Abstract
In recent years, there has been growing interest in exploring the therapeutic potential of Phlomis species, prompting numerous scientific studies on their pharmacological properties. However, the specific therapeutic applications of Phlomis remain underexplored, warranting further investigation. Iran, as one of the primary centers of diversity for the Phlomis genus in Asia, is home to 20 species, 9 of which are endemic to the region. This study aimed to conduct a comprehensive investigation and comparison of aerial part extracts from 56 Phlomis samples across 6 distinct Iranian species, focusing on their unique phenolic composition, antioxidant properties, and therapeutic potential. The analysis included a detailed assessment of total phenolics, flavonoids, tannin, phenylalanine ammonia-lyase activity, photosynthetic pigments, and ascorbic acid levels, along with measurements of their antioxidant activity. UHPLC-HRMS was also employed to identify unique chemical fingerprints. To interpret the extensive dataset, multivariate data analysis was applied, revealing correlations and distinctions among the different Phlomis species. Results showed that each species contains distinct polyphenols with known bioactivities, anti-inflammatory, antitumor, antimicrobial, cardiovascular, and neuroprotective properties, suggesting the potential for targeted therapeutic applications of specific Phlomis species. In addition, the study found that variations in polyphenol profiles and antioxidant capabilities among Phlomis species are primarily driven by genetic factors rather than environmental conditions, highlighting the critical role of species selection in advancing plant-derived nutraceutical research and applications.
Similar content being viewed by others
Introduction
The genus Phlomis, belonging to the Lamiaceae family with over 100 species, comprises a diverse group of plants known for their rich phytochemical profiles and notable medicinal properties. These species are widely distributed across various ecological niches, particularly in the Mediterranean and Middle Eastern regions, where they have adapted to a range of environmental conditions. Phlomis species have demonstrated a wide range of pharmacological activities, including antidiabetic, anticancer, and anti-inflammatory effects1,2,3. The cytotoxic effects of certain Phlomis extracts on cancer cell lines underscore their potential as adjunct therapies in cancer treatment2. Moreover, the traditional use of these plants in treating ailments, such as gastrointestinal and respiratory disorders, further emphasizes their importance in ethnomedicine and the need for scientific validation of their efficacy4,5. Interest in Phlomis species has grown due to their documented antioxidant and antimicrobial properties, as highlighted in numerous studies6,7,8,9. Phlomis species have been traditionally utilized in various cultures for their therapeutic effects, including anti-inflammatory, antimicrobial, and antioxidant activities10,11,12. Studies have also shown that Phlomis extracts can inhibit cancer cell proliferation, reinforcing their potential as anticancer agents13,14.
The genus Phlomis is distinguished by its rich diversity of phytochemical compounds, including flavonoids, phenolic acids, and phenolic compounds. Various studies have identified several flavonoids, such as luteolin, quercetin, and apigenin, along with their glycosides4,15. P. tuberosa has been shown to contain significant amounts of luteolin-7-O-glycoside and myricetin, which contribute to its antioxidant and anti-inflammatory properties4,16. P. bruguieri has been reported to contain flavonoids like kaempferol and chrysoeriol, which exhibit cytotoxic effects against cancer cells13,17. The presence of these flavonoids is associated with various biological activities, including antioxidant, anti-inflammatory, and antimicrobial effects4,9. Phenolic acids represent another important group of compounds found in Phlomis species. Commonly identified phenolic acids include gallic acid, vanillic acid, and sinapic acid18,19. Notably, P. stewartii has been shown to contain significant levels of p-coumaric and salicylic acids, which contribute to its health-promoting properties18,20. The antioxidant activities of these phenolic acids are crucial for the plant’s defense mechanisms and have important implications for their use in traditional medicine21. Furthermore, phenolic compounds like verbascoside, a phenylethanoid glycoside, are prevalent in many Phlomis species and are associated with various pharmacological effects, including antinociceptive and antimicrobial activities10,21. The presence of verbascoside in Phlomis extracts has also been associated with effective metal chelating properties, further enhancing their antioxidant potential16,21. Moreover, other phenolic compounds, such as phenylethyl alcohols and tannins, have been identified, enriching the phytochemical profile of these plants4,21. The essential oil composition of Phlomis species, particularly sesquiterpenes, like germacrene D and β-caryophyllene, has been extensively studied, revealing a rich diversity of volatile compounds that contribute to their aromatic and medicinal properties.
The phytochemical composition of the genus Phlomis can vary significantly depending on the species and environmental factors such as soil type, altitude, and climate22,23. These environmental conditions influence the biosynthesis of bioactive compounds, which in turn affect the plants overall health and therapeutic efficacy24. Understanding these relationships is essential for optimizing the cultivation and use of Phlomis species in both traditional and modern medicine. Additionally, genetic diversity among different Phlomis species contributes to the variability in their phytochemical profiles. This genetic variability not only increases the potential for discovering novel therapeutic agents but also underscores the importance of conservation efforts to protect these valuable plant resources25,26.
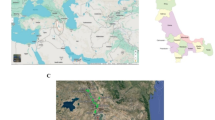
In this study, we adopted a comprehensive approach to analyze the phytochemical and antioxidant properties of various Phlomis species, while considering the influence of environmental factors. By integrating phytochemical analysis with antioxidant assays, the research aims to provide a deeper understanding of how these variables interact to shape the medicinal value of Phlomis plants. The findings are expected to offer valuable insights into the sustainable use and conservation of these species, supporting their role in traditional and modern medicine. Exploring the effects of environmental conditions and species type on the phytochemical and antioxidant properties of Phlomis plants is not only timely but also essential for advancing our understanding of their medicinal potential. As demand for natural products continues to rise, this research will serve as a foundation for future studies, aiming to harness the therapeutic benefits of Phlomis species while promoting their conservation and sustainable use. The genus Phlomis represents a key component of Iran’s botanical heritage, emphasizing the importance of preserving and exploiting these species for their pharmacological attributes. Through a focused investigation of Phlomis species native to Iran, we provide critical insights on the diversity and bioactivity inherent to the flora of this region. Specifically, 56 Phlomis specimens representing six distinct species (illustrated in Fig. 1 and detailed in Table S1), were collected from various sites within the West Azerbaijan province of Iran (as shown in Fig. 2) and analyzed for their phytochemical characteristics to identify the most promising species based on phytochemical indicators for prospective therapeutic applications.
Materials and methods
Sampling of Phlomis species
Between April and July 2022, a total of 56 Phlomis samples from the aerial parts were manually collected from different areas in West Azerbaijan. This province is a major center of diversity for genus Phlomis in Iran. The collected samples represent six distinct Phlomis species (as shown in Fig. 1 and Table S1), providing a comprehensive representation of the genus within this region. Additionally, even among samples belonging to the same Phlomis species, there are differences in collection sites and altitudes. These samples were gathered from different altitudes, leading to exposure to diverse meteorological conditions, potentially influencing the phytochemical composition and properties, even within the same species. The species identification of various Phlomis samples was conducted at University of Tehran by botanist Dr. Bahadori. The collected species were P. olivieri, P. herba venti, P. kurdica, P. persica, P. tuberosa, and P. rigida, which are kept in the Department of Horticultural Sciences, Urmia University, with voucher numbers 1532, 1533, 1534, 1535, 1536, and 1537, respectively.
Preparation of methanolic extracts
The aerial parts organs of the selected Phlomis samples were carefully segregated and thoroughly washed under running water to remove surface debris. The cleaned samples were air-dried for several days, then finely ground into powder, and stored in plastic bags for future use. To prepare extracts, plant powders (0.1 g) were placed into test tubes, and 10 mL of methanol was added for soaking, followed by vigorous shaking. The resulting mixtures were filtered through filter paper, and the resulting filtrates were collected individually for subsequent phytochemical analysis. Methanol was chosen as the extraction solvent based on findings by Bajkacz et al.27. , who demonstrated that methanol exhibited superior efficacy compared to alternative solvents in the extraction of polyphenols. While methanol is not suitable for direct oral administration, it is commonly used in preliminary studies focused on chemical characterization and bioactivity screening. For translational applications, future research will focus on re-extracting the active components using non-toxic, biocompatible solvents, to ensure safety and suitability for potential therapeutic applications.
Total phenolic content (TPC)
The Folin-Ciocalteu colorimetric method was used to determine the total phenolic content in the methanolic extracts derived from the 56 Phlomis samples, following the protocol outlined by Ul-Haq et al.28. The concentration of the extracts was set at 20 µg/mL, and absorbance readings were recorded at 765 nm using a HALO DB-20 UV-VIS spectrophotometer (Dynamica Scientific LTD, Livingston, UK). A calibration curve was created using varying concentrations of gallic acid (10–50 µg/mL). The total phenolic content for each sample was expressed as gallic acid equivalents per gram of dry weight (mg GAE/g DW).
Total flavonoid content (TFC)
The total flavonoid content for each sample was assessed using the aluminum chloride colorimetric assay29. Briefly, 0.15 mL of each diluted extract was mixed with 1.5 mL of methanol, 0.1 mL of potassium acetate (1.0 M), 0.1 mL of aluminum chloride (10%, w/v), and 2.8 mL of distilled water. The mixture was incubated at room temperature for 35 min. After incubation, the absorbance of each sample was quantified at 415 nm. A calibration curve was established using quercetin as the standard, with concentrations ranging from 10 to 50 µg/mL. The total flavonoid content (TFC) was expressed in milligrams of quercetin equivalents per gram of dry weight (mg QUE/g DW).
Total tannin content (TTC)
The total tannin content of each extract was assessed using the Vanillin reagent method as described by Bharath et al.30. The reaction mixture consisted of 0.1 mL of extract, 2 mL of vanillin (4% w/v in methanol), and 1 mL of concentrated hydrochloric acid. The mixture was thoroughly agitated and incubated for 30 min at 30 °C. After incubation, the absorbance of each sample was measured at 500 nm. The total tannin content was calculated using a linear regression equation derived from a standard tannic acid curve and expressed as milligrams of tannic acid equivalents per gram of extract (mg TAE/g DW).
