Abstract
This study aims to identify and evaluate the most common drugs associated with the risks of autoimmune hepatitis (AIH) using the FDA Adverse Event Reporting System (FAERS) database. Adverse drug events (ADEs) associated with drug-induced AIH (DI-AIH) were retrieved from the FAERS database (January 2004–June 2024). Disproportionality analysis was performed to identify drugs significantly linked to DI-AIH, and time-to-onset (TTO) analyses were conducted to evaluate the timing and risk profiles of DI-AIH adverse reactions. Our study identified 2,511 ADEs linked to autoimmune hepatitis. Disproportionality analysis identified 22 drugs significantly associated with AIH risk, including 4 antibiotics, 3 antivirals, 4 cardiovascular drugs, 5 antitumor agents, 2 immunomodulators, 2 nonsteroidal anti-inflammatory drugs, and 1 drug each from the respiratory and nervous system categories. The highest DI-AIH risks were observed with minocycline (ROR = 53.97), nitrofurantoin (ROR = 57.02), and doxycycline (ROR = 16.12). Antitumor drugs had the shortest median TTO (77.00 days), whereas cardiovascular drugs exhibited the longest (668.30 days). Through a comprehensive analysis of the FAERS database, our study identified drugs strongly associated with AIH. Preventing DI-AIH requires careful drug selection and monitoring. This study provides evidence-based insights into implicated drugs, aiming to optimize clinical management and mitigate risks.
Similar content being viewed by others
Introduction
Drug-induced autoimmune hepatitis (DI-AIH), a rare but potentially life-threatening subtype of drug-induced liver injury (DILI), may progress to acute liver failure1. Also referred to as immune-mediated DILI2 or DI-AIH-like injury3, DI-AIH accounts for 2–18% of autoimmune hepatitis (AIH) cases4,5,6,7,8 and 2.9–8.8% of DILI cases9,10. The clinical, biochemical, and pathological features of DI-AIH closely resemble idiopathic AIH, including the presence of AIH-specific antibodies and elevated immunoglobulin G levels, complicating diagnosis11. Consequently, accurate identification requires thorough evaluation of patients’ medical histories and laboratory findings to inform diagnosis and treatment. Drugs commonly implicated in DI-AIH include minocycline, nitrofurantoin, methyldopa, hydralazine, and tienilic acid9. Statins are another significant contributor, with immune-mediated DILI or DI-AIH, representing 8.5–27.2% of statin-related DILI cases12,13. Nonsteroidal anti-inflammatory drugs (NSAIDs) have also been implicated14. More recently, anti-tumor necrosis factor (TNF), along with statins and NSAIDs, have emerged as primary agents associated with DI-AIH10,14,15. Despite these findings, systematic research on DI-AIH remains limited. Certain medications significantly increase the risk of DI-AIH, underscoring the need for heightened clinical awareness and vigilance. Enhanced understanding can support the development of personalized treatment strategies, improving patient safety and therapeutic outcomes.
This study evaluated the iatrogenic risk of DI-AIH using large-scale real-world data from the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS) database. To the best of our knowledge, this is the first investigation to extract and evaluate FAERS data specifically for assessing drug-related risks of DI-AIH. The findings aim to enhance clinical awareness, support personalized medication strategies, and provide additional insights into potential adverse reactions not explicitly addressed in drug labeling.
Materials and methods
Data source
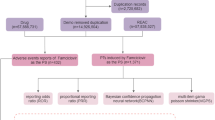
This study analyzed adverse event data from the FAERS database (January 2004–June 2024) [source: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html], encompassing seven datasets: patient demographics, drug information, adverse events, outcomes, report sources, treatment durations, and indications for use. During this period, the database recorded 21,035,995 entries. In the data cleaning process, we first standardize the drug names in the FAERS database to ensure that all drug names meet the uniform pharmacopoeia standards. Irregular and non-standardized drug names are corrected through comparison with official drug databases, such as the FDA Drug Database. The reports were then identified and duplicate reports were eliminated, specifically, we applied a de-weighting algorithm based on patient ID and the time of the reported event, ensuring that each event was only counted once, and ended up retaining 17,785,793 entries. Adverse events associated with DI-AIH were screened, and related drugs were analyzed to characterize the epidemiology and drug distribution of DI-AIH. This process yielded 2,511 patients and 743 associated drugs. After consolidating redundant drug names caused by brand variations, the top 30 drugs were ranked, with 22 unique drugs retained. The data cleaning workflow is illustrated in Fig. 1.
Identification of adverse drug events
In this study, adverse drug events (ADE) were coded according to the Medical Dictionary for Regulatory Activities (MedDRA, version 20.0, http://www.meddra.org/), using MedDRA Preferred Terms (PTs). DI-AIH related PTs were identified through standardized queries, with the term “drug-induced autoimmune hepatitis” selected for analysis. Cases linked to this PT were extracted from the DEMO files using their PRIMARYID, and duplicate reports were excluded to ensure accurate case counts.
