Abstract
The synergistic effect of nanosilver fluoride (NSF) with L-arginine on early carious lesions was evaluated. NSF was synthesized from chitosan, acetic acid, silver nitrate, sodium borohydride, and sodium fluoride. NSF + Arg was synthesized by adding L-arginine. After demineralization the enamel slabs from extracted molar, remineralization agents were applied by randomly dividing them into five groups (n = 15): sodium fluoride varnish (NaF), silver diamine fluoride (SDF), NSF, NSF + Arg, and control. The surface microhardness (SMH), remineralization effects using microcomputed tomography and color changes using a spectrophotometer were measured before and after pH cycling. SMH was analyzed by Kruskal–Wallis test with Dunn’s test. Remineralization effects, and color changes were analyzed using the one-way analysis of variance with Duncan’s test; p-value < 0.05 was considered significant. SMH recovered to similar levels in all groups (p > 0.05), except in the control group after pH cycling. The NSF + Arg and SDF groups showed a higher remineralization than the NaF and NSF groups (p < 0.05). SDF caused the largest discoloration (p < 0.05). The other groups showed no difference in discoloration. NSF + Arg could be an alternative to SDF given its ability to remineralize early caries lesions without discoloration.
Similar content being viewed by others
Introduction
Early caries lesions, or incipient caries lesions, are characterized by the demineralization of the enamel without cavitation1. These lesions appear as white spots on the enamel surface and indicate subsurface mineral loss. Because early lesions do not exhibit surface breakage in the enamel, they can be reversibly restored by applying appropriate remineralization therapies to restore the mineral content of the enamel and halt the progression of carious lesions2.
There are various strategies and materials to remineralize early caries lesion materials including, such as the use of nano-hydroxyapatite, peptides, and natural products. Nano-hydroxyapatite closely resembles the mineral composition of natural tooth enamel, acts as a calcium and phosphate reservoir to promote remineralization, and exhibits antimicrobial properties by disrupting bacterial adhesion and reducing biofilm formation3. Some bioactive peptides, such as antimicrobial peptides, offer dual benefits by reducing bacterial activity while supporting remineralization. Specific examples include P11-44 peptide and Histatin-53. Natural products, such as flavonoids5 derived from propolis or the milk-derived compound casein phosphopeptide-amorphous calcium phosphate6, are also among the various strategies that can be used to remineralize early caries lesions.
Over the past decades, fluoride has been the most widely used to treat early carious lesions7. Fluoride promotes remineralization by enhancing the deposition of calcium and phosphate ions into the demineralized enamel. It forms a protective layer of fluorapatite, which is more resistant to acid attacks than hydroxyapatite8,9. In clinical practice, fluorine is used as sodium fluoride (NaF), stannous fluoride (SnF2), and acidulated phosphate fluoride. These are available in various forms such as toothpastes, mouth rinses, gels, and varnishes. Some professionally applied fluoride agents are 1.23% acidulated phosphate fluoride gel, 5% sodium fluoride varnish, and 5% silver diamine fluoride (SDF)10.
SDF halts the caries process and simultaneously prevents new caries formation. This is due to the antibacterial effect of silver and the remineralization effect of fluoride contained in SDF11. However, SDF has a critical drawback because it causes black staining following the precipitation of silver phosphate on carious lesions. This is caused by the oxidative properties of ionic silver present in the formulation12. The addition of potassium iodide SDF reduces staining by converting silver oxide to silver iodide, a less visible compound13. However, the effect was also limited because silver iodide darkens when exposed to light because of its photosensitivity14.
To address these issues, a nanosilver fluoride (NSF) formulation containing chitosan, fluoride, and silver nanoparticles (AgNPs) was developed15. Chitosan is a biocompatible carrier with antimicrobial properties, fluoride aids in remineralization, and AgNPs exert antibacterial effects16. Given their antimicrobial properties, AgNPs have been added to some products such as glass ionomer cement, resin-modified glass ionomer cement17, and dentin bonding agents18. NSF has demonstrated remineralization effects on early carious lesions15,19; however, other studies have reported its efficacy to be similar to or even lower than that of SDF or fluoride varnish20,21,22,23, making its effectiveness controversial. Therefore, research on methods to enhance the efficacy of NSF is necessary.
One approach to enhance the efficacy of NSF is to add arginine (Arg). Arginine ensures uniform nanoparticles, promotes particle size reduction, and acts as a stabilizer and a reducing agent24,25. In addition to its potential role in nanoparticle synthesis, arginine has demonstrated caries-preventive potential in several in vitro and clinical studies, whether used alone or in combination with fluorides. The caries-preventive effect of fluoride has been shown to be superior when used synergistically with arginine compared to fluoride used alone26,27,28,29,30.
