Abstract
The Magnesium Depletion Score (MDS) is a practical tool used to assess magnesium deficiency. Studies have indicated that MDS is associated with various urological conditions, such as kidney stones and the prognosis of chronic kidney disease. However, the relationship between MDS and prostate cancer (PCa) remains unclear. Therefore, this study aims to investigate the association between MDS and PCa. This study conducted a cross-sectional analysis of 16,043 participants from the 2005–2018 NHANES database. Subgroup analysis, restricted cubic splines (RCS), and multivariable logistic regression were employed to examine the association between MDS and the prevalence of PCa. A total of 16,043 participants were included in this study, of whom 511 had PCa. After adjusting for all variables using multivariable logistic regression, each 1-unit increase in MDS was associated with a 26% higher prevalence of PCa (OR = 1.26, 95% CI: 1.05, 1.50). Additionally, compared to an MDS of 0, an MDS of 3 or higher was associated with a 3.04-fold increase in PCa prevalence (OR = 3.04, 95% CI: 1.53, 6.01). RCS analysis demonstrated a significant linear positive correlation between MDS and PCa prevalence. Subgroup analysis indicated that the positive association between MDS and PCa was generally consistent across different population groups. This study indicates a significant association between MDS and the risk of PCa, with higher MDS linked to an increased prevalence of PCa. These findings highlight the potential role of MDS in PCa. Further research is needed to determine whether a causal relationship exists between MDS and PCa, which would help assess the appropriateness of potential interventions.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is one of the most common cancers among men in the United States. According to the American Cancer Society, approximately 1.9 million new cancer cases are expected in the U.S. in 2024, with prostate cancer accounting for 29%, making it the most prevalent cancer among men1. As the global population ages and lifestyle patterns change, the incidence and mortality rates of prostate cancer continue to rise annually. PCa has become a major public health concern, posing significant disease and economic burdens on society, families, and individuals2. The development of prostate cancer is a complex, multi-stage process likely influenced by a combination of factors, including genetic predisposition, lifestyle, and environmental exposures3. However, current evidence and the number of well-established risk factors remain limited.
Magnesium ions (Mg²⁺) are essential divalent cations in the human body, playing a critical role in maintaining cellular function and physiological homeostasis4. Magnesium is involved in various physiological processes, including nerve conduction, muscle contraction, cardiovascular function, energy metabolism, and the synthesis of proteins and nucleic acids5. Deficiency or metabolic dysregulation of magnesium has been linked to the development of numerous diseases6. Recently, a research team from the University of Basel, Switzerland, reported in Cell that magnesium influences tumor immunity by regulating cytotoxic T lymphocytes (CD8 + T cells), suggesting that magnesium levels are closely related to tumorigenesis7. Furthermore, magnesium plays a critical role in maintaining genomic stability and regulating apoptosis, with its deficiency potentially increasing genetic instability and promoting tumorigenesis8. For example, the study by Dai and colleagues demonstrated that total magnesium intake is significantly associated with a reduced risk of colorectal adenoma9. Despite this, the relationship between magnesium levels and PCa incidence remains complex and not fully understood. Moreover, most existing studies exploring this relationship have used serum magnesium as the primary indicator of magnesium status in the body10.
The Magnesium Tolerance Test (MTT) is widely regarded as the most reliable method for assessing overall magnesium levels and tolerance11. However, its clinical utility is limited by its invasive and cumbersome nature, requiring intravenous magnesium administration followed by 24-hour urine collection to measure magnesium excretion. Some studies have shown that the magnesium depletion score (MDS) has been used in depression12, COPD13, Parkinson’s disease14, periodontitis15 and metabolic dysfunction associated steatotic liver disease (MASLD)16. MDS is a composite score based on four risk factors—diuretic use, proton pump inhibitor (PPI) use, alcohol consumption, and declining kidney function—providing a more accurate assessment of systemic magnesium levels compared to serum and urine magnesium concentrations17. A higher MDS indicates a more severe magnesium deficiency. MDS has been associated with certain urological conditions, including the occurrence of kidney stones18 and long-term mortality in chronic kidney disease19. However, studies evaluating the relationship between MDS and the risk of PCa remain limited. Given the high prevalence of prostate cancer and its substantial impact on men’s health, investigating whether magnesium depletion could increase the risk of prostate cancer is of significant importance. This study aims to explore the association between the Magnesium Depletion Score (MDS) and prostate cancer, providing new insights into the relationship between magnesium status and tumorigenesis.