Identification of polyphenolic compounds by UHPLC-HRMS
The profiling of polyphenolic compounds was performed using an ultra-high-performance liquid chromatography (UHPLC) system (Dionex UltiMate 3000, Thermo Fisher Scientific, Waltham, MA, USA) coupled with a Q-Exactive Orbitrap mass spectrometer (UHPLC, Thermo Fisher Scientific, Waltham, MA, USA)31. Chromatographic separation was performed on a Luna Omega PS 1.6 μm column (50 × 2.1 mm, Phenomenex, Torrance, CA, USA) with the temperature meticulously regulated at 25 °C. The mobile phase was comprised of solvent A (water containing 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). Polyphenolic compounds were eluted using the following gradient program: 0–1 min, 0% B; 1–2 min, 0–95% B; 2–2.5 min, 95% B; 2.5–5 min, 95–75% B; 5–6 min, 75 − 60% B. The gradient was then reverted to 0% B within 0.5 min and maintained for 2.5 min for column re-equilibration. The flow rate was kept constant at 0.4 mL/min, and the injection volume was precisely set at 5 µL. The autosampler temperature was maintained at 10 °C.
The mass spectrometer was operated in negative ion mode (ESI-) due to the acidic nature of the phenolic hydroxyl groups. In this mode, the molecules (M) lose a proton (H), resulting in the formation of anions (M-H)-. This configuration enhances sensitivity and improves signal stability for polyphenols, which typically form stable negative ions. Two types of scan events were employed for all targeted compounds: Full ion mass spectrometry and all ion fragmentation, AIF. Full scan data were acquired with a resolving power set at 35,000 FWHM (full width at half maximum) at m/z 200, while AIF scan events were recorded at a resolving power of 17,500 FWHM with collision energy values of 10, 20, and 45 eV. The mass parameters for both scenarios included: Spray Voltage, − 3.5 kV; Sheath Gas Flow Rate, 45 arbitrary units; Auxiliary Gas Flow Rate, 10 arbitrary units; Capillary Temperature, 275 °C; Auxiliary Gas Heater Temperature, 350 °C; S-lens RF level, 50; Scan Range m/z, 80–1200. Data acquisition and processing were conducted utilizing Quan/Qual Browser Xcalibur software, v. 3.1.66.10 (Xcalibur, Thermo Fisher Scientific, Waltham, MA, USA). The individual phenolic compounds were identified by comparison with available standards. Detection was based on calculated exact mass with a mass error below 5 ppm and on the retention time of the molecular ion, while regarding the fragments on the intensity threshold of 1000 and a mass tolerance of 5 ppm. Analyses were performed in triplicate.
Evaluation of phenylalanine ammonia lyase (PAL) enzyme activity
The evaluation of the phenylalanine ammonia lyase (PAL) enzyme activity was conducted following the methodology established by Karthikeyan et al.32. One gram of aerial parts of plant specimens was homogenized in 3 mL of a 0.1 M ice-cold sodium borate buffer, which contained 1.4 mM of 2-mercaptoethanol and 0.1 g of insoluble polyvinylpyrrolidone, adjusted to pH 7.0. The resultant extract was filtered using cheesecloth and subsequently centrifuged at 16,000 g at 40 °C for 15 min. The supernatant obtained served as the source of the enzyme. PAL activity was assessed by measuring the conversion rate of L-phenylalanine into trans-cinnamic acid. The reaction mixtures consisted of 0.4 mL of the enzyme extract, 0.5 mL of 0.1 M borate buffer at pH 8.8, and 0.5 mL of 12 mM L-phenylalanine, all incubated for 30 min at 30 °C. The optical density of the reaction was measured at 290 nm, and the amount of trans-cinnamic acid produced was calculated using its extinction coefficient of 9630 M− 1·cm− 1. The enzymatic activity was expressed as nanomoles of trans-cinnamic acid produced per minute per milligram of protein.
Antioxidant activity by DPPH assay
The free radical scavenging activity was assessed by quantifying the elimination of DPPH radicals, following the methodology described by Shimada et al.33. , with some modifications. Various concentrations of each extract were systematically added to 4 mL of a 0.004% DPPH methanol solution. The mixture was shaken and incubated for 30 min at room temperature in the dark. Absorbance was measured at 517 nm, and all experimental determinations were performed in triplicate to ensure reliability. Antioxidant activity was quantified as the percentage inhibition of DPPH formation, attributable to the hydrogen-donator activity of each sample, calculated using the following equation: Inhibition (%) = (1 - absorbance of the sample/absorbance of the blank) × 100. Results are expressed as micrograms of ascorbic acid equivalent per milliliter (µg AAE/mL). The percentage inhibition for each sample was incorporated into the standard curve of ascorbic acid, enabling the calculation of DPPH concentration based on AAE.
Antioxidant activity by FRAP assay
The FRAP assay was performed according to the protocol established by Miao et al.34. , with minor modifications. Briefly, the FRAP reagent was prepared by mixing 300 mmol/L acetate buffer (pH 3.6), 20 mmol/L ferric chloride (FeCl3) solution, and 10 mmol/L 2,4,6-tripyridyl-triazine (TPTZ) solution, in a 10:1:1 (v/v) ratio. The FRAP reagent was freshly prepared and preheated to 37 °C in a water bath before use. An aliquot of 100 µL from each plant extract was mixed with 3 mL of the FRAP reagent. The mixture was vortexed, and the absorbance of the solution was measured at 593 nm after a 35-minute incubation at 37 °C. A standard curve was constructed using varying concentrations of Fe2+ solution (from 50 to 600 µmol/L). Results were expressed as micromoles of Fe2+ per gram of dry weight (µmol Fe2+/g DW).
Ascorbic acid content (AAC)
The AAC was determined following the method established by Klein and Perry35. Briefly, 0.1 g of sample powder was extracted with 3 mL of a 1% metaphosphoric acid solution, incubating the mixture at -4 °C for 35 min, followed by filtration at room temperature. The resulting filtrate was then combined with 2,6-dichloroindophenol (2 mL), and the absorbance was recorded at 520 nm within a 30-minute timeframe against a blank reference. The ascorbic acid content was quantified using a standard calibration curve constructed with known concentrations of L-ascorbic acid (10–50 mg/mL). Results were expressed as milligrams of ascorbic acid per gram of dry weight (mg AA/g DW).
Photosynthetic pigments content
Chlorophylls (Chla and Chlb), total carotenoids (TCC), and β-carotene were quantified spectrophotometrically using plant extracts prepared in an 80% acetone/distilled water (v/v) mixture, following the methodologies described by Lichtenthaler36 and Nagata et al.37. The concentrations of these compounds were calculated using the following formulas:
Chla = 15.65 A662 − 7.340 A645.
Chlb = 27.05 A645 − 11.21 A662.
TCC = 1000 A470 − 2.860 Chla − 129.2 Chlb/245.
β-Carotene = 0.854 A470 − 0.312 A645 + 0.039 A662 − 0.005.
where A662, A645, and A470 are the absorbance values determined at the wavelengths of 662, 645, and 470 nm, respectively.
Statistical analysis
The acquired data were analyzed using one-way ANOVA (with three replicates) integrated in SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). The means were evaluated with Duncan’s Multiple Range Test (DMRT). For multivariate data analysis, the raw data obtained from UHPLC-HRMS analysis were organized according to the m/z values and retention times of the ion signals, resulting in a data matrix with 56 rows (samples) and 37 columns (polyphenolic data derived from UHPLC-HRMS analysis). The matrix was completed by adding other 11 columns containing the results of TPC, TFC, TTC, AAC, PAL, photosynthetic pigment content (chlorophyll a and b, total carotenoid concentration, and β-carotene), and antioxidant activity (FRAP and DPPH). This data matrix (51 variables x 56 samples) was subsequently imported into MATLAB R2015b (The Mathworks Inc., Natick, MA, USA) for further multivariate analysis. A principal component analysis (PCA) was performed using the PLS Toolbox 8.6.1 (Eigenvector Research Inc., Wenatchee, WA, USA) within the MATLAB environment. Prior to conducting PCA, the data were standardized to unit variance (autoscaling), which incorporates the standard deviation as a scaling factor, allowing all metabolites to have an equal influence on the model, along with mean-centering, which is essential for PCA computation.
Results and discussion
Phenolic contents of Phlomis species (TPC, TFC, and TTC)
The TPC, TFC and TTC of various Phlomis species from different habitats are summarized in Table 1. Significant differences in these parameters were observed among the 56 genotypes (p < 0. 01). The TPC ranged from 25.29 to 83.90 mg GAE/g DW, with the highest value recorded in the P3 (P. olivieri) population and the lowest in the P30 population (P. olivieri). The TFC varied from 7.88 to 14.35 mg QUE/g DW; the P16 population (P. herba venti) exhibited the highest TFC, while the P50 population (P. kurdica) showed the lowest. TTC also varied considerably among the tested genotypes, with the P36 population (P. herba venti) having the highest content at 13.94 mg TAE/g DW and P28 population (P. olivieri) the lowest at 2.50 mg TAE/g DW.
Phlomis is renowned for its rich phytochemical profile, particularly regarding phenolic compounds, flavonoids, and tannins. The TPC, TFC, and TTC in these species are significantly influenced by genetic background and environmental conditions. Environmental conditions play a crucial role in determining the phytochemical profiles of Phlomis species. Soil composition, moisture levels, and climatic factors are crucial in determining the phytochemical profiles of Phlomis species. For example, studies on P. crinita demonstrated that environmental factors significantly impact phenolic content and antioxidant activity21. Adverse conditions, such as drought and temperature fluctuations, may lead to increased concentrations of protective compounds, including flavonoids and tannins, as a response to stress38, suggesting an adaptive response that enhances medicinal properties. Furthermore, the genetic and ecological diversity among Phlomis species contributes to variations in TPC, TFC, and TTC. For instance, the P. aurea and P. caucasica exhibit distinct phytochemical profiles attributable to their specific ecological niches and adaptations11,39. The presence of various classes of flavonoids and phenolic compounds among species indicates a complex interplay between genetic factors and environmental conditions that shapes the phytochemical landscape of the genus10. Genetic instructions for enzyme production in phenolic compounds biosynthesis are regulated by both internal and external factors40. Transcription factors control the expression of these genes based on environmental signals, growth stages, and hormonal cues41. Genetic differences in these regulatory regions can affect gene activity levels and the subsequent accumulation of phenolic compounds in Phlomis species. Environmental conditions critically influence enzyme and gene activity across different Phlomis species42,43,44,45, resulting in genetic diversity that leads to variations in phenolic compound production. Genetic factors govern the expression and regulation of enzymes like phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H), 4-coumarate CoA ligase (4CL), which are essential for the biosynthesis of various phenolic subclasses. Variations in the genes coding for these enzymes can lead to differences in phenolic compound content among Phlomis species46,47,48,49,50,51,52,53.