The ecological effect of arginine on oral microbiota has been shown to be effective against the initiation and progression of dental caries31. In oral biofilms, arginine is metabolized by arginolytic bacteria (Streptococcus sanguinis and Streptococcus gordonii) through the arginine deiminase system. This results in the production of ammonia, which neutralizes glycolytic acids and inhibits the growth of cariogenic microflora, ultimately preventing tooth demineralization32. A recent study by Bijle et al.33 found that arginine contributes to enhancing enamel remineralization on early enamel carious lesions, as it encourages fluoride uptake into the demineralized enamel lesion.
The synergistic effect of existing formulation of NSF and arginine (NSF + Arg) has not been investigated yet, and This study aimed to synthesize an NSF solution added with L-arginine (NSF + Arg) and evaluate the remineralization effect of the synthesized NSF + Arg on demineralized enamel lesions.
Results
Characterization of NSF and NSF + Arg
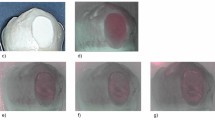
Transmission electron microscopy (TEM) was employed to characterize NSF and NSF + Arg formulations. Figure 1 reveals the successful formation of AgNPs in both NSF and NSF + Arg groups. The nearly spherical-shaped nanoparticles with a diameter of 3–18 nm in both groups are shown. The size distribution histogram of the related nanoparticles is shown in Fig. 1d, h for NSF and NSF + Arg, respectively. In NSF, the mean particle size of the AgNPs was 9.65 nm, with a variance of 14.03, whereas in NSF + Arg, the mean particle size was 7.20 nm, with a variance of 7.80. When NaBH4 is added to an AgNO3 solution, the color changes from colorless to light red, forming an NSF solution. In the NSF + Arg solution, a more intense red color is observed (Fig. 1i). This indicates that Ag+ ions are reduced to Ag0, leading to the formation of silver nanoparticles.
Transmission electron microscopy images and histogram distributions of AgNPs in NSF and NSF + Arg solutions. (a–c) NSF solution, scaled at 50, 20, and 10 nm, respectively. (d) Particle size distribution of AgNPs in the NSF solution, (e–g) NSF + Arg solution, scaled at 50, 20, and 10 nm, respectively. (h) Particle size distribution of AgNPs in the NSF + Arg solution. AgNPs, silver nanoparticles; NSF, nanosilver fluoride, (i) Visual appearances of synthesized aqueous solution of NSF (left) and NSF + Arg (right).
Scanning electron microscopy
Figure 2 shows a representative SEM image of the enamel surface. Rough and porous enamel surfaces were observed in the demineralized enamel and control groups. In the NaF varnish and SDF groups, agglomerated precipitates that fill the irregularities of the enamel and reduce the porosities are observed on the enamel surface. NSF and NSF + Arg images showing prisms and interprism gaps were covered with mineral depositions. The enamel surface is relatively smooth compared with the control group, particularly in NSF + Arg, NSF, and NaF varnish groups.
SEM images of the enamel surface morphology after pH cycling. Loss of surface integrity and high surface porosities are shown in (a) demineralized enamel surface and (b) control group (deionized water). The remineralized samples reveal less microporosity and a relatively smooth surface (c), SDF (d), NaF varnish (e), NSF (f), NSF + Arg. AgNPs, silver nanoparticles; NSF, nanosilver fluoride, SEM, scanning electron microscope.
Surface microhardness
After demineralization, surface microhardness (SMH) decreased in all groups (Table 1). After pH cycling, a significant increase in microhardness values was found in all experimental groups; however, no significant difference was found among the experimental groups, except for the control group (p > 0.05).
Mineral density analysis with micro-CT
The mean and standard deviation of the mineral density (MD) of the enamel is presented in Table 2, and representative images are shown in Fig. 3. The MD of all groups at T1 was significantly lower than that at T0. At T2, the MD of all groups, except the control group, was significantly higher than that at T1. Mineral gain (MG) of the NSF + Arg group was higher than those of the NaF and control groups (p < 0.05); however, no significant difference was found between the SDF and NSF groups (p > 0.05). The NSF + Arg group showed a higher percent remineralization than the NaF, NSF, and control groups (p < 0.05), and a similar level to the SDF group (p > 0.05).
Color change
Table 3 presents the mean values with standard deviation for ΔL, Δa, Δb, and ΔE of all groups, and representative images are shown in Fig. 4. The SDF group showed the greatest variation of the ΔE value and only significant color difference among all groups (p < 0.05).