Patient age (AGE) and reporting country (REPORTER_COUNTRY) were also retrieved. For drug involvement analysis, only cases labeled as “primary suspect drug” were included, excluding entries marked as “secondary suspect drug”, “concomitant drug”, or “interacting drug” to minimize result uncertainty.
Statistical analysis
This study employed four disproportionality analysis methods to identify potential drug-event associations: Reporting Odds Ratio (ROR)16, Proportional Reporting Ratio (PRR)17, Bayesian Confidence Propagation Neural Network (BCPNN)18, and Multi-item Gamma Poisson Shrinker (MGPS)19. These methods leverage a fourfold table to compare target events and drugs with other events and drugs, generating signals of potential associations (see Supplementary Tables 1 and 2). Criteria for defining positive signals are detailed in Supplementary Table 2. Drugs meeting the criteria of all four methods were included, indicating a significant association with DI-AIH. The BCPNN method was further used to assess the risk of DI-AIH, analyze drug usage duration, and assess Time to Onset (TTO) of related ADE. Using the BCPNN algorithm, drugs were classified according to DI-AIH risk, and ADE were stratified into quartiles to analyze the distribution of onset times.
Results
Baseline subject information
Between 2004 and 2024, 2,511 subjects reported DI-AIH adverse reactions in the FAERS database. The average age was 53.82 ± 18.43 years, with females representing 59.26% of cases. Female subjects were predominantly aged 50–80 years, while males were concentrated in the 55–75 years range (Fig. 2A). A steady increase in DI-AIH cases was observed, peaking in 2023. The female incidence rate consistently exceeded that of males until 2023, after which male cases slightly surpassed female cases in the first half of 2024 (Fig. 2B). The primary outcomes were “Other serious (important medical events)” (43.53%) and “Hospitalization (initial or prolonged)” (39.03%). Most reports originated from the Europe (49.64%) and Asia (16.95%). Further details are provided in Table 1.
Characteristics of reports involved in drug-related autoimmune hepatitis from the FAERS database. Notes: Distribution of demographic data for drug-induced autoimmune hepatitis. (A) Population pyramid of subjects with drug-induced autoimmune hepatitis categorized by gender and age. (B) Bar chart showing the annual reporting counts of drug-induced autoimmune hepatitis by gender.
Distribution of drugs causing DI-AIH
Through disproportionality analysis, 22 drugs were identified from a total of 743, meeting all four positive signal screening criteria. These drugs were categorized by therapeutic class as follows: Antibiotics (4): Minocycline, Nitrofurantoin, Doxycycline, Azithromycin; Antivirals (3): Pegylated interferon α-2a/α-2b, Ribavirin; Cardiovascular drugs (4): Atorvastatin, Simvastatin, Rosuvastatin, Olmesartan; Antitumor drugs (5): Nivolumab, Atezolizumab, Pembrolizumab, et al.; Immune system drugs (2): Glatiramer acetate, Alemtuzumab; NSAIDs (2): Naproxen, Meloxicam; Other drugs: Bosentan (respiratory system), Levetiracetam (neurological system). Further details are provided in Fig. 3; Table 2.
The identification and classification of drugs associated with drug-induced autoimmune hepatitis. Notes: Drugs associated with AIH are predominantly distributed among immune system drugs, respiratory system medications, nervous system medications, antibiotic, antivirus drugs, antitumor drug, cardiovascular system medications, nonsteroidal anti-inflammatory drugs.
Drug risk and latency of DI-AIH
The potential risk of DI-AIH for each drug was evaluated based on their ROR values, and their drug-induced latency periods were analyzed. The drugs with the highest DI-AIH risk, as indicated by their ROR values, were Nitrofurantoin (ROR = 57.02), Minocycline (ROR = 53.97), Doxycycline (ROR = 16.12), Atorvastatin (ROR = 12.70), and Meloxicam (ROR = 11.11). The drugs with the shortest median latency periods were Doxycycline (6.40 days), Pemetrexed (16.00 days), Azithromycin (25.40 days), Ipilimumab (55.07 days), and Nivolumab (68.75 days). Detailed results are provided in Fig. 4; Table 3.
Comparison of onset time across drug categories
Drugs were categories based on their clinical use into groups such as cardiovascular drugs, antitumor drugs, immune system drugs, antibiotics, antivirals, NSAIDs, respiratory drugs, and neurological drugs. Cumulative risk curves were used to assess differences in TTO. The analysis revealed that antitumor drugs had the shortest median TTO (77.0 days), while cardiovascular drugs exhibited the longest median TTO (668.3 days). The median TTO for anti-infective drugs was 500.7 days, for antibiotics 491.1 days, for antiviral drugs 143.3 days, for immune system drugs 352.1 days, for the neurological drug Levetiracetam 373.5 days, and for respiratory drugs 420.0 days. Data on the TTO for NSAIDs were unavailable. Further details are provided in Fig. 5.