The total contents of phenolics, flavonoids, and tannins in Phlomis species are collectively influenced by both species type and the environmental conditions. Understanding these relationships is essential for harnessing the medicinal potential of these plants and ensuring their conservation in changing environmental contexts.
Identification of phytochemicals by UHPLC-HRMS and their potential applications
A total of 40 phytochemical compounds (Table 2) were analyzed in 56 Phlomis populations from various habitats, using UHPLC-HRMS. Isoverbascoside, yunnaneic acid E, and salvianolic acid C were notably absent in all analyzed Phlomis populations. In contrast, caffeoyl quinic acids, verbascoside, rosmarinic acid and forsythoside A were the most abundant compounds found in the aerial parts of each Phlomis population studied. The highest concentrations of these major compounds were found in specific populations: caffeoyl quinic acids in P34 (P. herba venti), verbascoside in P49 (P. tuberosa), rosmarinic acid in P43 (P. olivieri), and forsythoside A in P49 (P. tuberosa).
These compounds have been widely studied in various contexts, highlighting their significant biological activities and therapeutic potential. Verbascoside, a common phenylethanoid glycoside in many Phlomis species, is particularly notable for its antioxidant properties, which play a crucial role in mitigating oxidative stress and its associated diseases. Studies have shown that verbascoside exhibits potent free radical scavenging activity, contributing to its potential as a protective agent against oxidative damage in cells19,54. Furthermore, it has been demonstrated to have anti-inflammatory and anti-cancer activities, with research indicating that verbascoside can inhibit the growth of certain cancer cell lines2,20. Its widespread occurrence in Phlomis species emphasizes its importance within the genus, as it both in terms of chemotaxonomy and the potential therapeutic value of these plants10,55. Forsythoside A, another significant phenylethanoid glycoside, has also been regognized for its notable biological activities. Like verbascoside, it exhibits antioxidant properties and is believed to contribute to the protective effects of Phlomis extracts against oxidative stress16,56. Forsythoside A is often found alongside other phenolic compounds that enhance the overall bioactivity of extracts from Phlomis species57,58,59. Its use in traditional medicine, particularly in the treatment of various ailments, is supported by its documented effects on cellular health and its potential to modulate inflammatory responses60. Rosmarinic acid, a well-known polyphenolic compound, is another key component in Phlomis species. With its potent antioxidant and anti-inflammatory properties, rosmarinic acid has garnered significant attention in pharmacological research61. Studies have shown that rosmarinic acid can protect cells from oxidative damage, with potential applications in the treatment of conditions related to inflammation and oxidative stress60,61. Its presence in Phlomis species contributes to the overall therapeutic profile of these plants, highlighting their utility in traditional and modern medicine61. Additionally, caffeoyl quinic acids, including compounds such as chlorogenic acid, are also abundant in Phlomis species. These compounds are renowned for their antioxidant activities and have been linked to various health benefits, including anti-diabetic and anti-cancer properties20,57,58. Research indicates that caffeoyl quinic acids can modulate glucose metabolism and exhibit cytotoxic effects against certain cancer cell lines, further highlighting the pharmacological relevance of these compounds20,57,58.
PAL enzyme activity
The results of PAL enzyme activity across different populations are presented in Table 3. Significant variations in PAL enzyme activity of Phlomis species were observed among 56 genotypes (p < 0. 01). The highest PAL enzyme activity (7.91 nM min− 1 mg− 1 protein) was recorded in the P33 population (P. tuberosa), while the lowest (2.49 nM min− 1 mg− 1 protein) was found in the P15 population (P. persica). Phenylalanine ammonia-lyase (PAL), a key enzyme in the phenylpropanoid biosynthetic pathway, plays a critical role in producing of phenolic compounds essential for plant defense, growth, and development. The high phenolic and flavonoid content in P33 may be linked to the elevated PAL activity. PAL activity in Phlomis species is strongly influenced by environmental conditions such as temperature, light, and stress factors, which are common in their natural habitats. Abiotic stressors like low temperatures and UV radiation are known to stimulate PAL expression, leading to increased production of flavonoids and other phenolic compounds, which protect plants from oxidative stress62,63. This enzyme’s activity is also essential in oxidative stress responses, helping Phlomis species survive harsh environments by activating antioxidant systems to effectively scavenge reactive oxygen species (ROS)6. Species-specific responses to environmental conditions also contribute to variations in PAL activity. Different Phlomis species may exhibit distinct levels of PAL activity in response to the same environmental stimuli due to genetic diversity and evolutionary adaptations64. Additionally, the ecological niches these species occupy may result in variations in their metabolic pathways, including differences in PAL activity and the production of secondary metabolites9. Understanding these dynamics provides valuable insights into the adaptability and ecological roles of Phlomis species.
Antioxidant activity (AA) by DPPH and FRAP assays
The results of antioxidant activity (AA) obtained by DPPH and FRAP assays are presented in Table 3. They indicate a statistically significant variation in AA among the studied Phlomis species and populations (p < 0. 01). The P49 population (P. tuberosa) exhibited the highest AA value (56.64 µg AAE /mL) based on the DPPH assay, while the P13 population (P. olivieri) had the lowest (10.43 µg AAE /mL). In the FRAP assay, the P16 population (P. herba venti) recorded the highest AA value (35.03 µM Fe2+/g DW), and the P21 population (P. olivieri) showed the lowest (12.22 µM Fe2+/g DW).
The antioxidant activity of various Phlomis species is influenced by several factors, including phytochemical composition, genetic diversity, environmental conditions, and species-specific traits. Understanding these factors is crucial for harnessing their therapeutic potential in medicinal and food applications. A major contributor to the antioxidant activity in Phlomis species is their rich phenolic content, particularly phenylethanoid glycosides like verbascoside, which has been identified in species such as P. sieheana, P. samia, and P. monocephala. Verbascoside is known for its potent antioxidant, anti-inflammatory, and photoprotective properties10. Antioxidant activity in Phlomis is often assessed through assays such as DPPH, which reveal varying IC50 values across different extracts, indicating the diverse range of antioxidant capacities65. For example, the essential oils from P. leucophracta demonstrated significant antioxidant activity, linked to their volatile compounds66, while P. floccosa essential oil did not show such activity67. These variations underscore the role of genetic factors in shaping the antioxidant potential of different Phlomis species. Genetic diversity within and between species leads to differences in phytochemical profiles, which directly influence antioxidant activity6,68. For instance, P. fruticosa exhibits both antioxidant and antimicrobial activities, suggesting that genetics influences the biosynthesis of these beneficial compounds69,70. Phylogenetic studies also highlight the significance of genetic relationships in understanding the chemical diversity and potential health benefits of Phlomis species67. Environmental factors further impact the antioxidant activity of Phlomis species. Plants exposed to oxidative stress, such as those growing in harsh climatic conditions, often upregulate their antioxidant defense mechanisms, increasing the production of enzymes and secondary metabolites that scavenge free radicals6. For example, P. aurea and other endemic species from the Egypt’s Saint Catherine Mountain showed enhanced antioxidant activity in response to environmental stressors7. The interaction between environmental factors and genetic predisposition results in notable variations in antioxidant profiles among Phlomis species. Overall, the antioxidant activity of Phlomis species is a complex interplay of phytochemical composition, genetic diversity, environmental influences, and species-specific adaptations. Future research should aim to further clarify these relationships, to maximize the potential of Phlomis species in therapeutic and food applications.
Antioxidant defense systems in different species are crucial for mitigating oxidative stress caused by environmental factors such as climatic conditions and various stress. These systems consist of both enzymatic and non-enzymatic components. Enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), and peroxidases (POD), which play significant roles in detoxifying reactive oxygen species (ROS)71,72. SOD catalyzes the dismutation of superoxide radicals into hydrogen peroxide, which is subsequently decomposed by CAT and POD, thereby preventing oxidative damage72. The non-enzymatic components, such as ascorbate (ASC) and glutathione (GSH), also contribute significantly by directly scavenging ROS73. The interplay between these components plays a key role for maintaining cellular redox homeostasis and protecting plants from oxidative damage under stress conditions74,75. Moreover, the accumulation of phytochemicals, particularly phenolic compounds (flavonoids, phenolic acids) has been shown to enhance the antioxidant capacity of different plant species. Flavonoids not only act as ROS scavengers but also play a role in signaling pathways that activate the antioxidant defense mechanisms76. A study by Yu-Ying et al.77. highlights that the overexpression of specific flavonoid biosynthetic genes increases antioxidant activity in plants, enhancing their tolerance to oxidative stress. This is corroborated by findings from Zhao et al.78. , which indicate that modulating flavonoid levels improves stress tolerance in plants exposed to salinity.
Ascorbic acid content (AAC)
The AAC of Phlomis species across different sampling habitats is presented in Table 3. Significant differences were observed among 56 different populations (p < 0. 01), with AAC ranging from 4.45 to 22.02 mg AA/ g DW. The highest AAC was recorded in the P53 population (P. rigida), while the lowest was found in the P56 population (P. olivieri).