Discussion
In the treatment of early carious lesions, remineralization is crucial because it helps restore the mineral content of the enamel, reversing the effects of demineralization and preventing caries progression. The most widely used agents for remineralization are fluoride-based products, among which SDF is notable for containing both fluoride for remineralization and silver particles for antibacterial effects. However, SDF causes severe discoloration. Thus, this study evaluated the remineralization effect and discoloration by NSF, a different form of fluoride-based agent containing silver, and assessed the synergistic effect of adding arginine to NSF.
In the TEM analysis, NSF and NSF + Arg were successfully synthesized15, and nearly spherical AgNPs with diameters ranging from 3 to 18 nm were identified. The addition of arginine did not change the shape of the NPs but affected the uniformity of the distribution and size, thereby promoting a more stable formulation24,25. Arginine exhibits a strong affinity for silver ions34, allowing it to bind at various electron-rich sites such as the nitrogen atoms in the α-amino groups and guanidino side chains, as well as the carboxyl groups at the C-terminus, resulting in the formation of stable silver–arginine complexes35. In contrast to colloidal AgNPs, immobilized AgNPs are more physicochemically stable because they are less susceptible to aggregation and oxidation in aqueous environments36. This stability ensures their long-term antibacterial effectiveness and reduces potential toxic effects37.
The remineralization of NSF and NSF + Arg on artificial enamel caries lesions was evaluated using several indicators. SMH measurements revealed that NSF + Arg did not significantly improve the remineralization of artificial enamel caries lesions compared with other groups (p > 0.05) but tended to successfully remineralize. This finding indicates that arginine not only enhances nanoparticles stability but also increases fluoride uptake, facilitating more effective remineralization38. The MG and remineralization percentage using micro-CT analysis supported these findings, showing higher percent remineralization values in the NSF + Arg group after remineralization treatment than in the NSF group.
NSF + Arg demonstrated enhanced performance, which can be explained by the unique properties of arginine. Arginine promotes the uniform distribution of nanoparticles and reduces particle size, which increases the surface area for interaction with the enamel24,25. Furthermore, arginine acts as a stabilizer and a reducing agent, preventing nanoparticles agglomeration and maintaining their activity over time39. Arginine aids in fluoride uptake in enamel lesions38 contributing to the formation of a more robust and acid-resistant fluorapatite layer and remineralization of early enamel caries33. Various in vitro40 and clinical studies28 have demonstrated the synergistic effect of arginine and fluoride, showing superior performance to fluoride alone. Arginine has an overall positive charge because of the positively charged guanidinium group attached to the alpha carbon of the amino acid. This guanidinium segment attracts electronegative components, including fluoride, inducing the formation of arginine–F complexes41,42. In addition, in oral biofilms, arginine is metabolized by arginolytic bacteria such as Streptococcus sanguinis and Streptococcus gordonii through the arginine deiminase system, preventing tooth demineralization31,32.
Notably, NSF and NSF + Arg groups demonstrated a significant reduction in discoloration. In the measurements of color change (ΔE) after remineralization, the SDF group showed the largest color change with a noticeable black stain on the enamel surface, whereas the NSF and NSF + Arg group showed significantly lower color change than the SDF group (p < 0.05) (Table 3). NSF also contains silver, which is the major cause of discoloration in SDF; however, it only causes less discoloration because its shapes and properties are different from those of SDF. The silver particles in NSF have nanosizes (Fig. 3), and these nanoparticles have a large surface area, providing a high antibacterial effect while having a milder discoloration through oxidation and precipitation15. In the aqueous medium, chitosan tends to agglomerate and adhere to surfaces43. Therefore, after applying NSF, a yellowish stain that can be easily removed may appear on the enamel surface. In this study, such a film-like stain was removed by the subsequent pH cycling after NSF application, resulting in the lack of significant difference in the measured values after pH cycling compared with the NaF varnish or the control group. In addition, arginine stabilized the AgNPs while maintaining a smaller particle size distribution, reducing oxidation reactions24,25 and thereby lessening black staining.
In this study, NSF + Arg did not cause enamel discoloration but did not show significant SMH or MG values compared with other groups and showed improved effects than NaF, NSF, and control groups only in percent normalization. Several factors can explain this limited effect. One of the main factors is the difference in the application time for each experimental group. Fluoride formulations tend to have a longer-lasting anti-caries effect with prolonged contact time with teeth. A longer contact allows fluoride ions to penetrate deeper into the enamel, aiding in remineralization and formation of a protective layer of calcium fluoride on the tooth surface44. NaF has a higher viscosity than SDF, NSF, and NSF + Arg. To reflect this in the experimental design, the NaF group had a contact time of 24 h with the remineralizing agent, whereas the other groups had a contact time of only 3 min. As a result, its efficacy was sufficiently demonstrated because it exhibited comparable remineralization effects despite a much shorter contact time, which is consistent with previous studies22,23,45.