Therefore, to determine the association between MDS and the risk of PCa in the U.S. population, we utilized data from the 2005–2018 National Health and Nutrition Examination Survey (NHANES) cycles for the first time. These findings may offer new strategies for the prevention and treatment of prostate cancer in clinical practice.
Methods
Study participants
The National Health and Nutrition Examination Survey (NHANES) is a continuous, stratified, multi-stage sampling program designed to assess the health and nutritional status of adults and children in the United States. It provides comprehensive data on various aspects of health and nutrition. Each year, NHANES conducts a nationally representative sampling of approximately 5,000 individuals, consisting of interviews and physical examinations20. The interview component collects demographic, socioeconomic, dietary, and health-related information, while the physical examination includes physiological measurements and laboratory tests. Written informed consent is obtained from each participant. As such, NHANES offers high-quality, nationally representative data suitable for determining disease prevalence and risk factors. The NHANES program is reviewed and approved by the Research Ethics Review Board of the National Center for Health Statistics (NCHS). This study was conducted in accordance with the Declaration of Helsinki (2013 revision).
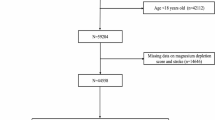
We used NHANES data from 2005 to 2018 (7 cycles) for male participants, which included relevant information on prostate cancer and MDS. Exclusion criteria were: (I) female participants (n = 20,499) and those aged < 20 years (n = 30,441); (II) incomplete MDS data (n = 2,106); and (III) missing data on prostate cancer (n = 1,101). Ultimately, 16,043 individuals were eligible for analysis (Fig. 1).
Outcome assessment
NHANES utilizes self-administered questionnaires to collect information regarding the presence of prostate cancer. A survey question, “Have you ever been told by a doctor or health professional that you have prostate cancer?” is used to determine the respondents’ prostate health status. Participants who answered “yes” were classified as having prostate cancer, while those who declined to answer or responded “don’t know” were excluded from the analysis21,22,23.
Exposure variable
The Magnesium Depletion Score (MDS) predicts magnesium deficiency by assessing the kidneys’ ability to reabsorb magnesium, calculated by summing scores from four risk factors24: (1) Current Diuretic Use: 1 point for “yes,” 0 points for “no.“. (2) Current Proton Pump Inhibitor (PPI) Use: 1 point for “yes,” 0 points for “no.“. (3) Kidney Function: Estimated glomerular filtration rate (eGFR) ≥ 90 mL/min/1.73 m² receives 0 points; eGFR < 90 but ≥ 60 mL/min/1.73 m² receives 1 point; eGFR < 60 mL/min/1.73 m² receives 2 points. (4) Heavy Alcohol Consumption: Defined as more than two drinks per day for men and more than one drink per day for women, receiving 1 point. All other drinking habits (never, rarely, lightly, moderately) receive 0 points. In-home face-to-face interviews were used to collect self-reported intake of diuretics and proton pump inhibitors throughout the previous 30 days. The eGFR was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations. 25,26. Each component contributes to the overall score, reflecting the severity of magnesium deficiency. Due to the small sample sizes for total scores of 3, 4, and 5, participants were categorized into four groups: 0, 1, 2, and ≥ 3. This classification is consistent with previous research27.
Covariables
The covariates in this study included age, race, marital status, educational attainment, poverty income ratio (PIR), obesity, smoking, alcohol consumption, physical activity, hypertension, diabetes, and self-reported hypercholesterolemia. For detailed information regarding these covariates, please refer to Table S1.