The genus Phlomis, known for its diverse species and medicinal properties, exhibits varying ascorbic acid levels influenced by environmental factors and species-specific traits. Ascorbic acid (vitamin C) is a vital antioxidant that protects plants from oxidative stress, enhancing their overall antioxidant capacity. Understanding the factors that regulate ascorbic acid synthesis in Phlomis species can provide insights into their ecological adaptability and potential health benefits. Environmental conditions, particularly light intensity, significantly impact ascorbic acid synthesis. High light levels stimulate photosynthesis, which in turn promotes ascorbic acid production79. This is relevant for Phlomis species growing in different light environments, as those exposed to higher light intensities tend to have elevated ascorbic acid levels. Temperature fluctuations also play a role; cooler temperatures can enhance ascorbic acid accumulation by triggering stress responses80.
Species-specific traits are equally important in determining ascorbic acid content. Different Phlomis species have distinct genetic and metabolic pathways that influence their ability to synthesize ascorbic acid. Some species may have evolved higher levels of this antioxidant as a defense against herbivory or environmental stressors81.
The correlation between ascorbic acid content and antioxidant activity is well-documented in various plant species, including Phlomis. Research shows a positive correlation between ascorbic acid levels and antioxidant activity, as measured by assays like DPPH82. This suggests that ascorbic acid plays a crucial role in the antioxidant defense system of these plants, and higher ascorbic acid levels contribute to greater overall antioxidant activity83.
Photosynthetic pigments content (Chla, Chlb, TCC, and β-Carotene)
Significant differences in the levels of Chla, Chlb, TCC, and β-Carotene were observed (p < 0.01) as shown in Table 4. The P26 population (P. herba venti) exhibited the highest content of these pigments with values of 1.59 mg/g DW for Chla, 0.55 mg/g DW for Chlb, 131.02 mg/100 g DW for TCC, and 7.08 mg/100 g DW for β-carotene. In contrast, the P33 population (P. tuberosa) showed the lowest levels: 0.46 mg/g DW for Chla, 0.21 mg/g DW for Chlb, 44.27 mg/100 g DW for TCC, and 2.58 mg/100 g DW for β-carotene.
Environmental conditions play a crucial role in determining the photosynthetic pigment content in Phlomis species. Factors such as light intensity, soil composition, and moisture levels can significantly influence chlorophyll and carotenoid synthesis, highlighting their role in shaping biochemical pathways for pigment production84. Geographic distribution, influenced by ecological variables, can lead to variations in pigment content among Phlomis species. For instance, studies have shown that the distribution of Phlomis aurea is affected by soil calcium levels and human activities, which alter the plant’s physiological responses and pigment composition7,85. Additionally, niche differentiation among congeneric species suggests that different ecological adaptations can result in variations in photosynthetic efficiency and pigment content86. Genetic diversity also contributes to variations in pigment levels among Phlomis species. Differences in genes responsible for producing enzymes involved in chlorophyll and carotenoid formation pathways can lead to discrepancies in pigment accumulation. This genetic diversity is shaped by natural selection and genetic drift, resulting in distinct pigment compositions among populations adapted to different habitats87,88. Furthermore, light availability directly impacts photosynthetic pigments synthesis. Phlomis species in high-light environments may exhibit increased levels of chlorophyll and carotenoids to optimize light absorption and enhance photosynthetic effectiveness89,90.
The genus Phlomis, renowned for its ecological significance, exhibits variation in photosynthetic pigment content, including chlorophyll a, chlorophyll b, total carotenoid content (TCC), and β-carotene, influenced by species type and environmental conditions. Understanding these variations is essential for ecological research and application of these plants in food and pharmaceutical industries. Different species of Phlomis display distinct biochemical profiles, which can affect their photosynthetic pigment content. Studies have shown significant differences in the chemical compositions of various Phlomis species, which may correlate with variations in pigment concentrations91,92,93. Additionally, specific phytochemicals, such as phenolic compounds and flavonoids, have been linked to the overall health and photosynthetic efficiency of these plants, suggesting that species with higher concentrations of these compounds may also exhibit enhanced pigment levels94.
Collectively, the interplay between species type and environmental conditions significantly influences the photosynthetic pigment content in the genus Phlomis. Future research should focus on elucidating the specific biochemical pathways involved in pigment synthesis across different species and environmental contexts to enhance our understanding of their ecological and medicinal significance.
Correlation analysis
The results of the correlation analysis between the studied characteristics are presented in Fig. 3. Positive correlations are depicted in blue, while negative correlations are in red, with color intensity indicating correlation strength of the coefficients. The strongest correlations were found among caffeoyl quinic acids, PAL enzyme activity, verbascoside, forsythoside A, 6-hydroxyluteolin-7-O-glucuronide, kaempferol glucuronide, and luteolin glucuronide (Fig. 3). These positive correlations can be explained by the interconnected biochemical pathways involved in phenolic compound biosynthesis. PAL is a key enzyme in the phenylpropanoid pathway, catalyzing the conversion of phenylalanine to cinnamic acid, a precursor for flavonoids and hydroxycinnamic acids95,96,97. Numerous studies have shown that increased PAL activity is often positively correlated with the accumulation of phenolic compounds, particularly in response to different stressors or environmental conditions98,99. This relationship is critical for plant defense, as PAL induction typically enhances phenolic compound production to protect against pathogens and abiotic stresses like wounding or UV exposure100,101,102,103. The correlation between PAL activity and phenolic compounds, such as verbascoside and forsythoside A, is particularly relevant because these compounds exhibit strong antioxidant properties and have potential therapeutic benefits104,105. Their biosynthesis is closely tied to PAL activity, reflecting the plant’s ability to respond metabolically to environmental challenges by producing secondary metabolites106,107,108. This finding underscores the importance of the phenylpropanoid pathway in plant defense and metabolism, highlighting the dynamic role PAL plays in the accumulation of bioactive compounds that benefit both plant health and human applications. Additionally, the antioxidant activity measured through DPPH and FRAP assays results was strongly correlated with bioactive components, specifically syringic acid, forsythoside A, verbascoside, TPC, TFC and TTC (Fig. 3). The significant positive correlation between antioxidant activity and these compounds to the well-established antioxidant properties of flavonoids and phenolics, known for their ability to neutralize free radicals through their redox properties109,110,111. Specifically, flavonoids with their hydroxyl (-OH) groups exhibit strong radical scavenging activity by donating electrons, thus reducing oxidative damage112,113. Research consistently supports a positive correlation between antioxidant activity and total phenolic and flavonoid content in plant extracts114,115,116. This highlights the integral role of phenolic compounds in enhancing antioxidant defenses, both in plants and their potential health applications.
Correlation analysis of phytochemical properties of Phlomis species. Positive and negative correlations are shown in blue and red colors, respectively. The color intensity is proportional to the correlation coefficients. Martynoside/isomartynoside (M-im); Caffeic acid hexosides (Ca-a-h); Lipedoside_A (L-A); Caffeoyl_quinic_acids (Ca-q-a); Phenylalanine ammonia lyase (PAL); Verbascoside (V); Forsythoside_A (Fo-a); 6-Hydroxyluteolin_7-O-glucuronide (H-7-O-g); Kaempferol-glucuronide (K-g-u); Luteolin-glucuronide (L-g-u); Hispidulin_glucuronide (H-g); DPPH; Syringic acid (Sy-a); Caffeoyl_malic_acid (C-m-a); Ferulic_acid (F-a); Apigenin_7-O-glucoside (A-7-O-g); Genistein (G); Apigenin (A); Ascorbic acid content (AAC); Total phenolic content (TPC); FRAP; Total flavonoid content (TFC); Total tannin content (TTC); Total carotenoids content (TCC); β-carotene (Bcar); Chlorophyll a (Chla); Chlorophyll a (Chlb); Salvianolic_acid_A (S-a-A); Tuberonic_acid_glucosides (T-a-g); Eriodictyol-O-glucuronide (E-O-g); Myricitrin (M); Hyperoside (Hy); Apigenin_acetyl_glucoside (A-a-g); Sagecoumarin (S); Salvianolic_acid_F_isomers (S-a-F-i); Salvianolic_acid_K (S-a-K); Rosmarinic_acid (R-A); Salvianolic_acid_B (S-a-B); Yunnaneic_acid_F (Y-a-F); Lityhospermic_acid_isomers (L-a-i); Luteolin (L); Kaempferol (K); Hispidulin (Hi); Kaempferide (Ka); Sagerinic_acid (Sa-a); Luteolin_acetyl_glucoside (L-a-g); Luteolin-glucoside (L-g-o); Kaempferol-glucoside (K-g-o).
Chemometric analysis of Phlomis populations by HCA and PCA
In the hierarchical clustering analysis (HCA) (using the Ward’s method) of 56 Phlomis populations, using 48 key traits, including chemical compositions, antioxidant activity, UHPLC-HRMS profiling, and photosynthetic pigment content, five distinct species groups were identified (Fig. 4). The results indicate that species identity has a more substantial role than environmental factors in differentiating these populations based on phytochemical characteristics. Group 1 contained all populations of P. tuberosa (P33, P39, P49, P29, P22, P27, P40, and P38) characterized by high levels of luteolin glucuronide, hispidulin glucuronide, kaempferol glucuronide, 6-hydroxyluteolin-7-O-glucuronide, genistein, apigenin, verbascoside, forsythoside A, PAL, and caffeoyl quinic acids. Group 2 consisted of all populations of P. persica (P17, P18, P15, and P8), which displayed high levels of caffeoyl malic acid and apigenin-7-O-glucoside. This group exhibited lower concentrations of caffeoyl quinic acids, PAL, forsythoside A, and verbascoside. Group 3 included P. kurdica populations (P9, P6, P5, P50, and P11), which were rich in phenolic compounds such as kaempferide, hispidulin, kaempferol, luteolin, sagerinic acid, luteolin acetyl glucoside, kaempferol glucoside, and luteolin glucoside. Group 4 comprising all P. herba venti populations (P26, P10, P24, P4, P36, P16, P37, and P34), showed high concentrations of photosynthetic pigments such as Chla, Chlb, TCC, and β-Carotene. Group 5 contained the P. olivieri and P. rigida populations, which were characterized by a diverse phytochemical profile. This group was distinguished by high concentrations of eriodictyol-O-glucuronide, hyperoside and myricitrin compounds.