Time to event onset of drug-induced autoimmune hepatitis elicited by various drugs. Notes: Figure 5A shows a cumulative risk timeline of drug induction for seven group induction drugs. Figure 5B exhibits a violin plot for time disparities in seven group induction. Figure 5C presents the number of patients at risk for developing drug-induced autoimmune hepatitis.
Discussion
This study utilized the FAERS database to examine the epidemiological characteristics of DI-AIH, a condition with a consistent annual increase since 2004, peaking in 2023. A total of 743 drugs associated with DI-AIH were identified, with the top 30 selected based on the frequency of reported cases. Disproportionality analysis identified 22 drugs that significantly increase the risk of DI-AIH. Risk values and drug-induced incidence for this subset were assessed. This study provides empirical evidence to support individualized treatment for DI-AIH patients and offers supplementary information on potential DI-AIH related adverse reactions for inclusion in drug labels. To our knowledge, this is the first large-scale, real-world study on DI-AIH using the FAERS database.
The two models adopted in this study, frequency model and Bayesian model, have been relatively common technologies in pharmacovigilance signal detection. However, considering that the two models have been widely used in existing literature and have strong reliability and effectiveness18, as well as their applicability and theoretical basis in pharmacovigilance data, to be specific, The frequentist approach provides intuitive and easy-to-understand results through classical statistical inferences such as hypothesis testing and confidence intervals, while the Bayesian approach makes more flexible inferences using prior knowledge and provides a more comprehensive assessment of uncertainty. Therefore, by combining these two methods, we can compare them under different statistical frameworks in order to improve the accuracy and robustness of signal detection. While these approaches may not be entirely novel on their own, their combined use and real-world research in drug-induced autoimmune hepatitis provide new perspectives and insights.
Clinically, drug-induced DI-AIH is associated with a broad range of medications, and understanding their mechanisms is crucial. AIH is an immune-mediated, non-self-limiting inflammatory liver disease, characterized by elevated transaminases and immunoglobulin G (IgG), the presence of antinuclear antibodies or smooth muscle antibodies, and interface lymphoplasmacytic hepatitis20. Its immune pathogenesis involves autoreactive CD4 and CD8 T cells, which are activated when environmental factors disrupt self-tolerance4. DI-AIH is specifically linked to certain drugs, with studies indicating that the median TTO for antibiotic induced DI-AIH is 491.1 days. This study identifies nitrofurantoin, minocycline, and doxycycline as the top three drugs with the highest risk of DI-AIH. Nitrofurantoin, a nitrofuran-class antimicrobial, is primarily used for the treating acute uncomplicated urinary tract infections. Minocycline, a broad-spectrum tetracycline antibiotic commonly prescribed for acne and skin infections, has been frequently associated with DI-AIH. A prospective study of 1,322 DILI cases identified 88 cases of DI-AIH, 42 of which were attributed to nitrofurantoin and 28 to minocycline9. Similarly, a retrospective analysis of 261 AIH patients found 24 DI-AIH cases, 11 of which were linked to nitrofurantoin and 11 to minocycline7. Several case reports have further documented minocycline induced DI-AIH21,22,23. The median duration of DI-AIH induced by nitrofurantoin and minocycline was reported to be 24 months and 12 months24. Doxycycline, another tetracycline antibiotic, is associated with fewer cases of DI-AIH but has the shortest median TTO at 6.4 days. Notably, a 22-year-old woman developed DI-AIH following doxycycline use for acne treatment25, and a similar case was reported in a 50-year-old woman26. Azithromycin, a macrolide antibiotic, has also been associated with AIH, including a case of autoimmune liver injury in an asthma patient with a positive antinuclear antibody (ANA) titer of 1:320 following azithromycin use. Liver pathology in this case revealed mild portal inflammation and interface hepatitis27. The mechanisms by which these antibiotics induce DI-AIH remain unclear. It is hypothesized that nitrofurantoin, or its breakdown product, when presented via the class I HLA complex, activates CD8 + cytotoxic T cells, contributing to drug-induced liver injury28. Minocycline induced liver injury has been linked to the rare HLA allele B*35:02; however, most cases do not exhibit this allele, and clinical features remain consistent regardless of HLA association29. The precise mechanisms by which doxycycline and azithromycin induce DI-AIH have yet to be fully elucidated, but clinical evidence underscores the need for heightened awareness of their potential to cause autoimmune liver injury.