Statistical analysis
To ensure that the data were representative of the national population, all analyses incorporated sampling weights. The weight variable used in our study was “WTMEC2YR,” and new weights for the period from 2005 to 2018 were calculated as 1/7 × “WTMEC2YR.” Continuous variables are presented as means ± standard deviation (SD), while categorical variables are expressed as frequencies (percentages). The weighted t-test was used to compare continuous variables, and the weighted chi-square test was employed for categorical variables. Weighted logistic regression was utilized to explore the relationship between MDS and PCa. Three logistic regression models were established: Model 1 was unadjusted for potential confounding factors; Model 2 adjusted for covariates including age, educational attainment, marital status, PIR, and race; and Model 3 further adjusted for obesity, smoking, alcohol consumption, physical activity, hypertension, diabetes, and hypercholesterolemia in addition to the covariates from Model 2. Furthermore, including alcohol intake could result in overadjustment because it is a component of the MDS. We performed a sensitivity analysis that removed alcohol intake from the covariates to address this. Moreover, in Model 3, MDS was treated as a continuous variable, and RCS plots were used to illustrate the linear or nonlinear association between MDS and PCa. Subsequently, stratified subgroup analyses and interaction analyses based on Model 3 were conducted to explore potential variations in associations between subgroups. Data processing was performed using R statistical software (version 4.3.1). A two-sided p-value of less than 0.05 was considered statistically significant.
Result
Baseline characteristics
Among the 16,043 individuals in our study, representing approximately 90.37 million US adults, 511 (2%) were diagnosed with PCa, and the mean MDS was 1.58 (SD = 0.9). Table 1 presents the baseline characteristics of respondents categorized by prostate cancer status. Significant associations (P < 0.05) were observed between PCa status and age, race, marital status, PIR, smoking, alcohol consumption, hypertension, diabetes, hypercholesterolemia, and MDS. Compared to non-PCa patients, those with PCa were more likely to be older, non-Hispanic white, married, and non-poor, and exhibited higher rates of smoking, alcohol consumption, hypertension, hypercholesterolemia, and elevated MDS levels.
Relationship between MDS and PCa
As shown in Table 2, three different models were employed to assess the association between MDS and prostate cancer (PCa). In the model adjusted for all covariates, individuals in the high MDS group (MDS ≥ 3) had an odds ratio (OR) of 3.04 (95% CI: 1.53, 6.01) compared to the MDS = 0 group. Furthermore, as alcohol consumption is a component of the MDS, its inclusion may lead to overadjustment. To address this, we conducted a sensitivity analysis excluding alcohol consumption from the covariates, and the results remained robust (Model 4). Restricted cubic spline (RCS) analysis (Fig. 2) further indicated a significant positive correlation between MDS and the prevalence of PCa (P for overall = 0.019; P for nonlinear = 0.375). The results of the subgroup analysis are illustrated in Fig. 3. A positive association between MDS and PCa was observed in most subgroups (P < 0.05), with no significant interactions detected.
Discussion
The current study utilized a large, nationally representative cross-sectional design to investigate the relationship between MDS and PCa. Our findings indicate a significant positive correlation between MDS and PCa. This association remained significant in models adjusted for multiple covariates, particularly evident in individuals with an MDS of 3 or higher.
Previous studies examining the association between magnesium and prostate cancer (PCa) have yielded contradictory results. Some research indicates that magnesium levels in blood and diet are not associated with a reduced risk of prostate cancer28. This analysis included only 708 Black, and 651 White participants recruited from urology clinics in Nashville, Tennessee, and Durham, North Carolina. Conversely, other studies suggest that magnesium intake can decrease the risk of various cancers29,30, including prostate cancer, which aligns with our findings. The absorption and excretion of dietary magnesium may be influenced by several factors. Alcohol consumption can reduce food intake, thereby decreasing magnesium intake, while also potentially increasing magnesium excretion, leading to lower magnesium levels in the body31. Long-term use of proton pump inhibitors (PPIs) has been shown to decrease TRPM6 activity, resulting in reduced magnesium absorption32. Diuretics, particularly loop diuretics (e.g., furosemide) and thiazide diuretics (e.g., hydrochlorothiazide), increase urine output and subsequently enhance magnesium excretion, contributing to magnesium deficiency33. The kidneys maintain magnesium balance in the body by regulating magnesium filtration, reabsorption, and excretion34. As a reflection of magnesium bioavailability, the Magnesium Deficiency Score (MDS) integrates all of these factors, serving as an indicator for assessing individual magnesium deficiency status. This makes MDS a valuable tool for predicting disease risk associated with magnesium deficiency and plays a critical role in the early identification of magnesium deficiency risks and guiding clinical interventions for prostate cancer.