Dendrogram of hierarchical clustering analysis (HCA) and heatmap of phytochemical properties of Phlomis species. Caffeic acid hexosides (Ca-a-h); Lipedoside_A (L-A); Martynoside/isomartynoside (M-im); DPPH; Syringic acid (Sy-a); Apigenin_acetyl_glucoside (A-a-g); Sagecoumarin (S); Salvianolic_acid_A (S-a-A); Yunnaneic_acid_F (Y-a-F); Salvianolic_acid_F_isomers (S-a-F-i); Total phenolic content (TPC); FRAP; Total flavonoid content (TFC); Total carotenoids content (TCC); Rosmarinic_acid (R-A); Salvianolic_acid_B (S-a-B); Salvianolic_acid_K (S-a-K); Myricitrin (M); Hyperoside (Hy); Eriodictyol-O-glucuronide (E-O-g); Tuberonic_acid_glucosides (T-a-g); Luteolin-glucoside (L-g-o); Kaempferol-glucoside (K-g-o); Luteolin_acetyl_glucoside (L-a-g); Sagerinic_acid (Sa-a); Lityhospermic_acid_isomers (L-a-i); Apigenin_7-O-glucoside (A-7-O-g); Caffeoyl_malic_acid (C-m-a); Ascorbic acid content (AAC); Ferulic_acid (F-a); Luteolin (L); Kaempferol (K); Hispidulin (Hi); Kaempferide (Ka); Total tannin content (TTC); β-carotene (Bcar); Chlorophyll a (Chla); Chlorophyll a (Chlb); Luteolin-glucuronide (L-g-u); Hispidulin_glucuronide (H-g); Kaempferol-glucuronide (K-g-u); 6-Hydroxyluteolin_7-O-glucuronide (H-7-O-g); Genistein (G); Apigenin (A); Verbascoside (V); Forsythoside_A (Fo-a); Phenylalanine ammonia lyase (PAL); Caffeoyl_quinic_acids (Ca-q-a).
Principal Component Analysis (PCA) is a widely used statistical method in metabolomics for reducing the dimensionality of datasets with numerous interrelated variables, simplifying the visualization of key sources of variation and the identification of significant patterns117,118. PCA achieves this by converting the original correlated variables into a smaller set of uncorrelated variables, known as principal components (PCs). These PCs form new axes in a coordinate system, with the first capturing the most variance, followed by the second, and so on. The results are usually displayed in two plots: the “scores plot “, which shows the similarity or differences between samples, and the “loadings plot”, which reveals the variables responsible for those differences119,120. In this study, PCA was performed to extract the maximum useful information from the extensive amount of acquired data (56 samples x 51 variables). In particular, a relative quantification of polyphenols was conducted, as this approach is commonly considered adequate in metabolomics for investigating similarities and differences among analyzed samples through PCA, which functions by comparing samples. The PCA (Fig. 5) corroborated the HCA results, showing correlations between the detected phytochemicals, antioxidant activity, and the different Phlomis populations. Notably, the PCA revealed a clear separation of samples when colored based on their species classification (Fig. 5), indicating strong species-specific chemical profiles. Additionally, PCA was used to better visualize how the metabolome was differentially affected by species compared to environmental factors. To explore this, samples were further categorized into five groups based on their harvesting areas (Supplementary Figure S1). However, when the samples were grouped by harvesting areas (Supplementary Figure S1), the separation was less distinct, suggesting that geographical location had a lesser impact on the phytochemical variation compared to species identity. These observations are consistent with other studies that highlight the predominance of species-specific traits over environmental influences in determining phytochemical content and biological activities121,122,123.
Potential applications of different Phlomis species in treating different diseases
Each Phlomis species contains significant amounts of compounds belonging to the same chemical class or involved in similar biological pathways (as shown in Table 2), but the observed differences indicate that each species is characterized by its own class of polyphenols (Figs. 4 and 5). These variations in phenolic composition could prove valuable for the development of targeted treatments for various diseases.
For instance, all populations of P. tuberosa species, positioned in the right part of the scores plot (Fig. 5), showed high levels of luteolin glucuronide, hispidulin glucuronide, kaempferol glucuronide, 6-hydroxyluteolin-7-O-glucuronide, genistein, apigenin, verbascoside, forsythoside A, caffeoyl quinic acids. Genistein, a phytoestrogen, is a precursor for antimicrobial phytoalexins and phytoanticipins in plants and has attracted attention due to its diverse pharmacological effects, including anti-cancer, neuroprotective, cardiovascular, anti-inflammatory, antioxidant, and anti-obesity properties124. Hispidulin-glucuronide inhibits mast cell-mediated allergic inflammation by down-regulating histamine release and inflammatory cytokines, making it a potential therapeutic target for allergic conditions125. Luteolin and its glucuronides, such as luteolin 7-O-β-glucuronide, exhibit antioxidant properties, protecting against oxidative stress-related diseases126. Apigenin, another flavonoid, shows antibacterial and anti-leukemic activities, as well as anti-cancer potential through autophagy induction127. Forsythoside A has demonstrated anti-cancer activity, particularly in esophageal squamous cell carcinoma, where it influences apoptosis-related pathways128. Similarly, kaempferol glucuronide offers anti-inflammatory and anti-cancer benefits, by modulating cell survival pathways129. Caffeoyl quinic acids have wide therapeutic applications, including anti-Alzheimer, antibacterial, antiviral, and antioxidant activities130. Additionally, verbascoside, a phenylethanoid glycoside prevalent in many Phlomis species16,21, is linked to antinociceptive, antimicrobial, and metal-chelating antioxidant properties10,21.
In P. kurdica populations, positioned in the upper left part of the scores plot, high concentrations of phenolics like kaempferide, hispidulin, kaempferol, luteolin, sagerinic acid, luteolin acetyl glucoside, kaempferol glucoside, and luteolin glucoside were found. Kaempferide, a flavonoid found in numerous plants, shows significant antioxidant effects and prevents UVB-induced photoaging, by regulating reactive oxygen species (ROS) signaling pathways131. The glycosylated forms of these flavonoids, particularly kaempferol glucoside and luteolin acetyl glucoside, offer anti-obesity and anti-diabetic benefits by regulating energy metabolism and inhibiting lipid accumulation in hepatocytes132,133. Furthermore, the high concentration of sagerinic acid with renowned anti-inflammatory and neuromodulatory properties through interactions with enzymes such as MAO-A, MAO-B, and COX-2, suggests a significant therapeutic potential of these specimens134.
P. herba venti populations, positioned in the lower left part of the scores plot, exhibited high levels of TCC and β-Carotene. Carotenoids, including β-carotene, are well-documented for their health benefits, particularly their antioxidant properties. β-Carotene serves as a precursor to vitamin A and has been associated with a reduced risk of various cancers, including breast and prostate cancer135. The antioxidant capacity of carotenoids helps in neutralizing free radicals, thereby reducing oxidative damage to cells136. Moreover, carotenoids have been shown significant anti-inflammatory effects, which can further contribute to their protective role against chronic diseases137. Recent advancements in nanotechnology have also enhanced the bioavailability and therapeutic efficacy of carotenoids, allowing for improved delivery systems that maximize their health benefits138.
The key characteristic of P. olivieri and P. rigida populations is the high concentration of eriodictyol-O-glucuronide, hyperoside and myricitrin in their aerial parts. Flavonoids like eriodictyol-O-glucuronide and hyperoside exhibit anti-inflammatory and antioxidant effects, which could enhance their therapeutic potential in cancer treatment139. Additionally, myricitrin, a flavonoid glycoside, has been shown to inhibit HIV-1 and to possess broader anti-cancer properties140. In P. persica populations, high levels of caffeoyl malic acid, salvianolic and rosmarinic acids were detected, along with lower amounts of caffeoyl quinic acids, forsythoside A, verbascoside and martynoside/isomartynoside. Caffeoyl malic acid, a malic acid derivative, shows anticancer potential due to its antioxidant properties141, although further research is needed to clarify on its specific effects. Salvianolic acid and rosmarinic acid, both polyphenolic compounds with potent antioxidant effects, are known for their broad biological activities and are commonly found in plants of the Lamiaceae family. These compounds contribute to the overall antioxidant defense system in plants, potentially offering health benefits such as anti-inflammatory, cardioprotective, and neuroprotective effects142.
Conclusion
In recent years, there has been increasing interest in exploring the potential therapeutic potential of Phlomis species, leading to a growing number of scientific studies highlighting their pharmacological properties. However, despite this attention, the full therapeutic applications of Phlomis remain underutilized. In this study, a comprehensive analysis of 56 samples from 6 distinct Phlomis species native to Iran was performed, with the aim of better understanding of their polyphenol content, antioxidant capabilities, and potential therapeutic uses. The study revealed that each Phlomis species exhibited a unique polyphenolic profile, with significant variations in their phenolic compositions. These differences suggest that each species holds distinct potential for treating various diseases. Results of both HCA and PCA analyses suggest that the variation in polyphenol profiles and antioxidant capabilities among Phlomis species is largely driven by genetic factors rather than environmental conditions, reinforcing the importance of species selection in studies and applications of plant-derived nutraceuticals. Specifically, P. tuberosa, with its high concentration of key bioactive compounds such as genistein, apigenin, verbascoside, and caffeoyl quinic acids, which are well-known for their antimicrobial activity, suggests potential applications in the development of antimicrobial adjuvants. Additionally, the elevated levels of forsythoside A, luteolin-glucuronide, and hispidulin-glucuronide, which are recognized for their anti-inflammatory and antitumor properties, indicate that P. tuberosa could also be particularly valuable in therapeutic applications aimed at combating inflammation-related disorders. On the other hand, P. kurdica, with its phytochemical profile rich in polyphenols such as sagerinic acid, kaempferol, and kaempferide, offers significant potential for nutraceutical formulations aimed at providing antioxidant support, anti-inflammatory benefits, promoting cardiovascular health, cancer prevention, and offering neuroprotective effects. P. herba venti, which is particularly rich in TCC and β-Carotene, well-known for their powerful antioxidant properties, may hold significant potential for preventing and managing chronic diseases linked to oxidative stress. In conclusion, our findings suggest significant potential for developing innovative nutraceuticals from the investigated Phlomis species, which could help address gaps in the limited range of available Phlomis-derived products on the market.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Safamansouri, H. et al. α-Amylase inhibitory activity of some traditionally used medicinal species of Labiatae. J. Diabetes Metab. Disord. 13, 114 (2014).