Additionally, this study identifies three antiviral drugs—pegylated interferon α-2a/α-2b and ribavirin—as strongly associated with DI-AIH, with a median TTO of 143.3 days. Interferon α has been implicated in various autoimmune diseases. One case involved a 34-year-old woman who, after receiving pegylated interferon α-2a and ribavirin for chronic hepatitis C, developed Graves’ disease and AIH in the 32nd and 44th weeks of treatment, respectively30. Another case described a 56-year-old male who developed IgG4-related AIH one year after liver transplantation, following pegylated interferon α-2a and ribavirin treatment for a hepatitis C relapse31. A third case involved a 56-year-old female who developed multiple positive autoantibodies, AIH, and chronic hepatitis C after 9 months of treatment with pegylated interferon α-2b32. While evidence linking interferons to DI-AIH largely derives from case reports, it is crutical to monitor AIH markers and liver function regularly when administering interferons to mitigate associated risks.
This study identifies cardiovascular drugs, including atorvastatin, simvastatin, rosuvastatin, and olmesartan, as potential risk factors for DI-AIH, with a median TTO of 668.3 days. Statins, HMG-CoA reductase inhibitors, are commonly used to prevent cardiovascular diseases and lower cholesterol levels. Although rare, statin-induced DI-AIH has been reported in several studies33,34,35,36,37,38. The latency period of statin induced liver damage varies widely, ranging from months to years, with a maximum of more than 10 years, using the time of initial identification of abnormal liver tests as the definition33. Some studies suggest that statins may trigger autoantibody production through pro-apoptotic effects in genetically predisposed individuals, contributing to AIH39. Olmesartan, an antihypertensive agent that blocks angiotensin II receptors, has also been linked to liver injury with AIH-like features. Symptoms typically improve following drug discontinuation and glucocorticoid treatment40,41. In conclusion, while the risk is rare, atorvastatin, simvastatin, rosuvastatin, and olmesartan may induce DI-AIH. Clinicians should assess individual risks when prescribing these medications, especially in patients with a history of DILI or AIH risk factors.
This study identifies several biologic agents potentially associated with DI-AIH, including nivolumab, atezolizumab, pembrolizumab, and ipilimumab. These monoclonal antibodies enhance T-cell function by inhibiting immune checkpoint signals (e.g., PD-1/PD-L1 or CTLA-4/CD80/CD86), thereby exerting anti-tumor effects. However, their immune-related adverse events, including AIH, have raised concern. A case report described a patient with malignant melanoma who developed AIH symptoms 8 months after nivolumab treatment42. Another study reported immune-mediated liver injury in 4 patients with malignant melanoma and 1 with tongue squamous cell carcinoma treated with nivolumab, as well as in 2 patients treated with ipilimumab, all within 41 days43. Additionally, a randomized study of 483 postoperative melanoma patients treated with pembrolizumab found 7 cases of AIH-related adverse events44. While no reports link atezolizumab to DI-AIH, the potential for liver injury with immune checkpoint inhibitors warrants close monitoring. The mechanisms by which these agents induce AIH remain unclear. Given the potential for severe liver damage, baseline immunological testing and liver function assessments are recommended before initiating treatment. Furthermore, pemetrexed, a folate antagonist, may also be associated with DI-AIH. Although some studies suggest that pemetrexed may mitigate experimental AIH liver injury by inhibiting the NF-κB signaling pathway45, current evidence is insufficient to confirm its direct relationship with DI-AIH, warranting further investigation. Notably, antitumor drugs exhibited the shortest median TTO of DI-AIH (77.0 days).
The study identifies a close association between immune-modulating drugs, such as glatiramer acetate and alemtuzumab, and DI-AIH, with a median TTO was 352.1 days. Glatiramer acetate, commonly used for multiple sclerosis due to its favorable safety profile, has been implicated in over 50 reported cases of liver injury in theFAERS database. Among these, 3 cases involved classic AIH requiring prolonged immunosuppressive therapy46,47,48, and 5 cases presented with transient elevations in antinuclear antibodies or smooth muscle antibodies, indicative of immune-mediated liver injury49,50,51,52. The exact mechanism remains unclear, but potential pathways include Th2 cell induction or mitochondrial damage53,54. Routine liver function monitoring is recommended during the initial 6 months of treatment, particularly for elderly patients, those with chronic liver disease, or those with a history of DILI46. Alemtuzumab, an anti-tumor necrosis factor agent widely used in autoimmune disease treatment, has an AIH incidence of 10.7 cases per 10,000 treatments55,56. Two cases of alemtuzumab induced acute liver failure, both immune-mediated AIH, have been reported57,58, alongside a fatal AIH case documented in the EudraVigilance database59. The proposed mechanism involves B-cell proliferation; rapid reconstitution of CD19 + B cells, in the absence of effective T-cell regulation, may increase the risk of secondary autoimmunity60.