Currently, compelling explanations for the potential mechanisms linking magnesium to prostate cancer remain insufficient. Mg²⁺ primarily inhibits tumor growth through various pathways, including the suppression of tumor cell proliferation, promotion of apoptosis, inhibition of migration and invasion, and modulation of tumor inflammation35. Magnesium ions regulate intracellular calcium levels via TRPM7, indirectly influencing apoptosis; a deficiency in magnesium may lead to excessive accumulation of intracellular calcium, triggering either apoptosis or necrosis10. Additionally, magnesium exhibits antioxidant properties, allowing it to neutralize excess free radicals in the body and reduce oxidative stress-induced cellular damage36. Oxidative stress is recognized as a critical mechanism in the onset and progression of cancer37; thus, magnesium may play a role in prostate cancer prevention by alleviating oxidative stress. Some studies have also indicated that PPM1D (protein phosphatase, magnesium-dependent 1D), an enzyme whose activity relies on magnesium ions, plays a crucial role in regulating the cell cycle, cell proliferation, DNA damage response, and apoptosis38.
As the population ages exponentially and life expectancy continues to rise, particularly in developing countries, the burden of prostate cancer among the elderly is expected to increase dramatically in the future. Age is a significant risk factor for prostate cancer, with its incidence significantly rising as individuals grow older. In subgroup analyses, the prevalence of prostate cancer in individuals aged 41 to 60 is 1.52 times higher than in those without prostate cancer (OR = 1.52), and the odds ratio for those over 60 is 1.22. With advancing age, most organs and tissues undergo atrophy, whereas the prostate typically enlarges, leading to increased urinary frequency, bladder overactivity, incontinence, and, in severe cases, kidney failure. Magnesium deficiency is common among the elderly, and the Western diet often lacks sufficient magnesium due to excessive consumption of high-fat meats and processed foods, alongside a low intake of whole grains and leafy vegetables, resulting in deficiencies in trace elements, vitamins, and minerals. Some studies suggest that magnesium may help maintain telomere length by influencing telomerase activity, potentially delaying cellular aging and apoptosis39. This finding could have positive implications for human health. Maintaining optimal magnesium levels through dietary intake and lifestyle modifications may represent a safe and cost-effective strategy to help mitigate aging. Future studies should be meticulously designed to further validate this hypothesis.
Following the demonstration that magnesium deficiency can induce tumors, recent advancements in materials science have explored the application of magnesium alloys in cancer treatment, primarily based on the principle that these alloys generate locally elevated concentrations of magnesium ions (Mg²⁺) during their degradation within the body, thereby inhibiting tumor growth and proliferation. For instance, researchers have developed magnesium anastomosis staples for use after colorectal tumor surgeries, which not only suppress tumor cell proliferation but also effectively promote wound healing40. Additionally, pH-sensitive polymer-coated magnesium nanoflowers have been developed to release Mg²⁺ in the tumor microenvironment, directly targeting breast cancer cells41. This approach enhances therapeutic efficacy while minimizing systemic side effects. The potential of magnesium alloys for treating prostate cancer is also being actively investigated35. For example, magnesium alloys have been utilized as link materials for radioactive iodine-125 particles implanted in prostate cancer patients, which may improve prognosis. Overall, due to their dual functions of antitumor activity and tissue regeneration, magnesium alloys exhibit significant potential in cancer treatment.
According to a study conducted in the United States, nearly 50% of Americans do not meet the recommended daily intake of magnesium42. This deficiency is primarily attributed to advancements in modern agriculture, the use of chemical fertilizers, and the increasing proportion of processed foods in the diet. A high-magnesium diet, achieved through the consumption of magnesium-rich foods or appropriate supplements, exceeds the normal daily intake levels for adults. Numerous epidemiological studies, including prospective studies, retrospective case-control analyses, and meta-analyses, have identified a significant impact of high-magnesium diets on cancer incidence and mortality. Researchers such as Yang et al.43 and Chiu et al.44 have conducted a series of retrospective case-control studies that reveal an association between the magnesium ion content in drinking water and the mortality risk of various malignancies, including prostate, breast, esophageal, and ovarian cancers. Long-term consumption of drinking water with high magnesium ion content may reduce mortality rates associated with these cancers. However, some studies have indicated that magnesium intake does not significantly affect the incidence of bladder cancer45. These findings suggest that the impact of high-magnesium diets on cancer incidence and mortality remains controversial and warrants further investigation.