Kurman, Y. & Pasin, O. Anticancer activity of endemic Phlomis extracts in HCT116 human Colon cancer cells. Sağlık Bilimlerinde Değer. 13, 227–233 (2023).
Sarkhail, P. et al. Effect of Phlomis Persica on glucose levels and hepatic enzymatic antioxidants in streptozotocin-induced diabetic rats. Phcog Mag. 6, 219 (2010).
Guven, L., Erturk, A., Koca, M. & Gulcin, I. Phenolic compounds of Phlomis tuberosa by LC–MS/MS-Determination of antioxidant activity, Molecular Docking, and enzyme inhibition profiles. Chem. Select 8 (2023).
Okur, M. E. et al. Vivo Wound Healing and in Vitro anti-inflammatory activity evaluation of Phlomis Russeliana Extract Gel formulations. Molecules 25, 2695 (2020).
Dawood, S., Moursy, M., Sharaf, A. E. M. & Fouda, H. Ecophysiological studies on the oxidative stress responses of Phlomis aurea, Ballota undulata and Nepeta septemcrenata endemic plants in Saint Katherine Mountain, Egypt. EAJBSC 14, 13–23 (2022).
Erecevit Sönmez, P., Kirbag, S. & Çakilcioğlu, U. Reviewing Phlomis Rigida Labill from Turkey as a Antimicrobial efficacy. SAUJS 24, 1265–1271 (2020).
Ferrante, C. et al. Protective effects induced by alcoholic Phlomis fruticose and Phlomis herba‐venti extracts in isolated rat colon: focus on antioxidant, anti‐inflammatory, and antimicrobial activities in vitro. Phytother. Res. 33, 2387–2400 (2019).
Eruygur, N., Kirci, D., Ayaz, F., Doğu, S. & Bağci, Y. Biological activities of three Phlomis species. J. Res. Pharm. 26, 255–262 (2022).
Sarkhail, P. et al. Quantification of verbascoside in medicinal species of Phlomis and their genetic relationships. Daru J. Pharm. Sci. 22 (2014).
Sarkhail, P., Salimi, M., Sarkheil, P., Heidarnezhad, F. & Saeidnia, S. Evaluation of anti-melanogenic and cytotoxic activities of Phlomis Caucasica on Human Melanoma SKMEL-3 cells. Int. J. Cancer Manag. 10 (2017).
Kepekçi, R. A. & Pehlivan, M. Preliminary analysis of phenolic acid composition of Phlomis Syriaca. Glob J. Bot. Sci. 9, 46–50 (2021).
Ghannadian, M., Sajjadi, S., Delazari, Z. & Aghaei, M. Flavone constituents of Phlomis Bruguieri Desf. With cytotoxic activity against MCF-7 breast cancer cells. Res. Pharma Sci. 13, 422 (2018).
Demirci, B., Toyota, M., Demirci, F., Dadandi, M. Y. & Can Baser, K. H. Anticandidal pimaradiene diterpene from Phlomis essential oils. Comptes Rendus Chim. 12, 612–621 (2008).
Aghakhani, F., Kharazian, N. & Lori Gooini, Z. Flavonoid constituents of Phlomis (Lamiaceae) Species using Liquid Chromatography Mass Spectrometry. Phytochem. Anal. 29, 180–195 (2017).
Kondeva-Burdina, M., Shkondrov, A., Popov, G., Manov, V. & Krasteva, I. In Vitro/In vivo hepatoprotective and antioxidant effects of Defatted Extract and a phenolic fraction obtained from Phlomis tuberosa. IJMS 24, 10631 (2023).
Stefanakis, M. K., Tsiftsoglou, O. S., Mašković, P. Z., Lazari, D. & Katerinopoulos, H. E. Chemical constituents and Anticancer activities of the extracts from Phlomis × commixta rech. f. (P. Cretica × P. Lanata). IJMS 25, 816 (2024).
Rasheed, M. U., Naqvi, S. A. R., Al-Asmari, F., Rahim, M. A. & Ramadan, M. F. Phytochemicals, health-promoting effects, and enzyme inhibition traits of Phlomis stewartii extracts. Molecules 29, 1049 (2024).
Zhang, Y. & Wang, Z. Phenolic composition and antioxidant activities of two Phlomis species: a correlation study. C.R. Biol. 332, 816–826 (2009).
Rasheed, M. U., Naqvi, S. A. R., Rasool, N., Shah, S. A. A. & Zakaria, Z. A. Anti-diabetic and cytotoxic evaluation of Phlomis stewartii plant phytochemicals on cigarette smoke inhalation and alloxan-induced diabetes in Wistar rats. Metabolites 12, 1133 (2022).
Derafa, I., Amira, S., Benchikh, F., Mamache, W. & Kaoudoune, C. Phenolic content and antioxidant activity of hydromethanolic and aqueous extracts of aerial parts of Phlomis Crinita. Turkish JAF Sci. Tech. 10, 2061–2066 (2022).
Moshari-Nasirkandi, A., Alirezalu, A., Alipour, H. & Amato, J. Screening of 20 species from Lamiaceae family based on phytochemical analysis, antioxidant activity and HPLC profiling. Sci. Rep. 13 (2023).
Khaledi, M., Khaledi, F., Asadi-Samani, M., Gholipour, A. & Mahmoodi Kouhi, A. Phytochemical evaluation and antibacterial effects of Medicago sativa, Onosma Sericeum, Parietaria judaica L., Phlomis Persica and Echinophora platyloba. On Enterococcus faecalis. Biomed. Res. Ther. 5, 1941–1951 (2018).
Abbasi, B. & Anjum, S. Biomimetic synthesis of antimicrobial silver nanoparticles using in vitro-propagated plantlets of a medicinally important endangered species: Phlomis bracteosa. IJN 1663 (2016).
Lu, S. et al. Iridoid Glycosides from Phlomis Medicinalis Diels: Optimized Extraction and Hemostasis Evaluation. Chem. Biodivers. 19 (2022).
Mohammadi, S. et al. Taxonomic revision and clinical importance of Phlomoides genus: a comprehensive review. Pharm. Sci. 30, 21–35 (2023).
Bajkacz, S., Baranowska, I., Buszewski, B., Kowalski, B. & Ligor, M. Determination of flavonoids and phenolic acids in plant materials using SLE-SPE-UHPLC-MS/MS method. Food Anal. Methods 11, 3563–3575 (2018).
Ul-Haq, I. et al. Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran. J. Pharm. Res. 11, 241–249 (2012).
Chang, C. C., Yang, M. H., Wen, H. M. & Chern, J. C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 10 (2020).
Bharath, B., Pavithra, A. N., Divya, A. & Perinbam, K. Chemical composition of ethanolic extracts from some seaweed species of the south Indian coastal zone, their antibacterial and membrane-stabilizing activity. Russ J. Mar. Biol. 46, 370–378 (2020).
Moshari-Nasirkandi, A. et al. Chemometrics-based analysis of the phytochemical profile and antioxidant activity of Salvia species from Iran. Sci. Rep. 14 (2024).
Karthikeyan, M. et al. Induction of phenolics and defense-related enzymes in coconut (Cocos nucifera L.) roots treated with biocontrol agents. Braz. J. Plant. Physiol. 18, 367–377 (2006).
Shimada, K., Fujikawa, K., Yahara, K. & Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 40, 945–948 (1992).
Miao, J. et al. Chemical composition and bioactivities of two common Chaenomeles fruits in China: Chaenomeles speciosa and Chaenomeles sinensis. J. Food Sci. 81 (2016).
Klein, B. P. & Perry, A. K. Ascorbic acid and vitamin a activity in selected vegetables from different geographical areas of the United States. J. Food Sci. 47, 941–945 (1982).
Lichtenthaler, H. K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382 (1987).
Nagata, M., Noguchi, Y., Imanishi, S. & Sugiyama, K. A simple method for the estimation of alpha- and beta-carotene in carrots. Acta Hortic. 768, 565–569 (2008).
Tharayil, N. et al. Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: climatic stress-induced tannins are more reactive. New Phytol. 191, 132–145 (2011).
Serag, M., Moustafa, A. & Qiqa, S. Impact of climate change on surviving of phlomis aurea as an endemic species growing in southern Sinai, Egypt. Catrina. Int. J. Environ. Sci. 17, 33–39 (2018).
de la Rosa, L. A., Moreno-Escamilla, J. O., Rodrigo-García, J. & Alvarez-Parrilla, E. Phenolic compounds. Postharvest. Physiol. Biochem. Fruits Veg. 253–271 (2019).
Marchiosi, R. et al. Ferrarese-Filho, O. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 19, 865–906 (2020).
Marchica, A. et al. The biosynthesis of phenolic compounds is an integrated defence mechanism to prevent ozone injury in Salvia officinalis. Antioxidants 9, 1274 (2020).
Ma, X. H. et al. The Biosynthetic pathways of Tanshinones and phenolic acids in Salvia miltiorrhiza. Molecules 20, 16235–16254 (2015).
Shi, M., Huang, F., Deng, C., Wang, Y. & Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 59, 953–964 (2018).
Wang, J. et al. Chemistry, and Pharmacology of polyphenols from Chinese Salvia species: a review. Molecules 24, 155 (2019).
Lattanzio, V. Phenolic compounds: introduction. Nat. Prod. 1543–1580 (2013).
Li, S. et al. SmMYB111 is a key factor to Phenolic Acid Biosynthesis and interacts with both SmTTG1 and SmbHLH51 in Salvia miltiorrhiza. J. Agric. Food Chem. 66, 8069–8078 (2018).
Ma, D. et al. Accumulation of phenolic compounds and expression profiles of phenolic acid biosynthesis-related genes in developing grains of white, purple, and red wheat. Front. Plant. Sci. 7 (2016).
