents the first report and formal record, with geovisualisation and spatial modelling, of B. pfeifferi within Chikwawa District. Better predicting and noting the presence and abundance of B. pfeifferi in future is an important step towards developing locally appropriate interventions to reduce intestinal schistosomiasis transmission.
Data availability
The genetic sequences generated and/or analysed during the current study are available in the GenBank repository, [https://www.ncbi.nlm.nih.gov/genbank/ and Accession numbers OR880274 - OR880281]. The survey dataset that supports the findings of this study are available in the supplementary materials.
References
Colley, D. G., Bustinduy, A. L., Secor, W. E. & King, C. H. Human schistosomiasis. Lancet 383, 2253–2264 (2014).
Alharbi, M. H. et al. Biomphalaria pfeifferi Snails and Intestinal Schistosomiasis, Lake Malawi, Africa, 2017–2018. Emerg. Infect. Dis. 25, 613–615 (2019).
Bu, L. et al. A genome sequence for Biomphalaria pfeifferi, the major vector snail for the human-infecting parasite Schistosoma mansoni. PLoS Negl. Trop. Dis. 17, e0011208 (2023).
Coelho, P. R. S. et al. Identification of risk areas for intestinal Schistosomiasis, based on malacological and environmental data and on reported human cases. Front. Med. 8, 642348 (2021).
Stanton, M. C. et al. Intestinal schistosomiasis in Uganda at high altitude (> 1400 m): Malacological and epidemiological surveys on Mount Elgon and in Fort Portal crater lakes reveal extra preventive chemotherapy needs. Infect. Dis. Poverty. 6, 34 (2017).
Magero, V. O., Kisara, S. & Wade, C. M. Geographical Distribution of Biomphalaria snails in East Africa. Preprint at (2021). https://doi.org/10.1101/2021.11.04.467236
Wiselka, M., Robinson, M., Clipsham, K. & Weddon, S. The epidemiology of schistosomiasis in central Malawi. (1988).
Makaula, P. et al. An assessment of implementation and effectiveness of mass drug administration for prevention and control of schistosomiasis and soil-transmitted helminths in selected southern Malawi districts. BMC Health Serv. Res. 22, 517 (2022).
Alharbi, M. H. et al. Biomphalaria pfeifferi (Gastropoda: Planorbidae) in Lake Malawi and Upper Shire River, Mangochi District, Malawi: Distribution, genetic diversity and pre-patent schistosome infections. Trop. Med. Infect. Dis. 8, 126 (2023).
Poole, H. et al. Schistosomiasis in pre-school-age children and their mothers in Chikhwawa district, Malawi with notes on characterization of schistosomes and snails. Parasit. Vectors. 7, 153 (2014).
Reed, A. L. et al. A geospatial analysis of local intermediate snail host distributions provides insight into schistosomiasis risk within under-sampled areas of southern Lake Malawi. Parasit. Vectors. 17, 272 (2024).
Ministry of Health and Population. National Schistosomiasis and STH control programme report of 2018 MDA Campaign. (2018).
Ministry of Health, Republic of Malawi. Malawi neglected tropical diseases master 2015 to 2020; Master Plan for Malawi. (2014).
Stensgaard, A. S. et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: Does climate matter? Acta Trop. 128, 378–390 (2013).
Manyangadze, T., Chimbari, M. J., Gebreslasie, M., Ceccato, P. & Mukaratirwa, S. Modelling the spatial and seasonal distribution of suitable habitats of schistosomiasis intermediate host snails using Maxent in Ndumo area, KwaZulu-Natal Province, South Africa. Parasit. Vectors. 9, 572 (2016).
Glidden, C. K. et al. Climate and urbanization drive changes in the habitat suitability of Schistosoma mansoni competent snails in Brazil. Nat. Commun. 15, 4838 (2024).
Pedersen, U. B. et al. Modelling climate change impact on the spatial distribution of fresh water snails hosting trematodes in Zimbabwe. Parasit. Vectors. 7, 536 (2014).
Malawi Government. Chikwawa District Physical Development Plan. (2020).
Gondwe, S. V. C. & Shukla, S. H. Mapping flood risk of Nsanje District in Malawi using sentinel-1 data. J. Remote Sens. GIS. 11, 11–34 (2021).
Government of Malawi. Nsanje Disaster Impact Assessment & Transitional Recovery Framework Report. (2012).
National Statistical Office. Malawi Population and Housing Census Main Report (Government of Malawi, 2018).
Olkeba, B. K. et al. Environmental and biotic factors affecting freshwater snail intermediate hosts in the Ethiopian Rift Valley region. Parasit. Vectors. 13, 292 (2020).
Madsen, H., Daffalla, A. A., Karoum, K. O. & Frandsen, F. Distribution of freshwater snails in irrigation schemes in the Sudan. J. Appl. Ecol. 25, 853 (1988).
WHO. Field use of molluscicides in schistosomiasis control programmes: An operational manual for programme managers. (2017).
Hairston, N. G., Hubendick, B., Watson, J. M. & Olivier, L. J. An evaluation of techniques used in estimating snail populations. Bull. World Health Organ. 19, 661–672 (1958).
Hoverman, J. T. et al. Environmental gradients and the structure of freshwater snail communities. Ecography 34, 1049–1058 (2011).
Utzinger, J. & Tanner, M. Microhabitat preferences of Biomphalaria pfeifferi and Lymnaea natalensis in a natural and a man-made habitat in Southeastern Tanzania. Mem. Inst. Oswaldo Cruz. 95, 287–294 (2000).
Min, F. et al. Environmental factors affecting freshwater snail intermediate hosts in Shenzhen and adjacent region, South China. Trop. Med. Infect. Dis. 7, 426 (2022).