This study presents several significant advantages. First, it is the first to investigate the association between MDS and PCa. MDS has demonstrated its unique predictive accuracy in several studies such as depression12, COPD13, Parkinson’s disease14, periodontitis15 and metabolic dysfunction associated steatotic liver disease (MASLD)16. We investigated the association between MDS and PCa, and our results provide preliminary evidence of a potential relationship between MDS and PCa. While this study does not establish that changes in MDS directly reduce the risk of PCa, it offers initial insights into the possible role of MDS as a risk factor for prostate cancer. Second, the research is based on the large national NHANES database from 2005 to 2018, characterized by substantial sample size, good representation, and standardized data collection, ensuring the reliability of the findings. In terms of statistical methods, we thoroughly controlled for potential confounding factors and validated the robustness of the results through subgroup analyses. We offer new perspectives and strategies for the prevention and management of PCa, with these discoveries directly translatable to clinical and public health practices, guiding the development of individualized magnesium deficiency interventions and community prevention strategies. Therefore, future research could include prospective cohort studies or animal experiments to investigate whether actual magnesium intake can reduce the risk of prostate cancer.
This study also has several limitations. The primary limitation is inherent to the study design; as a cross-sectional study, it cannot establish causal relationships between variables or assess temporal changes. Secondly, there are limitations related to measurement tools; the questionnaire data may be subject to recall bias, particularly concerning unclear characteristics of prostate cancer, as diagnosis largely relies on self-reporting. Additionally, although we adjusted for multiple confounding factors in our analyses, unmeasured confounders may still influence the study results. The generalizability of the findings is also somewhat limited, as the study population primarily consists of Americans, which may not fully represent other racial or regional groups. Additionally, the absence of biomarker data means this study could not fully elucidate the underlying mechanisms. These limitations highlight the need for future research to conduct prospective cohort studies, incorporate more biomarker assessments, broaden the geographical and racial diversity of the study population, and employ more precise evaluation methods to further validate and refine the findings of this research. Furthermore, this study primarily focused on alcohol consumption as the exposure variable associated with MDS, based on the available NHANES data. However, NHANES also includes a wide range of dietary information, such as detailed magnesium intake and other dietary patterns, which were not incorporated into this analysis. This limitation may hinder a comprehensive assessment of the complex relationship between diet and magnesium deficiency. Future research should take advantage of the dietary data provided by NHANES to more systematically explore the potential interactions between dietary factors and prostate cancer risk, as well as their underlying mechanisms. Finally, despite providing preliminary evidence for an association between magnesium depletion score (MDS) and prostate cancer, this study has several limitations. First, the reliance on self-reported data for prostate cancer diagnosis may introduce information bias. Self-reported data may not capture all clinically diagnosed prostate cancer cases, particularly those that are mild or undiagnosed. Additionally, the study lacks detailed data on the clinical significance, disease progression, and mortality rates associated with prostate cancer, limiting our ability to further explore the impact of MDS on prostate cancer outcomes. Furthermore, prostate cancer screening and diagnosis methods, particularly PSA screening, are highly debated and vary significantly across regions and populations in the United States. These differences may result in substantial variability in prostate cancer reporting, which could influence the findings. As such, the conclusions of this study should be interpreted cautiously, particularly in the absence of clinical data linking MDS with prostate cancer mortality or clinically significant prostate cancer cases.
Conclusion
In summary, this study demonstrates a significant association between MDS and prostate cancer risk, with a higher MDS linked to increased prostate cancer prevalence. While MDS holds promise as a tool to assess prostate health, its ability to predict prostate cancer incidence requires further validation. Future studies should focus on exploring the applicability and clinical value of MDS in different populations to determine its potential as an early screening tool. These findings not only contribute to the understanding of the relationship between magnesium deficiency and prostate health but also provide important references for personalized prevention strategies and public health interventions.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Abbreviations
- PCa:
-
Prostate cancer
- MDS:
-
Magnesium depletion score
- RCS:
-
Restricted cubic splines
- NHANES:
-
National Health and Nutrition Examination Survey
References
Dizon, D. S. & Kamal, A. H. Cancer statistics 2024: all hands on deck. CA Cancer J. Clin. 74, 8–9 (2024).
Luo, L. S. et al. Estimating disparities of prostate cancer burden and its attributable risk factors for males across the BRICS-plus, 1990–2019: a comparable study of key nations with emerging economies. Prostate 84, 570–583 (2024).