However, this study used a chemical pH-cycling model to mimic clinical conditions, so it cannot fully replicate those conducted in vivo conditions such as saliva and dental plaque. Thus, further experiments in microcosmic biofilm models are needed rather than in environments that use only a single bacterium, or in vivo studies should be conducted.
Considering the aesthetic concerns associated with the dark staining effect of SDF, it is a very interesting issue to explore alternative treatment options that offer both safety and effectiveness. A study reported by Targino et al.15, the authors found that NSF exhibited significantly lower cytotoxicity towards human erythrocytes than SDF. The authors also stated that NSF is more biocompatible, does not cause discoloration, and has antibacterial effects at much lower doses15, more cost effective, and reliable46,47, which make it a possible alternative material to SDF. In our study, arginine was added to NSF formulation, which was reported to be not toxic on human gingival fibroblasts (HGF-1) in low concentration48. Arginine-containing NSF was successfully synthesized, and spherical-shaped AgNPs with diameters of 3–18 nm in this compound were identified. The NSF + Arg formulation could be an alternative to SDF owing to its ability to remineralize early caries lesions without causing black staining. However, in future studies is recommended to investigate the cytotoxic effect of NSF + Arg before its clinical application.
Methods
Preparation of NSF and NSF-Arginine
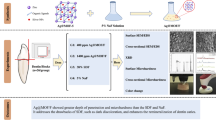
NSF was synthesized according to the protocol by Targino et al.15 Chitosan (1.0 g; Tokyo Chemical Industry Co., Ltd., Portland, OR, USA) was dissolved in 200 mL of 2% (v/v) acetic acid solution [CH₃COOH] (Daejung Co., Ltd., Busan, Korea). Then, 60 mL of the chitosan solution was transferred to an ice bath with continuous stirring and then added with 0.012 mol/L silver nitrate [AgNO₃] (Alfa Aesar, LLC, Haverhill, MA, USA). After 30 min of stirring, sodium borohydride [NaBH₄] (Sigma-Aldrich, Darmstadt, Hesse, Germany) was added dropwise maintaining an AgNO₃ to NaBH₄ mass ratio of 1:6. Subsequently, 11,310 ppm of 2.5% sodium fluoride [NaF] (Sigma-Aldrich, Darmstadt, Hesse, Germany) was added and stirred until completely dissolved. The colloidal solution was then stored at 4 °C.
NSF + Arg was synthesized by adding 5 mg/mL L-Arginine [C6H14N4O2] (Sigma-Aldrich, Darmstadt, Hesse, Germany) to the chitosan solution to enhance stability and control the nanoparticle size24,25. The concentration of arginine in NSF was determined based on the results of our pilot study, which tested various arginine concentrations in NSF.
Characterization of NSF and NSF + Arg
The shape, size, and shape of the AgNPs of NSF and NSF + Arg were evaluated by TEM (JEM-F200, Akishima, Tokyo, Japan) at 200 kV. A drop of NSF and NSF + Arg was placed separately on a carbon-coated copper grid and allowed to evaporate overnight at room temperature before TEM analysis. The diameter of each nanoparticle was measured using ImageJ2 (National Institutes of Health, Bethesda, MD, USA), and to obtain the average size of all particles in each sample, data were processed using Origin 2023 (version 10.5.113.50894).
Specimen preparation
This study was approved by the Ethics Committee of the Gangnam Severance Hospital (IRB approval no. 3–2023-0115). In total, 25 human sound permanent molars extracted for therapeutic purposes without early carious lesions, developmental anomalies, or any other defects were obtained from the Human Derivatives Bank at Gangnam Severance Hospital. Debris on the surface of all teeth was removed by perio-curette. Under a microscope (Olympus® BX40, Shibuya, Tokyo, Japan) at × 20, teeth were examined to check for the presence of crack lines and confirm the absence of cracks or any other defects. They were stored in 0.1% thymol solution to inhibit microbial growth.