Although NSAIDs rarely induce DI-AIH9,14, this study highlights a closer association between meloxicam and naproxen with DI-AIH. One case involved a 64-year-old male who developed elevated immunoglobulin levels and typical AIH features, including periportal inflammation and fibrosis, after 5 weeks of meloxicam use for lower back pain61. Another study found that patients with AIH induced by diclofenac did not experience liver function abnormalities after switching to naproxen62. Due to limited data, further research is needed to clarify naproxen’s role in inducing DI-AIH.
This study also identified the respiratory drug bosentan and the neurological drug levetiracetam as being associated with DI-AIH. Bosentan, an endothelin receptor antagonist used to treat pulmonary arterial hypertension, was linked to liver injury in two female patients, both diagnosed with AIH after 3 and 4 years of treatment, respectively63,64. Levetiracetam (median TTO 352.1 days), an anti-epilepsy drugs, has been associated with AIH in case reports, where patients exhibited positive ANA, anti-smooth muscle antibodies, and elevated IgG levels, with liver biopsies confirming the diagnosis of AIH65. Although cases of DI-AIH induced by bosentan and levetiracetam are rare, they warrant clinical attention.
In addition to clinical drugs, vaccines can also cause liver injury66, with some cases exhibiting characteristics of DI-AIH. A systematic review summarized the clinical features of 39 cases of DI-AIH induced by COVID-19 vaccines67. Another study suggested that liver injury caused by the SARS-CoV-2 vaccine is heterogeneous but primarily immune-mediated68. The mechanism underlying vaccine-induced DI-AIH remains unclear, though it is thought to involve immune system activation, destruction of immune tolerance, and the activation of specific immune cells. Related hypotheses propose that one potential mechanism contributing to vaccine-induced autoimmune disorders is molecular mimicry. This occurs when peptide sequences in vaccines closely resemble self-peptides in the human body69. Such similarities may trigger immune cross-reactions, where the immune response to foreign antigens inadvertently targets similar self-proteins, potentially leading to autoimmune diseases.
This study has several limitations. First, the FAERS database relies on spontaneous, voluntary reporting, which may result in underreporting of adverse events. Second, many reports lack sufficient evidence to establish a causal relationship between adverse events and drugs, and confounding factors such as age, gender, race, country, vaccination and underlying conditions were not controlled for. The inherent uncertainty in establishing causal links is a common limitation in pharmacovigilance and observational studies. Additionally, FAERS reports often contain incomplete information, with key details such as dosage, comorbidities, and TTO frequently missing. Therefore, the findings should be interpreted with caution, and conclusions should be drawn in conjunction with comprehensive, evidence-based assessments.
Conclusion
This study, utilizing a large dataset from the FAERS database, identified drugs associated with DI-AIH and assessed their risk values and induction time differences, providing valuable insights into the epidemiology of DI-AIH. These findings contribute critical data to reduce the incidence of DI-AIH and optimize clinical prescribing practices. The analysis of risk factors and occurrence patterns emphasizes the need for enhanced post-treatment monitoring to ensure the timely detection of liver injury, prompt intervention, and appropriate treatment adjustments. This evidence lays the groundwork for advancing clinical diagnosis, therapeutic strategies, and patient care. Future research should combine real-world data with experimental validation to further improve drug safety and support the development of safer treatment options.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Abbreviations
- DI-AIH:
-
Drug-induced autoimmune hepatitis
- DILI:
-
Drug-induced liver injury
- AIH:
-
Autoimmune hepatitis
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- TNF:
-
Anti-tumor necrosis factor
- FAERS:
-
The FDA’s Adverse Event Reporting System
- MedDRA:
-
Medical Dictionary for Regulatory Activities
- PTs:
-
Preferred Terms
- ROR:
-
Reporting Odds Ratio
- PRR:
-
Proportional Reporting Ratio
- BCPNN:
-
Bayesian Confidence Propagation Neural Network
- MGPS:
-
The Multi-Item Gamma Poisson Shrinker
- TTO:
-
Time-to-onset
References
Tan, C. K., Ho, D., Wang, L. M. & Kumar, R. Drug-induced autoimmune hepatitis: A minireview. World J. Gastroenterol. 28(24), 2654–2666 (2022).
Weiler-Normann, C. & Schramm, C. Drug induced liver injury and its relationship to autoimmune hepatitis. J. Hepatol. 55(4), 747–749 (2011).
Andrade, R. J. et al. Nomenclature, diagnosis and management of drug-induced autoimmune-like hepatitis (DI-ALH): An expert opinion meeting report. J. Hepatol. 79(3), 853–866 (2023).