Archer, J. et al. Population genetics and molecular xenomonitoring of Biomphalaria freshwater snails along the southern shoreline of Lake Malawi, Malawi. Parasit. Vectors. 17, 521 (2024).
Pennance, T. et al. Development of a molecular snail xenomonitoring assay to detect Schistosoma haematobium and Schistosoma Bovis infections in their Bulinus snail hosts. Molecules 25, 4011 (2020).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Evans, J. S., Murphy, M. A., Holden, Z. A. & Cushman, S. A. Modeling species distribution and change using random forest. in Predictive Species and Habitat Modeling in Landscape Ecology (eds Drew, C. A., Wiersma, Y. F. & Huettmann, F.) 139–159 (Springer New York, doi:https://doi.org/10.1007/978-1-4419-7390-0_8. (2011).
Luan, J., Zhang, C., Xu, B., Xue, Y. & Ren, Y. The predictive performances of random forest models with limited sample size and different species traits. Fish. Res. 227, 105534 (2020).
Liaw, A. & Wiener, M. Classification and regression by randomForest. (2002).
Dasgupta, A. Reptree: Representative trees from ensembles. (2004).
R Core Team. R: A language and environment for statistical computing. (2022).
Breiman, L. Out-of-bag estimation. (1996).
Bauer, E. & Kohavi, R. An empirical comparison of voting classification algorithms: Bagging, boosting, and variants. Mach. Learn. 36, 105–139 (1999).
Han, H. & Guo, X. & Hua Yu. Variable selection using Mean Decrease Accuracy and Mean Decrease Gini based on Random Forest. in 7th IEEE International Conference on Software Engineering and Service Science (ICSESS) 219–224 (IEEE, Beijing, China, 2016). (2016). https://doi.org/10.1109/ICSESS.2016.7883053
Martinez-Taboada, F. & Redondo, J. I. Variable importance plot (mean decrease accuracy and mean decrease Gini). (2020).
Powers, D. Evaluation: From precision, recall and F-measure to ROC, informedness, markedness & correlation. J. Mach. Learn. Technol. 2, 37–63 (2011).
De Kock, K., Wolmarans, C. & Bornman, M. Distribution and habitats of Biomphalaria pfeifferi, snail intermediate host of Schistosoma mansoni, in South Africa. Water SA. 30, 29–36 (2004).
Rusydi, A. F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 118, 012019 (2018).
Chimwanza, B., Mumba, P. P., Moyo, B. H. Z. & Kadewa, W. The impact of farming on river banks on water quality of the rivers. Int. J. Environ. Sci. Technol. 2, 353–358 (2006).
Monjerezi, M., Vogt, R. D., Aagaard, P. & Saka, J. D. K. Hydro-geochemical processes in an area with saline groundwater in lower Shire River valley, Malawi: An integrated application of hierarchical cluster and principal component analyses. Appl. Geochem. 26, 1399–1413 (2011).
Thomas, E. O. Effect of temperature on D.O and T.D.S: A measure of ground and surface water interaction. Water Sci. 35, 11–21 (2021).
Patil, P. Physico-chemical parameters for testing of water—A review. Int. J. Environ. Sci. 3, 1194–1207 (2012).
Rebello, L. R. B. & Drexler, S., Siepman, T. Correlations between TDS and electrical conductivity for high-salinity formation brines characteristic of South Atlantic pre-salt basins. Water SA 46, (2020).
Walton, N. R. G. Electrical conductivity and total dissolved solids—what is their precise relationship? Desalination 72, 275–292 (1989).
Chiepa, B. et al. A baseline epidemiological survey for malaria and schistosomiasis reveals an alarming burden in primary schools despite ongoing control in Chikwawa District, southern Malawi. Curr. Res. Parasitol. Vector-Borne Dis. 5, 100183 (2024).
Acknowledgements
We would like to thank the communities of Chikwawa and Nsanje for welcoming us and allowing this study.
Funding
This work was supported by the National Institute for Health Research (NIHR) (using the UK’s Official Development Assistance (ODA) Funding) and Wellcome [223660/Z/21/Z] under the NIHR-Wellcome Partnership for Global Health Research. CN is funded in whole by the Wellcome Trust [223660]. JAT is funded by the MRC Skills Development Fellowship [MR/T031743/1]. This UK funded award is part of the EDCTP2 programme supported by the European Union. The views expressed are those of the authors and not necessarily those of Wellcome, MRC, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
CN: Conceptualization of this study, funding acquisition, investigation (lead), analysis, project administration, visualization, method writing-original draft preparation, review and editing. RM: Supervision, writing-review and editing. JC: Supervision, writing – review and editing. MCS: Conceptualization of this study, supervision, validation, review-editing. CMJ: Supervision, writing-review and editing. PM: Investigation, data collection, resources, review-editing. GN: Investigation, data collection, resources and review. BC: Writing, review-editing. PK: Writing, review-editing. CB: Writing, review-editing. KZ: Writing, review-editing. AK: Investigation, data collection and review. CCK: Validation, writing-review and editing. JA: Methods, analysis, visualisation, writing-review and editing. AJ: Review-editing. LC: Methods, analysis. JAT: Supervision, writing –review and editing. JRS: Conceptualization of this study, investigation, data collection, resources, writing- review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Approval for this survey was received from College of Medicine Research Ethics Committee (COMREC) (Protocol number: P.02/23/3989), Chikwawa and Nsanje District Health Office research committees and ILLOVO Nchalo Estate.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nkolokosa, C., Mbewe, R., Chirombo, J. et al. A first report of Biomphalaria pfeifferi in the Lower Shire Valley, Southern Malawi, a major intermediate snail host species for intestinal schistosomiasis. Sci Rep 15, 5603 (2025). https://doi.org/10.1038/s41598-025-88930-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88930-4