The root part was removed up to approximately 1 mm above the cementoenamel junction. The smooth surface of the crown was longitudinally sectioned using IsoMet Low Speed (Bueller, Lake Bow, IL, USA) and Wafering Blade (Allied High-Tech Products, Inc., Compton, CA, USA) to obtain 3 enamel slabs approximately 2 mm thick, which was obtained 3 per tooth (Fig. 5). A total of 75 enamel slabs were embedded in acrylic resin using a Teflon mold (Polycoat EC304; Aektung Chemical Co., Ltd., Seoul, Korea). The enamel surface was polished gradually with a water-cooled rotating polishing machine (Ecomet 30, Buehler Ltd., Lake Bluff, IL, USA) using a series of sanding papers (600–2000 grit; SiC Sandpaper & Foil, R@B Inc., Daejeon, Korea) until a flat surface was achieved. The enamel specimens were covered with two layers of acid-resistant nail varnish, except for a window of 2 × 3 mm, and then cleaned and stored in 100% relative humidity at 4 °C to avoid dehydration.
Demineralization of the samples
The demineralization solutions were prepared according to the protocols described by 10 Cate and Duijsters49 using 2.2 mM Ca (NO3)2, 2.2 mM KH2PO4 and 0.1 ppm NaF, 50 mM acetic acid, and 1 M KOH, and the pH was adjusted to 4.5. Each specimen was immersed in 10 mL of the demineralizing solution for 96 h to create caries-like lesions and cleaned with deionized water. Each enamel surface sample was scanned once; one-half of the surface was demineralized, and the other was remineralized.
Application of remineralization agent
Enamel specimens were randomly allocated to five groups (n = 15) according to remineralization treatment agents (Table 4). Before the application of the remineralizing agent, the surface of the enamel of each specimen was dried.
Group I (NaF): 2.5% sodium fluoride varnish (FluoriMax, Elevate Oral Care, West Palm Beach, FL, USA) was applied with a microbrush to the enamel surface. Then, the samples were stored in a moist environment for 24 h. Afterward, taking care not to touch the enamel surface, the fluoride varnish was slowly removed with a scalpel blade and a cotton swab soaked in acetone and washed with deionized water for 1 min.
Group II (SDF): 5 μL of 12% SDF (Dengen Dental, Bahadurgarh, Haryana, India) was applied by a microbrush to dried surfaces of enamel specimens and left in contact for approximately 3 min. To remove any excess SDF, the specimen was washed with deionized water and gently dried with absorbent paper. The specimens were kept at 25 °C for 30 min.
Group III (NSF): The same protocol as group II was followed, and NSF was applied instead of SDF.
Group IV (NSF + Arg): The same protocol as group II was followed, and NSF + Arg was used instead of SDF.
Group V (control): The specimens were cleaned in deionized water, and no remineralizing agent was applied.
pH-cycling regime
After the application of the remineralizing agents, pH cycling was performed on the specimens under static conditions for 10 days50. They were immersed for 20 h in a remineralization solution composed of 1.5 mM CaCl₂·2H₂O, 0.9 mM KH₂PO₄, 150 mM KCl, and 20 mM HEPES. The pH of this solution was adjusted to 7 using KOH, approximating the neutral pH generally found in oral conditions. This was followed by 4 h of immersion in a demineralization solution consisting of 2.25 mM CaCl₂·2H₂O, 1.35 mM KH₂PO₄, 130 mM KCl, and 50 mM acetic acid, with the pH adjusted to 4.5 using KOH to mimic the acidic conditions that can lead to tooth demineralization (Table 5).
Moreover, pH levels were measured using a digital pH meter (Orion Star™ A211 Benchtop, Thermo Fisher Scientific Inc., Jakarta, Indonesia). Throughout the experiment, each sample was kept individually in separate containers at room temperature without stirring. To avoid the saturation or depletion of the solution, the demineralizing and remineralizing solutions were renewed daily. The composition of the solutions was designed to mimic the supersaturation of apatite minerals found in the saliva.
Scanning electron microscopy
After pH cycling, three specimens from each group were chosen to observe the enamel surface morphology. All specimens were dried and coated with a 100 Å layer of platinum using an ion coater (E-1010, Hitachi, Tokyo, Japan). The enamel surfaces were examined by SEM (S-3000N, Hitachi, Tokyo, Japan) with an accelerating voltage of 15 kV (× 10,000).
Surface microhardness
Specimens were set at least 30 min to dry to standardize the measurements. SMH assessment was taken using a Vickers microhardness measurement device (MMT-X, Matsuzawa Co., Ltd., Akita-shi, Akita Pref, Japan) at 200 g load for 15 s. Three indentations were made on the enamel surface at each test point spaced 100 μm apart. The mean SMH values of each sample were calculated at three time intervals:
SMH0: SMH of the sound enamel.
SMH1: SMH of the enamel after demineralization.