Mack, C. L. et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md.) 72(2), 671–722 (2020).
European Association for the Study of the Liver. EASL clinical practice guidelines: Autoimmune hepatitis. J. Hepatol. 63(4), 971–1004 (2015).
Gordon, V. et al. Diagnosis, presentation and initial severity of Autoimmune Hepatitis (AIH) in patients attending 28 hospitals in the UK. Liver Int. Off. J. Int. Assoc. Study Liver 38(9), 1686–1695 (2018).
Björnsson, E. et al. Drug-induced autoimmune hepatitis: Clinical characteristics and prognosis. Hepatology (Baltimore, Md) 51(6), 2040–2048 (2010).
Valgeirsson, K. B., Hreinsson, J. P. & Björnsson, E. S. Increased incidence of autoimmune hepatitis is associated with wider use of biological drugs. Liver Int. Off. J. Int. Assoc. Study Liver 39(12), 2341–2349 (2019).
de Boer, Y. S. et al. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 15(1), 103-112.e2 (2017).
Licata, A. et al. Clinical features and outcomes of patients with drug-induced autoimmune hepatitis: A retrospective cohort study. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 46(12), 1116–1120 (2014).
Aithal, G. P. et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Therapeutics 89(6), 806–815 (2011).
Meurer, L. & Cohen, S. M. Drug-induced liver injury from statins. Clin. Liver Dis. 24(1), 107–119 (2020).
Perdices, E. V. et al. Hepatotoxicity associated with statin use: Analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev. espanola de enfermedades digestivas 106(4), 246–254 (2014).
Weber, S., Benesic, A., Rotter, I. & Gerbes, A. L. Early ALT response to corticosteroid treatment distinguishes idiosyncratic drug-induced liver injury from autoimmune hepatitis. Liver Int. Off. J. Int. Assoc. Study Liver 39(10), 1906–1917 (2019).
Shelton, E. et al. New onset idiosyncratic liver enzyme elevations with biological therapy in inflammatory bowel disease. Aliment. Pharmacol. Therapeutics 41(10), 972–979 (2015).
Zhou, S. et al. Drug-induced fall risk in older patients: A pharmacovigilance study of FDA adverse event reporting system database. Front. Pharmacol. 13, 1044744 (2022).
Khouri, C. et al. A meta-epidemiological study found lack of transparency and poor reporting of disproportionality analyses for signal detection in pharmacovigilance databases. J. Clin. Epidemiol. 139, 191–198 (2021).
Bate, A. Bayesian confidence propagation neural network. Drug Saf. 30(7), 623–625 (2007).
Berlin, C. et al. Are all quantitative postmarketing signal detection methods equal? Performance characteristics of logistic regression and Multi-item Gamma Poisson Shrinker. Pharmacoepidemiol. Drug Saf. 21(6), 622–630 (2012).
Komori, A. Recent updates on the management of autoimmune hepatitis. Clin. Mol. Hepatol. 27(1), 58–69 (2021).
Angulo, J. M., Sigal, L. H. & Espinoza, L. R. Coexistent minocycline-induced systemic lupus erythematosus and autoimmune hepatitis. Semin. Arthritis Rheum. 28(3), 187–192 (1998).
Teitelbaum, J. E. et al. Minocycline-related autoimmune hepatitis: Case series and literature review. Arch. Pediatr. Adolesc. Med. 152(11), 1132–1136 (1998).
Rafa, O., Basile, E. J., Frankini, E. & Ahmed, A. Rapid development of autoimmune hepatitis secondary to minocycline. Cureus 14(3), e23038 (2022).
Fakhreddine, A. & Frenette, C. A cautionary report of doxycycline-induced autoimmune hepatitis. Hepatology (Baltimore, Md) 71(4), 1515–1517 (2020).
Pan, J. J. & Promrat, K. Doxycycline-induced autoimmune hepatitis. ACG Case Rep. J. 7(8), e00440 (2020).
Liang, R. & Ramdass, A. Azithromycin-induced liver injury in an asthma exacerbation patient with autoimmune features. Cureus 14(5), e25447 (2022).
Kelly, B. D. et al. Nitrofurantoin-induced hepatotoxicity mediated by CD8+ T cells. Am. J. Gastroenterol. 93(5), 819–821 (1998).
Hoofnagle, J. H. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. (National Institute of Diabetes and Digestive and Kidney Diseases, 2012).
Trikudanathan, G. V., Ahmad, I. & Israel, J. L. Concurrent autoimmune hepatitis and grave’s disease in hepatitis C during pegylated interferon α-2a and ribavirin therapy. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 17(5), 348–352 (2011).