Sekhoacha, M. et al. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules 27, 5730 (2022).
Saris, N. E., Mervaala, E., Karppanen, H., Khawaja, J. A. & Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta; Int. J. Clin. Chem. 294, 1–26 (2000).
Gröber, U., Schmidt, J. & Kisters, K. Magnesium in prevention and therapy. Nutrients 7, 8199–8226 (2015).
Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 24, 47–66 (2003).
Lötscher, J. et al. Magnesium sensing via LFA-1 regulates CD8 + T cell effector function. Cell 185, 585–602e29 (2022).
Wolf, F. I. & Cittadini, A. Magnesium in cell proliferation and differentiation. Front. Biosci. 4, d607 (1999).
Dai, Q. et al. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am. J. Clin. Nutr. 86, 743–751 (2007).
Dai, Q. et al. Blood magnesium, and the interaction with calcium, on the risk of high-grade prostate cancer. PLoS One. 6, e18237 (2011).
Arnaud, M. J. Update on the assessment of magnesium status. Br. J. Nutr. 99 (Suppl 3), S24–36 (2008).
Zhao, W. & Jin, H. Magnesium depletion score and depression: a positive correlation among US adults. Front. Public. Health. 12, 1486434 (2024).
Zhao, Y. et al. Exploring the association between magnesium deficiency and chronic obstructive pulmonary diseases in NHANES 2005–2018. Sci. Rep. 14, 25981 (2024).
Cen, Y. et al. Correlations between Dietary Magnesium Consumption and Magnesium Depletion score in relation to Parkinson’s Disease: a Population-based study. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-024-04428-6 (2024).
Wu, Q., Zhang, S. & Cao, R. Association between magnesium depletion score and periodontitis in US adults: results from NHANES 2009–2014. BMC Oral Health. 24, 1274 (2024).
Li, F. et al. Association between magnesium depletion score and the risk of metabolic dysfunction associated steatotic liver disease: a cross sectional study. Sci. Rep. 14, 24627 (2024).
Song, J. et al. Higher magnesium depletion score increases the risk of all-cause and Cardiovascular Mortality in Hypertension participants. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-024-04254-w (2024).
Xu, Y. et al. The magnesium depletion score is associated with increased likelihood of kidney stone disease among female adults. J. Trace Elem. Med. Biol. 84, 127432 (2024).
Yin, S., Zhou, Z., Lin, T. & Wang, X. Magnesium depletion score is Associated with Long-Term Mortality in chronic kidney diseases: a prospective Population-based Cohort Study. J. Nephrol. 36, 755–765 (2023).
Johnson, C. L., Dohrmann, S. M., Burt, V. L. & Mohadjer, L. K. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat. 2, 1–33 (2014).
Li, H. et al. Relationship between oxidative balance score and prostate cancer: a cross-sectional study of NHANES, 1999–2010. BMJ Open. 14, e084700 (2024).
Yan, W., Xiang, P., Liu, D., Zheng, Y. & Ping, H. Association between the serum uric acid levels and prostate cancer: evidence from National Health and Nutrition Examination Survey (NHANES) 1999–2010. Translational Cancer Res. 13, 2308 (2024).
Wang, L., Li, X., Liu, M., Zhou, H. & Shao, J. Association between monocyte-to-lymphocyte ratio and prostate cancer in the U.S. population: a population-based study. Front. Cell. Dev. Biology. 12, 1372731 (2024).
Fan, L. et al. Magnesium depletion score (MDS) predicts risk of systemic inflammation and Cardiovascular Mortality among US adults. J. Nutr. 151, 2226–2235 (2021).
Inker, L. A. et al. New Creatinine- and cystatin C-Based equations to Estimate GFR without Race. N Engl. J. Med. 385, 1737–1749 (2021).
Kuo, P. F., Huang, Y. T., Chuang, M. H. & Jiang, M. Y. Association of low-level heavy metal exposure with risk of chronic kidney disease and long-term mortality. PLOS One. 19, e0315688 (2024).
Tan, M. Y., Mo, C. Y. & Zhao, Q. The Association between Magnesium Depletion score and hypertension in US adults: evidence from the National Health and Nutrition Examination Survey (2007–2018). Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-023-04034-y (2023).