SMH2: SMH of the enamel after pH cycling.
The specimen was continuously stored at approximately 100% relative humidity at 4℃, except for the SMH measurement. The measured SMH value was expressed as the relative rate of the change of the sound enamel to SMH.
Micro-computed tomography
Specimens were scanned in a micro-CT (Skyscan 1076, Bruker/Skyscan, Kontich, Belgium) to assess the MD. Grayscale values obtained from the scans were converted to MD values using the hydroxyapatite phantom (1 g/cm3). Scan settings were as follows: 17-μm voxel size, 100 kV, 500 μA, 0.5° rotation angle, 360° scan, 0.5 mm aluminum filter, 1475 ms exposure/integration time, and frame average of 1. The total scan time per sample was approximately 21 min.
MD was evaluated at three different time points: before artificial caries-like lesion formation (T0, baseline), after caries-like lesion formation (T1, postdemineralization), and after treatment (T2, post pH cycling). Three distinct Sects. (9 μm each) for MD evaluation were randomly selected with approximately 77 μm depth at T0, T1 and T2.
The MG and percent remineralization were computed using the following Eq. 40.
(ΔZd – MD difference between T0 and T1, ΔZr – MD difference between T2 and T0).
Color change
Spectrophotometric color measurements were taken with the VITA Easyshade® advance V portable dental spectrophotometer (VITA Zahnfabrik GmbH, Bad Säckingen, Germany). Color measurements of the enamel surface were recorded at three time points (T0, T1, and T2). The International Commission on Illumination (CIE) L*, a*, and b* color coordinates of each specimen were measured. These coordinate values were measured to calculate the following: ΔL = L(T2) – L(T1); Δa = a(T2) – a(T1); and Δb = b(T2) – b(T1). Then, the following mathematical equation was used to determine the degree of the color difference ∆E = [(∆L)2 + (∆a)2 + (∆b)2]1/2.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the normality of SMH, MG, percent remineralization, and color change results. SMH data did not follow a normal distribution; thus, the Kruskal–Wallis test was applied, followed by Dunn’s test to evaluate between-group differences. The Wilcoxon signed-rank test was applied for within-group comparisons. Data for MG, percent remineralization, and color changes followed a normal distribution and were analyzed using a one-way analysis of variance with Duncan’s post-hoc test. A p-value of < 0.05 was considered significant. Statistical analyses were performed using IBM SPSS Statistics version 29.0 (SPSS Inc., Chicago, IL, USA). The level of significance was set at α = 0.05.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Machale, P., Shetiya, S. & Agarwal, D. The Incipient Caries. J. Contemp. Dent. 3, 20–24 (2013).
Walsh, L. & Brostek, A. Minimum intervention dentistry principles and objectives. Aust. Dent. J. 58(s1), 3–16 (2013).
Juntavee, A., Juntavee, N. & Sinagpulo, A. N. Nano-Hydroxyapatite Gel and Its Effects on Remineralization of Artificial Carious Lesions. Int. J. Dent. 2021(1), 7256056 (2021).
Welk, A., Ratzmann, A., Reich, M., Krey, K. & Schwahn, C. Effect of self-assembling peptide P11–4 on orthodontic treatment-induced carious lesions. Sci. Rep. 10(1), 6819 (2020).
Epasinghe, D., Yiu, C. & Burrow, M. Effect of flavonoids on remineralization of artificial root caries. Aust. Dent. J. 61(2), 196–202 (2016).
Agob, J. N., Aref, N. S. & Al-Wakeel, E. E. S. Effect of casein phosphopeptide-amorphous calcium phosphate on fluoride release and micro-shear bond strength of resin-modified glass ionomer cement in caries-affected dentin. Restor. Dent. Endod. 43, 4 (2018).
Weyant, R. J. et al. Topical fluoride for caries prevention. J. Am. Dent. Assoc. 144(11), 1279–1291 (2013).
Buzalaf, M. A. R., Pessan, J. P., Honório, H. M. & Ten Cate, J. M. Mechanisms of action of fluoride for caries control. Monogr. Oral Sci. 22(22), 97–114 (2011).
Featherstone, J. D. Prevention and reversal of dental caries: role of low level fluoride. Commun. Dent. Oral Epidemiol. 27(1), 31–40 (1999).
Chan, A. K. Y. et al. Clinical evidence for professionally applied fluoride therapy to prevent and arrest dental caries in older adults: A systematic review. J. Dent. 125, 104273 (2022).
Ainoosah, S. E., Levon, J., Eckert, G. J., Hara, A. T. & Lippert, F. Effect of silver diamine fluoride on the prevention of erosive tooth wear in vitro. J. Dent. 103, 100015 (2020).