Zhao, X. Y., Rakhda, M. I., Wang, T. I. & Jia, J. D. Immunoglobulin G4-associated de novo autoimmune hepatitis after liver transplantation for chronic hepatitis B- and C-related cirrhosis and hepatocellular carcinoma: A case report with literature review. Transpl. Proc. 45(2), 824–827 (2013).
Lörke, J., Erhardt, A. & Häussinger, D. Induction of autoimmune hepatitis by pegylated interferon alfa-2b in chronic hepatitis C. Clin. Gastroenterol. Hepatol. Off. Clin. Prac. J. Am. Gastroenterol. Assoc. 2(12), xx (2004).
Russo, M. W. et al. Spectrum of statin hepatotoxicity: Experience of the drug-induced liver injury network. Hepatology (Baltimore, Md.) 60(2), 679–686 (2014).
Tse, J., Natla, S., Mekala, R., Crumm, I. & Olken, M. H. Atorvastatin-induced autoimmune hepatitis: A case report. Cureus 15(10), e47807 (2023).
Khan, A. A., Ahmed, S., Mohammed, A. & Elzouki, A. Y. Autoimmune-like drug-induced liver injury caused by atorvastatin and demonstration of the safety profile of pravastatin: A case report and literature review. Cureus 12(3), e7299 (2020).
Patel, M., Fattah, A., Hussaini, H., Maneesha, F. & Ahmed, Z. Drug-induced autoimmune hepatitis: An unusual adverse event of atorvastatin therapy. Cureus 16(3), e55809 (2024).
Alla, V. et al. Autoimmune hepatitis triggered by statins. J. Clin. Gastroenterol. 40(8), 757–761 (2006).
Wolters, L. M. & Van Buuren, H. R. Rosuvastatin-associated hepatitis with autoimmune features. Eur. J. Gastroenterol. Hepatol. 17(5), 589–590 (2005).
Alvarado Cárdenas, M. et al. Estatinas y autoinmunidad [Statins and autoimmunity]. Medicina Clinica 145(9), 399–403 (2015).
Barge, S., Ziol, M. & Nault, J. C. Autoimmune-like chronic hepatitis induced by olmesartan. Hepatology (Baltimore, Md.) 66(6), 2086–2088 (2017).
Riado Minguez, D., Gutierrez Garcia, M. L. & Fernandez Rodriguez, C. Chronic autoimmune hepatitis triggered by olmesartan. Gastroenterologia y Hepatologia 43(10), 629–630 (2020).
Syed, T. et al. Nivolumab-induced delayed autoimmune hepatitis-A diagnostic and therapeutic challenge. Am. J. Therapeutics 27(3), e317–e319 (2020).
Zen, Y. & Yeh, M. M. Hepatotoxicity of immune checkpoint inhibitors: A histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc 31(6), 965–973 (2018).
Long, G. V. et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): Distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 23(11), 1378–1388 (2022).
Liu, Z., Bao, Z., Yu, B., Chen, L. & Yang, G. Pemetrexed ameliorates Con A-induced hepatic injury by restricting M1 macrophage activation. Int. Immunopharmacol. 125(Pt A), 111158 (2023).
Sinagra, E. et al. Does glatiramer acetate provoke hepatitis in multiple sclerosis?. Mult. Scler. Relat. Disord. 3(2), 266–268 (2014).
Neumann, H., Csepregi, A., Sailer, M. & Malfertheiner, P. Glatiramer acetate induced acute exacerbation of autoimmune hepatitis in a patient with multiple sclerosis. J. Neurol. 254(6), 816–817 (2007).
von Kalckreuth, V., Lohse, A. W. & Schramm, C. Unmasking autoimmune hepatitis under immunomodulatory treatment of multiple sclerosis–not only beta interferon. Am. J. Gastroenterol. 103(8), 2147–2148 (2008).
Deltenre, P., Peny, M. O., Dufour, A., Nady, M. E., & Henrion, J. Acute hepatitis induced by glatiramer acetate. BMJ Case Reports. 2009, bcr09.2008.0913 (2009).
Subramaniam, K., Pavli, P., Llewellyn, H. & Chitturi, S. Glatiramer acetate induced hepatotoxicity. Curr. Drug Saf. 7(2), 186–188 (2012).
Fernández Fernández, N. et al. Hepatitis asociada a acetato de glatirámero [Hepatitis induced by glatiramer acetate]. Gastroenterologia y Hepatologia 38(4), 280–281 (2015).
Almeida, J. et al. Liver injury and glatiramer acetate, an uncommon association: Case report and literature review. Therapeutic Adv. Neurol. Disord. 10(11), 367–372 (2017).
Biolato, M. et al. The disease-modifying therapies of relapsing-remitting multiple sclerosis and liver injury: A narrative review. CNS Drugs 35(8), 861–880 (2021).