Ma, P., Liu, J., Zhang, C. & Liang, Z. Regulation of water-soluble phenolic acid biosynthesis in Salvia miltiorrhiza bunge. Appl. Biochem. Biotechnol. 170, 1253–1262 (2013).
McCalla, D. R. & Neish, A. C. Metabolism of phenylpropanoid compounds in Salvia: II. Biosynthesis of phenolic cinnamic acids. Can. J. Biochem. Physiol. 37, 537–547 (1959).
Sun, M. et al. The biosynthesis of phenolic acids is positively regulated by the JA-responsive transcription factor ERF115 in Salvia miltiorrhiza. J. Exp. Bot. 70, 243–254 (2018).
Yang, D. et al. DNA methylation: a new regulator of phenolic acids biosynthesis in Salvia miltiorrhiza. Ind. Crops Prod. 124, 402–411 (2018).
Zhou, W. et al. Comprehensive transcriptome profiling of Salvia miltiorrhiza for discovery of genes associated with the biosynthesis of tanshinones and phenolic acids. Sci. Rep. 7 (2017).
Delazar, A. et al. Free-radical-scavenging principles from Phlomis Caucasica. J. Nat. Med. 62, 464–466 (2008).
Kondeva-Burdina, M., Shkondrov, A., Popov, G., Manov, V. & Krasteva, I. Antioxidant and hepatoprotective activity of an extract from the overground parts of Phlomis Russeliana lag. Ex Benth. Separations 11, 117 (2024).
López, V., Jäger, A. K., Akerreta, S., Cavero, R. Y. & Calvo M. I. antioxidant activity and phenylpropanoids of Phlomis Lychnitis L.: a traditional herbal tea. Plant. Foods Hum. Nutr. 65, 179–185 (2010).
Ersöz, T. et al. Physocalycoside, a new phenylethanoid glycoside from Phlomis physocalyx Hub.-Mor. Z. für Naturforschung. 58, 471–476 (2003).
Ersöz, T., Ivancheva, S., Akbay, P., Sticher, O. & Çalış, İ. Iridoid and phenylethanoid glycosides from Phlomis tuberosa L. Z. für Naturforschung. 56, 695–698 (2001).
Ersöz, T. et al. Iridoid and phenylethanoid glycosides from Phlomis longifolia var. Longifolia. Nat. Prod. Lett. 15, 345–351 (2001).
Martin-Nizard, F. et al. Natural phenylpropanoids protect endothelial cells against oxidized LDL-induced cytotoxicity. Planta med. 69, 207–211 (2003).
Amor, I. L. B. et al. Chekir-Ghedira, L. Phytochemistry and biological activities of Phlomis species. J. Ethnopharmacol. 125, 183–202 (2009).
Ma, R. F. et al. The phenylalanine ammonia-lyase gene family in Isatis Indigotica Fort.: molecular cloning, characterization, and expression analysis. CJNM 14, 801–812 (2016).
Lavola, A., Julkunen-Tiitto, R., de la Rosa, T. M., Lehto, T. & Aphalo, P. J. Allocation of carbon to growth and secondary metabolites in birch seedlings under UV‐B radiation and CO2 exposure. Physiol. Plant. 109, 260–267 (2000).
He, J. et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. 117, 271–277 (2019).
Kunter, İ. et al. New data for endemic Phlomis cypria post from north Cyprus: biological activities and LC MS/MS analysis. Ind. J. Pharm. Educ. Res. 57, 511–518 (2023).
Sarikurkcu, C. & Ćavar Zeljković, S. Chemical composition and antioxidant activity of Phlomis Leucophracta, an endemic species from Turkey. Nat. Prod. Res. 34, 851–854 (2018).
Lotfy, R. & Abd El-Moaty, H. Chemical composition and antioxidant activity of the essential oil and flavonoids of Phlomis Floccosa. Egypt. J. Desert Res. 65, 125–135 (2015).
El-Banhawy, A. & Al-Juhani, W. DNA barcoding and phylogeny of Phlomis aurea (Lamiaceae) endemic to Sinai Peninsula, Egypt. Pak. J. Bot. 51 (2019).
Zhao, W. et al. Complete chloroplast genome sequences of Phlomis fruticosa and phlomoides strigosa and comparative analysis of the genus Phlomis Sensu lato (Lamiaceae). Front. Plant. Sci. 13 (2022).
Stojković, D. et al. Phlomis fruticosa l. exerts in vitro antineurodegenerative and antioxidant activities and induces prooxidant effect in glioblastoma cell line. EXCLI J. 21, 387–399 (2022).
Wu, Q. S. et al. Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: changes in leaf antioxidant defense systems. Plant. Soil. Environ. 56, 470–475 (2010).
Hasanuzzaman, M. et al. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. IJMS 22, 9326 (2021).
Li, Q. S. et al. Arbuscular mycorrhizal fungi and endophytic fungi activate leaf antioxidant defense system of lane late navel orange. J. Fungi. 8, 282 (2022).
Hasanuzzaman, M. et al. Regulation of ROS metabolism in plants under environmental stress: a review of recent experimental evidence. IJMS 21, 8695 (2020).
Noctor, G., Reichheld, J. P. & Foyer, C. H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 80, 3–12 (2018).
Dias, M. C., Pinto, D. C. G. A. & Silva, A. M. Plant flavonoids: chemical characteristics and biological activity. Molecules 26, 5377 (2021).
Hou, Y. et al. A cinnamate 4-hydroxylase1 from safflower promotes flavonoids accumulation and stimulates antioxidant defense system in Arabidopsis. IJMS 24, 5393 (2023).
Zhao, G. et al. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. IJMS 19, 1912 (2018).
Ito, A. et al. Effect of different durations of root area chilling on the nutritional quality of spinach. Environ. Control Biol. 51, 187–191 (2014).
Brunele Caliman, F. R., da Silva, H., Stringheta, D. J., Fontes, P. C. R. & Moreira, P. C. R. Chartuni Mantovani, E. Quality of tomatoes grown under a protected environment and field conditions. Idesia 28, 75–82 (2010).
Bachir Nabti, Khadidja, T. A. I. B. A. O. U. I. & Ikram, S. I. S. A. L. A. H. Evaluation of the antioxidant activity of certain extracts from the leaves of Phlomis Crinita Cav from North western Algeria. World J. Bio Pharm. Health Sci. 16, 025–028 (2023).
Yilmaz, E. & Karadeniz, F. Effect of storage on the bioactive compounds and antioxidant activity of quince nectar. Int. J. Food Sci. Tech. 49, 718–725 (2013).
Lu, Q., Li, L., Xue, S., Yang, D. & Wang, S. Stability of Flavonoid, Carotenoid, Soluble Sugar and Vitamin C in ‘Cara Cara’ Juice during Storage. Foods 8, 417 (2019).
Habibi, E., Bakhshi, G. & Khaniki, G. Pretreatment with salicylic acid and nitric oxide mitigated silver nanoparticles toxicity and enhanced their removal in medicinal Phlomis tuberosa plants. Acta Biologica Cracov. Ser. Bot. 64, 35–48 (2022).
Shaltout, K. H., Ahmed, D. A. & Shabana, H. A. Distribution of the species associated with Phlomis aurea Decne along an elevation gradient in Southern Sinai, Egypt. ECMED 42, 65–77 (2016).
Khedr, A. H., El-Katony, T., Saad-Allah, K., Ahmed, F. & Kashlana, M. Niche differentiation of two congeneric Phlomis species in Egypt. Sci. J. Damietta Fac. Sci. 10, 45–57 (2020).
Rizi, M. R., Azizi, A., Sayyari, M., Mirzaie-Asl, A. & Conti, L. Increased phenylpropanoids production in UV-B irradiated Salvia verticillata as a consequence of altered genes expression in young leaves. Plant. Physiol. Biochem. 167, 174–184 (2021).
Valifard, M. et al. Effect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: from gene to metabolite. J. Hortic. Sci. Biotech. 94, 389–399 (2018).
Dou, H., Niu, G., Gu, M. & Masabni, J. G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 3, 36 (2017).
Saapilin, N. S., Yong, W. T. L., Cheong, B. E., Kamaruzaman, K. A. & Rodrigues, K. F. Physiological and biochemical responses of Chinese cabbage (Brassica rapa var. Chinensis) to different light treatments. Chem. Biol. Technol. Agric. 9 (2022).
Özcan, M. M. et al. Chemical composition of essential oils of Phlomis Grandiflora Thompson var. Grandiflora flowers and leaves of Turkish origin. J. Food Biochem. 35, 125–132 (2010).
Sarikurkcu, C., Uren, M. C., Kocak, M. S., Cengiz, M. & Tepe, B. Chemical composition, antioxidant, and enzyme inhibitory activities of the essential oils of three Phlomis species as well as their fatty acid compositions. Food Sci. Biotechnol. 25, 687–693 (2016).
Gostin, I. N. & Blidar, C. F. Glandular trichomes and essential oils variability in species of the genus Phlomis L. Rev. Plants 13, 1338 (2024).
Allafchian, A. R., Mirahmadi-Zare, S. Z., Jalali, S. A. H., Hashemi, S. S. & Vahabi, M. R. Green synthesis of silver nanoparticles using phlomis leaf extract and investigation of their antibacterial activity. J. Nanostruct. Chem. 6, 129–135 (2016).
Ghodsimaab, S. P., Ghasimi Hagh, Z., Makarian, H. & Gholipoor, M. Deciphering morphological and biochemical responses of Salvia leriifolia to seed cold plasma treatment, priming, and foliar spraying with nano-salicylic acid. Sci. Rep. 13, 18672 (2023).
Conselvan, G. B. et al. Biostimulant activity of humic substances extracted from leonardites. Plant. Soil. 420, 119–134 (2017).
Cheng, X. et al. Foliar phenylalanine application promoted antioxidant activities in cabernet sauvignon by regulating phenolic biosynthesis. J. Agric. Food Chem. 68, 15390–15402 (2020).