Almuqrin, A., Kaur, I. P., Walsh, L. J., Seneviratne, C. J. & Zafar, S. Amelioration strategies for silver diamine fluoride: moving from black to white. Antibiotics 12(2), 298 (2023).
Roberts, A. et al. Does potassium iodide application following silver diamine fluoride reduce staining of tooth?. Syst. Rev. Aust. Dent. J. 65(2), 109–117 (2020).
Zhao, I. S., Mei, M. L., Burrow, M. F., Lo, E.C.-M. & Chu, C.-H. Effect of silver diamine fluoride and potassium iodide treatment on secondary caries prevention and tooth discolouration in cervical glass ionomer cement restoration. Int. J. Mol. Sci. 18(2), 340 (2017).
Targino, A. G. R. et al. An innovative approach to treating dental decay in children A new anti-caries agent. J. Mater. Sci. Mater. Med. 25(8), 2041–2047 (2014).
Yin, I. X. et al. Use of silver nanomaterials for caries prevention: a concise review. Int. J. Nanomed. 2020(15), 3181–3191 (2020).
Qasim, S. S. B., Ali, D., Soliman, M. S. & Zafiropoulos, G.-G. The effect of chitosan derived silver nanoparticles on mechanical properties, color stability of glass ionomer luting cements. Mater. Res. Express 8(8), 085401 (2021).
Torres-Mendez, F. et al. Effects of silver nanoparticles on the bonding of three adhesive systems to fluorotic enamel. Dent. Mater. J. 36(3), 266–274 (2017).
Nozari, A., Ajami, S., Rafiei, A. & Niazi, E. Impact of nano hydroxyapatite, nano silver fluoride and sodium fluoride varnish on primary teeth enamel remineralization: an in vitro study. J. clin. diagn. res. 11(9), ZC97–ZC100 (2017).
Akyildiz, M. & Sönmez, I. S. Comparison of remineralising potential of nano silver fluoride, silver diamine fluoride and sodium fluoride varnish on artificial caries: an in vitro study. Oral Health Prev. Dent. 17(5), 469–477 (2019).
Ammar, N., El-Tekeya, M. M., Essa, S., Essawy, M. M. & Talaat, D. M. Antibacterial effect and impact on caries activity of nanosilver fluoride and silver diamine fluoride in dentin caries of primary teeth: a randomized controlled clinical trial. BMC Oral Health 22(1), 657 (2022).
El-Desouky, D. I. et al. Preventive potential of nano silver fluoride versus sodium fluoride varnish on enamel caries like lesions in primary teeth: in vitro study. BMC Oral Health 22(1), 244 (2022).
Silva, A. et al. In Vitro morphological, optical and microbiological evaluation of nanosilver fluoride in the remineralization of deciduous teeth enamel. Nanotechnol. Rev. 7(6), 509–520 (2018).
Wu, F., Liu, D., Wang, T., Li, W. & Zhou, X. Different surface properties of l-arginine functionalized silver nanoparticles and their influence on the conductive and adhesive properties of nanosilver films. J. Mater. Sci. Mater. Electron. 26, 6781–6786 (2015).
Hu, B., Wang, S.-B., Wang, K., Zhang, M. & Yu, S.-H. Microwave-assisted rapid facile “green” synthesis of uniform silver nanoparticles: self-assembly into multilayered films and their optical properties. J. Phys. Chem. C 112(30), 11169–11174 (2008).
Agnello, M. et al. Arginine improves pH homeostasis via metabolism and microbiome modulation. J. Dent. Res. 96(8), 924–930 (2017).
Huang, X. et al. Effect of arginine on the growth and biofilm formation of oral bacteria. Arch. Oral Biol. 82, 256–262 (2017).
Kraivaphan, P. et al. Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res. 47(6), 582–590 (2013).
Xue, Y. et al. Effect of toothpaste containing arginine on dental plaque—A randomized controlled in situ study. J. Dent. 67, 88–93 (2017).
Yin, W. et al. The anti-caries efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride as sodium monofluorophosphate assessed using quantitative light-induced fluorescence (QLF). J. Dent. 41, S22–S28 (2013).
Zheng, X. et al. Ecological effect of arginine on oral microbiota. Sci. Rep. 7(1), 7206 (2017).
Nascimento, M. M. & Burne, R. A. Caries prevention by arginine metabolism in oral biofilms: translating science into clinical success. Curr. Oral Health Rep. 1, 79–85 (2014).