Fowke, J. H. et al. Blood and dietary magnesium levels are not linked with lower prostate cancer risk in black or white men. Cancer Lett. 449, 99–105 (2019).
Castiglioni, S. & Maier, J. A. M. Magnesium and cancer: a dangerous liason. Magnesium Res. 24, 92–100 (2011).
Zhong, G. C. et al. Magnesium intake and primary liver cancer incidence and mortality in the prostate, lung, colorectal and ovarian cancer screening trial. Int. J. Cancer. 147, 1577–1586 (2020).
Rivlin RS. Magnesium deficiency and alcohol intake: mechanisms, clinical significance and possible relation to cancerdevelopment (a review). J. Am. Coll. Nutr. 13(5), 416–23. (1994).
William, J. H. & Danziger, J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J. Nephrol. 5, 152 (2016).
Gröber, U. Magnesium and drugs. Int. J. Mol. Sci. 20, 2094 (2019).
Blaine, J., Chonchol, M. & Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephro. 10, 1257 (2015).
Hsu, Y. et al. Magnesium alloys in tumor treatment: current research status, challenges and future prospects. J. Magnesium Alloys. 11, 3399–3426 (2023).
Barbagallo, M. & Dominguez, L. J. Chapter 16 - magnesium, oxidative stress, and aging muscle. in Aging (ed Preedy, V. R.) 157–166 (Academic, San Diego, doi:https://doi.org/10.1016/B978-0-12-405933-7.00016-0. (2014).
Khandrika, L., Kumar, B., Koul, S., Maroni, P. & Koul, H. K. Role of oxidative stress in prostate cancer. Cancer Lett. 282, 125–136 (2009).
Nahta, R. & Castellino, R. C. Phosphatase magnesium-dependent 1 δ (PPM1D), serine/threonine protein phosphatase and novel pharmacological target in cancer. Biochem. Pharmacol. 184, 114362 (2021).
Barani, M., Sabir, F., Rahdar, A., Arshad, R. & Kyzas, G. Z. Nanotreatment and nanodiagnosis of prostate cancer: recent updates. Nanomaterials 10, 1696 (2020).
Zhang, Y. et al. A biodegradable magnesium surgical staple for colonic anastomosis: in vitro and in vivo evaluation. Bioact Mater. 22, 225–238 (2022).
Patel, H. et al. In vitro evaluation of magnetic fluid hyperthermia therapy on breast cancer cells using monodispersed Mn0.5Zn0.5Fe2O4 nanoflowers. J. Magn. Magn. Mater. 587, 171275 (2023).
What we eat in. America, NHANES 2005–2006: usual nutrient intakes from food and water compared to 1997 Dietary Reference Intakes for vitamin D, calcium, phosphorus, and magnesium.
Yang, C. Y. et al. AND. Journal of Toxicology and Environmental Health, Part A 60, 17–26 (2000).
Chiu, H. F., Chang, C. C. & Yang, C. Y. Magnesium and calcium in drinking water and risk of death from ovarian cancer. Magnesium Res. 17, 28–34 (2004).
Brinkman, M. T. et al. Dietary intake of micronutrients and the risk of developing bladder cancer: results from the Belgian case-control study on bladder cancer risk. Cancer Causes Control: CCC. 22, 469–478 (2011).
Acknowledgements
We sincerely appreciate the NHANES database for all the data.
Funding
No specific funding for this research was provided by the government, business, or nonprofit sectors.
Author information
Authors and Affiliations
Contributions
H.G. contributed to the original draft, Methodology, and Formal analysis. X.L. contributed to Validation, Formal analysis, Resources, and Data curation. S.H. was involved in Writing – review & editing, Supervision, Project administration, and Investigation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The NCHS Ethics Review Board approved this study’s human subjects components, which followed the Declaration of Helsinki. For every subject, written informed permission was acquired.
Consent for publication
All participants gave informed consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gong, H., Lin, X. & Huang, S. Association between magnesium depletion score and prostate cancer. Sci Rep 15, 4801 (2025). https://doi.org/10.1038/s41598-025-89506-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89506-y
Keywords
This article is cited by
-
The Relationship Between Magnesium Depletion Score and Kidney Stone Risk in Gout Patients: a Mediation Analysis
Biological Trace Element Research (2025)