Tovar, M. J., Romero, M. P., Girona, J. & Motilva, M. J. L. Phenylalanine ammonia-lyase activity and concentration of phenolics in developing olive (Olea europaea L Cv Arbequina) fruit grown under different irrigation regimes. J. Sci. Food Agric. 82, 892–898 (2002).
Mohammadkhani, N. Effects of salinity on phenolic compounds in tolerant and sensitive grapes. Agric. for. 64 (2018).
Kang, H. & Saltveit, M. E. Wound-induced PAL activity is suppressed by heat‐shock treatments that induce the synthesis of heat‐shock proteins. Physiol. Plant. 119, 450–455 (2003).
Formica-Oliveira, A. C. et al. UV-C and hyperoxia abiotic stresses to improve healthiness of carrots: study of combined effects. J. Food Sci. Technol. 53, 3465–3476 (2016).
Campos-Vargas, R. & Saltveit, M. E. Involvement of putative chemical wound signals in the induction of phenolic metabolism in wounded lettuce. Physiol. Plant. 114, 73–84 (2002).
Ortega-García, F. & Peragón, J. Phenylalanine ammonia-lyase, polyphenol oxidase, and phenol concentration in fruits of Olea europaea L. Cv. Picual, Verdial, Arbequina, and Frantoio during Ripening. J. Agric. Food Chem. 57, 10331–10340 (2009).
Dalar, A., Bengu, A. S. & Allahverdiyev, O. Analysis of Phytochemical Composition and Biological activities of Verbascum cheiranthifolium var. Cheiranthifolium stem and flowers. IJSM 5, 233–242 (2018).
Li, X. et al. Unraveling the efficacy of verbascoside in thwarting MRSA pathogenicity by targeting sortase A. Appl. Microbiol. Biotechnol. 108, 360 (2024).
Olenichenko, N. A. & Zagoskina, N. V. Response of winter wheat to cold: production of phenolic compounds and L-phenylalanine ammonia lyase activity. Appl. Biochem. Microbiol. 41, 600–603 (2005).
Teklemariam, T. A. & Blake, T. J. Phenylalanine ammonia-lyase‐induced freezing tolerance in jack pine (Pinus banksiana) seedlings treated with low, ambient levels of ultraviolet‐B radiation. Physiol. Plant. 122, 244–253 (2004).
Becerra-Moreno, A., Redondo-Gil, M., Benavides, J., Nair, V. & Cisneros-Zevallos, L. Jacobo-Velázquez, D. A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant. Sci. 6, 837 (2015).
Jing, L., Ma, H., Fan, P., Gao, R. & Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement. Altern. Med. 15, 287 (2015).
Yao, Y., Sang, W., Zhou, M. & Ren, G. Phenolic composition and antioxidant activities of 11 celery cultivars. J. Food Sci. 75, 9–13 (2010).
Thusoo, S. et al. Antioxidant activity of essential oil and extracts of Valeriana jatamansi roots. Biomed. Res. Int. 2014, 1–4 (2014).
Glevitzky, I. et al. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 70, 3103–3107 (2019).
Kumar, S., Sandhir, R. & Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes. 7, 560 (2014).
Hadini, A., Azdimousa, A., Khoulati, A., El bekkaye, K. & Saalaoui, E. Valorization of Moroccan Pistacia lentiscus L. Leaves: phytochemical and in vitro antioxidant activity evaluation compared to different altitudes. Sci. World J.2022, 1–10 (2022).
Lu, Y., Chang, X. & Guo, X. Dynamic changes of ascorbic acid, phenolics biosynthesis and antioxidant activities in mung beans (Vigna radiata) until maturation. Plants 8, 75 (2019).
Ryu, J. et al. Comparison of phytochemicals and antioxidant activity in blackberry (Rubus fruticosus L.) fruits of mutant lines at the different harvest time. Plant. Breed. Biotech. 4, 242–251 (2016).
Saccenti, E., Hoefsloot, H. C. J., Smilde, A. K., Westerhuis, J. A. & Hendriks, M. M. W. B. reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 10, 361–374 (2013).
Iaccarino, N. et al. Impact of phytosterols on liver and distal colon metabolome in experimental murine colitis model: an explorative study. J. Enzyme Inhib. Med. Chem. 34, 1041–1050 (2019).
Granato, D., Santos, J. S., Escher, G. B., Ferreira, B. L. & Maggio, R. M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: a critical perspective. Trends Food Sci. 72, 83–90 (2018).
Laincer, F. et al. Characterization of monovarietal extra virgin olive oils from the province of Béjaïa (Algeria). Food Res. Int. 89, 1123–1133 (2016).
Ardila, J. A., Funari, C. S., Andrade, A. M., Cavalheiro, A. J. & Carneiro, R. L. Cluster analysis of commercial samples of Bauhinia spp. using HPLC-UV/PDA and MCR‐ALS/PCA without peak alignment procedure. PCA 26, 367–373 (2015).
Simion, I. M., Moţ, A. C. & Sârbu, C. Finding specific peaks (markers) using fuzzy divisive hierarchical associative-clustering based on the chromatographic profiles of medicinal plant extracts obtained at various detection wavelengths. Anal. Methods. 12, 3260–3267 (2020).
Kim, B., Han, S. R., Lee, H. & Oh, T. J. Insights into group-specific pattern of secondary metabolite gene cluster in Burkholderia Genus. Front. Microbiol. 14 (2024).
Coutinho, A. J., Pinheiro, M., Neves, A. R. & Pinto, M. M. M. Therapeutic potential of genistein: preclinical studies, clinical evidence, and nanotechnology application. CMC 30, 2480–2517 (2023).
Kim, D. E., Min, K., Kim, M. J., Kim, S. H. & Kwon, T. K. Hispidulin inhibits mast cell-mediated allergic inflammation through down-regulation of histamine release and inflammatory cytokines. Molecules 24, 2131 (2019).
Özgen, U. et al. Relationship between chemical structure and antioxidant activity of luteolin and its glycosides isolated from Thymus sipyleus subsp. sipyleus var. Sipyleus. Planta Med. 13, 1–12 (2010).
Muazzam, S., Harvey, J., Deviese, T., Farman, M. & McCullagh, J. Targeted and untargeted metabolite profiling of the ethnobotanical Martynia annua L. identifies bioactive compounds with medicinal properties. PMIO 5, e68–e78 (2018).
Yang, Y., Shen, J., Deng, P. & Chen, P. Mechanism investigation of Forsythoside a against esophageal squamous cell carcinoma in vitro and in vivo. Cancer Biol. Ther. 25, 2380023 (2024).
Wu, B., Xu, B. & Hu, M. Regioselective glucuronidation of flavonols by six human UGT1A isoforms. Pharm. Res. 28, 1905–1918 (2011).
Alcázar Magaña, A., Kamimura, N., Soumyanath, A., Stevens, J. F. & Maier, C. S. Caffeoylquinic acids: chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 107, 1299–1319 (2021).
Choi, J. K., Kwon, O. Y. & Lee, S. H. Kaempferide prevents photoaging of ultraviolet-b irradiated nih-3t3 cells and mouse skin via regulating the reactive oxygen species-mediated signalings. Antioxidants 12, 11 (2022).
Zang, Y., Zhang, L., Igarashi, K. & Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 6, 834–841 (2015).
Tie, F. et al. Kaempferol and Kaempferide Attenuate Oleic Acid-Induced lipid Accumulation and oxidative stress in HepG2 cells. Int. J. Mol. Sci. 22, 8847 (2021).
Zengin, G. et al. Di Giacomo, V. Novel perceptions on chemical profile and biopharmaceutical properties of mentha spicata extracts: adding missing pieces to the scientific puzzle. Plants 11, 233 (2022).
Kim, J. A., Jang, J. H. & Lee, S. Y. An updated comprehensive review on vitamin a and carotenoids in breast cancer: mechanisms, genetics, assessment, current evidence, and future clinical implications. Nutrients 13, 3162 (2021).
Bas, T. G. Bioactivity and bioavailability of carotenoids applied in human health: technological advances and innovation. Int. J. Mol. Sci. 25, 7603 (2024).
Genç, Y. et al. Oxidative stress and marine carotenoids: application by using nanoformulations. Mar. Drugs. 18, 423 (2020).
Jafari, Z. et al. Nanotechnology-abetted astaxanthin formulations in multimodel therapeutic and biomedical applications. J. Med. Chem. 65, 2–36 (2021).
Fredsgaard, M., Kaniki, S. E. K., Antonopoulou, I., Chaturvedi, T. & Thomsen, M. H. Phenolic compounds in Salicornia spp. and their potential therapeutic effects on H1N1, HBV, HCV, and HIV: a review. Molecules 28, 5312 (2023).
Tenfen, A. et al. Cechinel-Filho, V. effects of myricetin-3-O-α-rhamnoside (myricitrin) treatment on urinary parameters of Wistar rats. J. Pharm. Pharmacol. 71, 1832–1838 (2019).
Szajwaj, B. et al. Amides and esters of phenylpropenoic acids from the aerial parts of Trifolium pallidum. Nat. Prod. Commun. 6, 1293–1296 (2011).
Habtemariam, S. Molecular pharmacology of rosmarinic and salvianolic acids: potential seeds for Alzheimer’s and vascular dementia drugs. Int. J. Mol. Sci. 19, 458 (2018).
Acknowledgements
The present research was realized in the frame of the international agreement between the Department of Pharmacy of the University of Naples Federico II and the Department of Horticultural Science of the Urmia University (n. 2022/0059068).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.A. and J.A.; Methodology and investigation: S.A.G., N.I., F.R., H.S, and H.A.; Supervision: N.I., A.A., and J.A.; Manuscript writing: S.A.G., A.A., and J.A. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Guideline statement
Authors confirm that the use of plants in the present study complies with international, national and/or institutional guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gheibi, S.A., Alirezalu, A., Shirzad, H. et al. Phytochemical profiling, antioxidant potential, and UHPLC-HRMS analysis of Phlomis genus aerial parts for therapeutic applications. Sci Rep 15, 6732 (2025). https://doi.org/10.1038/s41598-025-89055-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89055-4