Bijle, M. N., Abdalla, M. M., Chu, C. H. & Yiu, C. K. Y. Synbiotic-fluoride synergism on enamel remineralization, cytotoxicity and genotoxicity. J. Dent. 128, 104356 (2023).
Shoeib, T., Siu, K. M. & Hopkinson, A. C. Silver ion binding energies of amino acids: use of theory to assess the validity of experimental silver ion basicities obtained from the kinetic method. J. Phys. Chem. A 106(25), 6121–6128 (2002).
Agnihotri, S., Bajaj, G., Mukherji, S. & Mukherji, S. Arginine-assisted immobilization of silver nanoparticles on ZnO nanorods: an enhanced and reusable antibacterial substrate without human cell cytotoxicity. Nanoscale 7(16), 7415–7429 (2015).
Lv, M. et al. Long-term antimicrobial effect of silicon nanowires decorated with silver nanoparticles. Adv. Mater. 48(22), 5463–5467 (2010).
Agnihotri, S., Mukherji, S. & Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver. Nanoscale 5(16), 7328–7340 (2013).
Bijle, M. N. et al. Concentration-dependent multi-potentiality of L-arginine: Antimicrobial effect, hydroxyapatite stability, and MMPs inhibition. Molecules 26(21), 6605 (2021).
Xu, L. et al. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 10(20), 8996 (2020).
Bijle, M. N. A., Ekambaram, M., Lo, E. C. & Yiu, C. K. Y. The combined enamel remineralization potential of arginine and fluoride toothpaste. J. Dent. 76, 75–82 (2018).
Bijle, M. N. A. et al. Enhancing the remineralization potential of child formula dentifrices: An in vitro study. J. Clin. Pediatr. Dent. 43(5), 337–344 (2019).
Favaro, J. C. et al. Anticaries agent based on silver nanoparticles and fluoride: characterization and biological and remineralizing effects—an in vitro study. Int. J. Dent. 2022(1), 9483589 (2022).
Espíndola-Castro, L., Rosenblatt, A., Galembeck, A. & Monteiro, G. Dentin staining caused by nano-silver fluoride: a comparative study. Oper. Dent. 45(4), 435–441 (2020).
Pichaiaukrit, W., Thamrongananskul, N., Siralertmukul, K. & Swasdison, S. Fluoride varnish containing chitosan demonstrated sustained fluoride release. Dent. Mater. J. 38(6), 1036–1042 (2019).
Teixeira, J. A. et al. Effects of a new nano-silver fluoride-containing dentifrice on demineralization of enamel and streptococcus mutans adhesion and acidogenicity. Int. J. Dent. 2018(1), 1351925 (2018).
dos Santos Jr, V. E. et al. A new “silver-bullet” to treat caries in children–nano silver fluoride: a randomised clinical trial. J. Dent. 42(8), 945–951 (2014).
Tirupathi, S., Nirmala, S., Rajasekhar, S. & Nuvvula, S. Comparative cariostatic efficacy of a novel Nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J. Clin. Exp. Dent. 11(2), e105 (2019).
Bijle, M. N., Ekambaram, M., Lo, E. C. & Yiu, C. K. Y. The enamel remineralization potential of fluoride varnishes containing arginine. J. Dent. 99, 103411 (2020).
Ten Cate, J. & Duijsters, P. Influence of fluoride in solution on tooth demineralization: I. Chem. data. Caries Res. 17(3), 193–199 (1983).
Lippert, F. & Juthani, K. Fluoride dose-response of human and bovine enamel artificial caries lesions under pH-cycling conditions. Clin. Oral Investig. 19(8), 1947–1954 (2015).
Author information
Authors and Affiliations
Contributions
A.S.A. contributed to conception, design, data analysis, and interpretation, drafted the manuscript; MJ.J. contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; JW.P. contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study was approved by the Ethics Committee of the Gangnam Severance Hospital (IRB approval no. 3–2023-0115). All biological samples were included after obtaining the informed consent from all subjects. All methods were conducted in accordance with Declarations of Helsinki.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Albahoth, A.S., Jeon, MJ. & Park, JW. Synergistic effect of nanosilver fluoride with L-arginine on remineralization of early carious lesions. Sci Rep 15, 5993 (2025). https://doi.org/10.1038/s41598-025-89881-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89881-6
Keywords
This article is cited by
-
Integrated salivary microbiome and metabolome profiling reveals ecological and functional alterations in severe early childhood caries
Journal of Translational Medicine (2025)
-
Preparation of silver-loaded metal-organic frameworks as a novel agent for arresting dental caries of primary teeth
Scientific Reports (2025)