Makhani, N., Ngan, B. Y., Kamath, B. M. & Yeh, E. A. Glatiramer acetate-induced acute hepatotoxicity in an adolescent with MS. Neurology 81(9), 850–852 (2013).
Hartung, H. P., Mares, J. & Barnett, M. H. Alemtuzumab: Rare serious adverse events of a high-efficacy drug. Mult. Sclerosis (Houndmills, Basingstoke, England). 26(6), 737–740 (2020).
Willis, M. D. et al. Alemtuzumab for multiple sclerosis: Long term follow-up in a multi-centre cohort. Mult. Sclerosis (Houndmills, Basingstoke, England). 22(9), 1215–1223 (2016).
El Sankari, S., Dahlqvist, G., Monino, L. & van Pesch, V. Auto-immune hepatitis in a patient with multiple sclerosis treated with alemtuzumab. Acta Neurol. Belgica 118(2), 331–333 (2018).
Bolte, F. J., Schmidt, H. H. & Schlevogt, B. Immune-mediated hepatitis induced by therapy with Alemtuzumab in a patient with multiple sclerosis. Hepatology (Baltimore, Md.) 73(1), 460–463 (2021).
Holmøy, T., Fevang, B., Olsen, D. B., Spigset, O. & Bø, L. Adverse events with fatal outcome associated with alemtuzumab treatment in multiple sclerosis. BMC Res. Notes 12(1), 497 (2019).
Baker, D. et al. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of Alemtuzumab. JAMA Neurol. 74(8), 961–969 (2017).
Martínez-Odriozola, P. et al. Meloxicam as a cause of drug-induced autoimmune hepatitis. Dig. Dis. Sci. 55(4), 1191–1192 (2010).
Scully, L. J., Clarke, D. & Barr, R. J. Diclofenac induced hepatitis. 3 cases with features of autoimmune chronic active hepatitis. Dig. Dis. Sci. 38(4), 744–751 (1993).
Naito, A. et al. Autoimmune hepatitis in a patient with pulmonary arterial hypertension treated with endothelin receptor antagonists. Intern. Med. (Tokyo, Japan) 53(7), 771–775 (2014).
de Araujo, A. et al. Bosentan inducing autoimmune hepatitis in a patient with idiopathic pulmonary arterial hypertension. J. Gastrointest. Liver Dis. JGLD 27(1), 89–92 (2018).
Hegazy, Y., Axley, P., Lee, G. & Gray, M. Levetiracetam liver injury: A benign antiepileptic agent?. ACG Case Rep. J. 10(3), e01003 (2023).
Lee, S. et al. Global burden of vaccine-associated hepatobiliary and gastrointestinal adverse drug reactions, 1967–2023: A comprehensive analysis of the international pharmacovigilance database. J. Med. Virol. 96(7), e29792 (2024).
Zhou, H. & Ye, Q. Clinical features of COVID-19 vaccine-associated autoimmune hepatitis: A systematic review. Diseases (Basel, Switzerland) 11(2), 80 (2023).
Barreira-Díaz, A. et al. Outcomes and factors associated with relapse of vaccine-induced liver injury after SARS CoV-2 immunization: A nationwide study. Ann. Hepatol. 29(3), 101489 (2024).
Segal, Y. & Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell Mol. Immunol. 15(6), 586–594 (2018).
Björnsson, E. et al. Drug-induced autoimmune hepatitis: Clinical characteristics and prognosis. Hepatology 51(6), 2040–2048 (2010).
Funding
This work was supported by Construction of Traditional Chinese Medicine Inheritance and Innovation Development Demonstration Pilot Projects in Pudong New Area - High-Level Research-Oriented Traditional Chinese Medicine Hospital Construction (YC-2023-0901) and Shanghai University of Traditional Chinese Medicine Science and Technology Development Project (20224Y0162).
Author information
Authors and Affiliations
Contributions
BZ: Writing-original draft, Writing-review & editing, Formal analysis, Investigation, Methodology. SC: Writing-original draft, Formal analysis, Investigation, Methodology, Resources. XL: Writing-original draft, Writing-review & editing, resources, Validation. SH: Writing-original draft, Resources, Validation. ZL: Writing-original draft, Validation. WZ: Conceptualization, Writing-review & editing, Conceptualization, Funding acquisition, Project administration, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
Since the FAERS database is publicly accessible and contains anonymized and de-identified patient records, this study does not require informed consent or ethical approval.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Bk., Chen, Sy., Li, X. et al. Real-world pharmacovigilance study of drug-induced autoimmune hepatitis from the FAERS database. Sci Rep 15, 4783 (2025). https://doi.org/10.1038/s41598-025-89272-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89272-x
Keywords
This article is cited by
-
Safety evaluation of etrasimod for ulcerative colitis based on the FAERS database
Scientific Reports (2025)